Abstract
Background: Elevated serum phosphorus is associated with all-cause mortality, but little is known about risk associated with dietary phosphorus intake.
Objective: We investigated the association between phosphorus intake and mortality in a prospective cohort of healthy US adults (NHANES III; 1998–1994).
Design: Study participants were 9686 nonpregnant adults aged 20–80 y without diabetes, cancer, or kidney or cardiovascular disease. Exposure to dietary phosphorus, which was assessed by using a 24-h dietary recall, was expressed as the absolute intake and phosphorus density (phosphorus intake divided by energy intake). All-cause and cardiovascular mortality was assessed through 31 December 2006.
Results: Median phosphorus intake was 1166 mg/d (IQR: 823–1610 mg/d); median phosphorus density was 0.58 mg/kcal (0.48–0.70 mg/kcal). Individuals who consumed more phosphorus-dense diets were older, were less often African American, and led healthier lifestyles (smoking, physical activity, and Healthy Eating Index). In analyses adjusted for demographics, cardiovascular risk factors, kidney function, and energy intake, higher phosphorus intake was associated with higher all-cause mortality in individuals who consumed >1400 mg/d [adjusted HR (95% CI): 2.23 (1.09, 4.5) per 1-unit increase in ln(phosphorus intake); P = 0.03]. At <1400 mg/d, there was no association. A similar association was seen between higher phosphorus density and all-cause mortality at a phosphorus density amount >0.35 mg/kcal [adjusted HR (95% CI): 2.27 (1.19, 4.33) per 0.1-mg/kcal increase in phosphorus density; P = 0.01]. At <0.35 mg/kcal (approximately the fifth percentile), lower phosphorus density was associated with increased mortality risk. Phosphorus density was associated with cardiovascular mortality [adjusted HR (95% CI): 3.39 (1.43, 8.02) per 0.1 mg/kcal at >0.35 mg/kcal; P = 0.01], whereas no association was shown in analyses with phosphorus intake. Results were similar by subgroups of diet quality and in analyses adjusted for sodium and saturated fat intakes.
Conclusions: High phosphorus intake is associated with increased mortality in a healthy US population. Because of current patterns in phosphorus consumption in US adults, these findings may have important public health implications.
See corresponding editorial on page 247.
INTRODUCTION
Phosphorus is an essential mineral for cell structure and function. In the United States, the average person consumes far more phosphorus than the Recommended Dietary Allowance (RDA)4 (700 mg/d for adults), although very few individuals exceed the tolerable upper limit (4000 mg/d for adults <70 y old) (1). The tolerable upper limit or the highest average phosphorus intake thought to be safe in a general population was extrapolated from studies that examined the effect of infusing a neutral phosphate solution intravenously to produce controlled hyperphosphatemia (2). Although it is well established that high concentrations of serum phosphorus are associated with increased risk of cardiovascular events and mortality (3–7), the evidence linking dietary phosphorus intake to serum phosphorus is sparse. Hence, to our knowledge, the long-term health risks associated with high dietary phosphorus intake are unknown.
Excessive dietary phosphorus intake may be harmful even in the absence of high serum phosphorus concentrations. Serum phosphorus concentrations are tightly regulated by parathyroid hormone and fibroblast growth factor-23 (FGF-23), which is a hormone that increases urinary phosphorus excretion. Individuals with normal kidney function are largely able to maintain serum phosphorus in a physiologic range, even in the setting of high phosphorus consumption because increased phosphorus consumption leads to physiologic increases in parathyroid hormone and FGF-23 (8–10). Over the long term, high FGF-23 concentrations may stimulate left ventricular hypertrophy (11), and epidemiologic studies have linked high FGF-23 concentrations with heart failure (12), cardiovascular events, chronic kidney disease (CKD) progression, and mortality (7, 13).
To investigate the potential health risks of high phosphorus intake, we evaluated the association of dietary phosphorus intake and mortality by using a nationally representative sample of healthy participants in the NHANES III. We evaluated for a threshold above which mortality risk increases and the consistency of the effect across subgroups of dietary quality (14, 15).
SUBJECTS AND METHODS
Study population
Participants were drawn from the NHANES III (1988–1994), which is a nationally representative sample of the civilian, noninstitutionalized US population. Details of the survey design have been reported previously (14). We included nonpregnant individuals aged 20–80 y with complete data on mortality status, diet, and relevant covariates (n = 12,366). Because dietary habits may change because of chronic illness, we excluded 2387 individuals with diabetes (on the basis of a self-report, the use of diabetes medications, or a fasting glucose concentration >126 mg/dL), CKD stage ≥3 [estimated glomerular filtration rate (eGFR) <60 mL · min−1 · 1.73 m−2) (15), or a self-reported history of myocardial infarction, congestive heart failure, stroke, or cancer. We also excluded 237 individuals who reported extreme energy intakes (<1st and >99th percentiles of energy intake/d), for a final sample of 9686 participants.
Dietary phosphorus measures
In-person 24-h dietary recalls were administered in mobile examination centers by trained interviewers, and dietary nutrient and energy components were determined from the USDA Survey Nutrient Database (14). Because it is unclear whether the absolute nutrient intake or nutrient density (nutrient intake divided by total energy intake) is optimal for defining relations between nutrients and outcomes (16), the exposure to dietary phosphorus was assessed as the absolute phosphorus intake and phosphorus density. The absolute phosphorus intake (mg/d) was log transformed to achieve a more normal distribution and modeled continuously by using linear splines with the mkspline command in STATA software (version 11.2; StataCorp). Linear splines were used to better characterize the shape of the association because the mortality risk association significantly changed at a knot of ln(1400 mg/d). We also modeled the absolute phosphorus intake as a binary variable that corresponded to the highest quartile [quartile 4 (≥1611 mg/d) compared with quartiles 1–3 (<1611 mg/d)]. Phosphorus density (mg/kcal) was modeled as a continuous variable by using linear splines (knot at 0.35 mg/kcal, which corresponded to 700 mg for a 2000-kcal diet) on the basis of a visual inspection of locally weighted smoothing plots, and a significant change in the mortality risk association at this knot.
A subsample of ∼8% of adult participants provided a second 24-h dietary recall. Correlations in the subset with a second dietary recall were 0.47 (P < 0.001) for the absolute phosphorus intake and 0.44 (P < 0.001) for phosphorus density.
Covariates
Age, sex, race, ethnicity, cigarette smoking (never, former, or current), physical activity, and family income were self-reported. Physical activity was assessed by the number of moderate to vigorous intensity activities (eg, walking, jogging, running, bicycling, swimming, dancing, or yard work) performed per week. Socioeconomic status was captured as the poverty income ratio (PIR) or the ratio of family income to the federal poverty threshold (specific to the year of the interview); poverty was defined as a PIR ≤1. The Healthy Eating Index (HEI) data file was used to quantify food-group consumption and the overall quality of diet according to nutritional guidelines at the time (HEI from 0 to 100, with 100 being the best-quality diet). Soda consumption (regular and diet) was quantified by using USDA food codes 9240000–9241162. Food items were classified as fast food if they were consumed at fast-food, take-out, or delicatessen locations or were menu items from name-brand major chains such as Arby's, McDonald's, Pizza Hut, Taco Bell, and White Castle.
Height and weight were measured by using standardized methods, and BMI (in kg/m2) was calculated as weight divided by height squared (14). Hypertension was defined as a self-reported history of hypertension, measured systolic blood pressure ≥140 mmHg, measured diastolic blood pressure ≥90 mmHg, or the self-reported use of blood pressure medications.
Serum creatinine, phosphorus, and vitamin D concentrations were measured as previously described (14). Low vitamin D was defined by the lowest quintile of serum vitamin D concentration (<16.2 ng/mL). the estimated glomerular filtration rate (eGFR) was calculated by using the Chronic Kidney Disease Epidemiology Collaboration equation (17) after calibrating serum creatinine values to Cleveland Clinic reference values (18). The urine albumin:creatinine ratio (ACR) was assessed from random urine samples; microalbuminuria was defined by using sex-specific cutoffs as an ACR ≥17 g/g in men and ≥25 mg/g in women (19). In regression models, the ACR was log transformed because of its skewed distribution.
Outcomes
All-cause and cardiovascular mortality data were abstracted from the NHANES III mortality file, which captured the vital status and cause of death of survey participants from survey participation (1988–1994) to 31 December 2006 (20). Cardiovascular mortality was defined by International Classification of Diseases, 10th Edition, Clinical Modification System codes I00–I78 derived from death-certificate data (21).
Statistical analysis
All analyses incorporated sample weights to account for the complex survey design according to NHANES analytic guidelines (14). Means and proportions of baseline characteristics were compared across quartiles by using logistic regression for categorical variables and linear regression for continuous variables. SEs were estimated by using Taylor series linearization. Baseline nutrient and food-group intakes were standardized by the total energy intake, and reported means (±SEs) were, for a 2000-kcal diet, approximately the median total energy intake in our sample. The associations between phosphorus intake and all-cause and cardiovascular mortality were investigated by using Cox proportional hazards regression in 2 nested models with the following covariates: model 1 included age, sex, race (black compared with white), ethnicity (Mexican American compared with non–Mexican American), PIR, and total energy intake; model 2 included model 1 covariates and BMI (knot at 20), systolic blood pressure, current and former smoking, physical activity, non–HDL cholesterol (knot at 100 mg/dL), log ACR, eGFR, and low vitamin D concentration. All analyses reported in Results reflect adjustment for covariates in model 2 unless otherwise stated.
Because high phosphorus intake may be a marker of an unhealthy diet, subgroup analyses were done by HEI score (above and below the median), soda consumption (any or none), and fast-food consumption (any or none). We formally tested interactions between high absolute phosphorus intake (≥1611 compared with <1611 mg) and all-cause mortality by HEI score, soda consumption, and fast-food consumption. Main analyses were also repeated with additional adjustment of model 2 for sodium and saturated fat intakes.
Finally, because of the relation between high phosphorus intake and all-cause mortality, we evaluated for mediation by serum phosphorus concentrations by comparing associations between dietary phosphorus measures and all-cause mortality with and without serum phosphorus as a covariate. All analyses were performed with STATA software (version 11.2; StataCorp).
RESULTS
Baseline characteristics of healthy NHANES participants by quartiles of absolute phosphorus intake
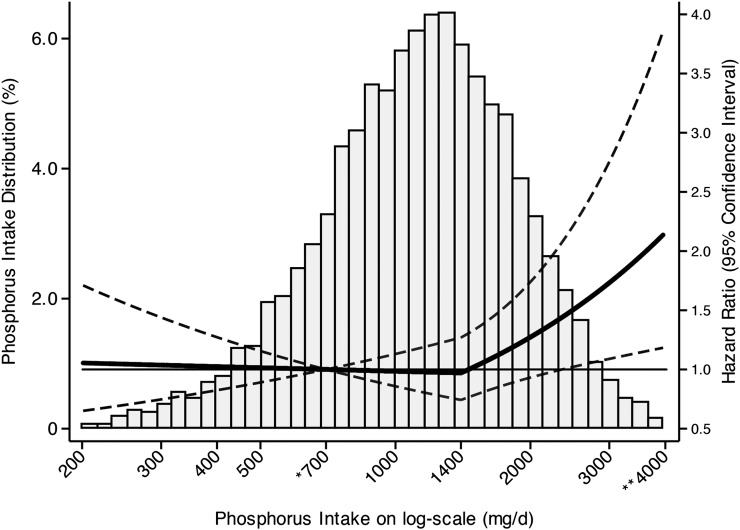
The median follow-up time was 14.7 y (IQR: 13.1–16.2 y). Most individuals (83.4%) consumed >700 mg P/d (RDA), whereas 35.1% of subjects consumed >2 times the RDA (1400 mg P/d), and only 0.2% of subjects consumed more than the tolerable upper limit (4000 mg P/d) (Figure 1). Median values of phosphorus consumption in the lowest to highest quartiles were 629, 993, 1356, and 1992 mg/d, respectively (Table 1). Individuals who consumed higher amounts of phosphorus were younger, more physically active, and more often male. Blacks were overrepresented in the lowest quartile of phosphorus consumption and underrepresented in the highest quartile [quartile 1 (14.9%) compared with quartile 4 (6.8%); P < 0.001]; in Mexican-Americans, the relation was reversed [quartile 1 (3.8%) compared with quartile 4 (6.4%); P < 0.001]. Higher phosphorus intake was associated with a higher vitamin D concentration, higher eGFR, and lower prevalent hypertension and microalbuminuria; however, only the relation with vitamin D persisted after adjustment for age, sex, and race and ethnicity (data not presented). Unadjusted serum phosphorus concentrations were not related to higher phosphorus intake although there was a positive association after adjustment for age, sex, race, and ethnicity (0.05 mg/dL per 1000 mg P/d; P < 0.001). Phosphorus consumption was directly associated with the total daily energy intake [quartile 1 (1309 kcal/d) compared with quartile 4 (3253 kcal/d); P < 0.001], higher intakes (as proportions of total energy intake) of sodium, calcium, protein, fat, dairy, meat, and legumes, and lower intakes of fruit, vegetables, and grains (P < 0.05 for all comparisons) (Table 2).
FIGURE 1.
Distribution and adjusted HRs (95% CIs) of death by absolute phosphorus intake. Cox proportional hazards regression was used to estimate HRs of mortality by absolute phosphorus intake by using linear splines with a knot at 1400 mg/d adjusted for age, sex, race, ethnicity, poverty:income ratio, total energy intake, BMI, systolic blood pressure, current and former smoking, physical activity, non–HDL cholesterol, log albumin:creatinine ratio, estimated glomerular filtration rate, and low vitamin D concentration. Values were centered at 700 mg/d, and the graph is truncated at 200 and 4000 mg/d for ease of presentation. *The Recommended Dietary Allowance (700 mg/d) represents the daily dietary intake of phosphorus considered sufficient by the Food and Nutrition Board to meet requirements for nearly all (97.5%) healthy adults; **the tolerable upper limit (4000 mg/d) is the highest average phosphorus intake that is likely to pose no adverse health effects to almost all individuals in a general population (1).
TABLE 1.
Baseline characteristics by absolute phosphorus intake quartiles1
| Quartile 1 (n = 2424) | Quartile 2 (n = 2422) | Quartile 3 (n = 2421) | Quartile 4 (n = 2419) | P | |
| Absolute phosphorus intake (mg/d) | 629 (503–737)2 | 993 (905–1078) | 1356 (1259–1471) | 1992 (1769–2355) | |
| Phosphorus density (mg/kcal) | 0.48 (0.40–0.58) | 0.55 (0.47–0.67) | 0.61 (0.51–0.71) | 0.67 (0.57–0.79) | <0.001 |
| Deaths/100 person-years3 | 2.51 ± 0.494 | 2.66 ± 0.71 | 2.43 ± 0.44 | 2.64 ± 0.64 | 0.5 |
| CVD deaths/100 person-years3 | 0.62 ± 0.08 | 1.58 ± 0.6 | 0.98 ± 0.4 | 0.68 ± 0.3 | 1.0 |
| Age (y) | 42.5 ± 0.5 | 42.6 ± 0.6 | 41.0 ± 0.5 | 38.5 ± 0.4 | <0.001 |
| M (%) | 23.5 | 38.2 | 53.0 | 75.6 | <0.001 |
| Black (%) | 14.9 | 10.6 | 8.6 | 6.8 | <0.001 |
| Mexican (%) | 3.8 | 4.9 | 5.0 | 6.4 | <0.001 |
| Poverty (%) | 13.8 | 11.5 | 9.8 | 10.1 | 0.001 |
| Less than high school education (%) | 23.7 | 20.8 | 17.8 | 18.2 | 0.001 |
| No. of moderate-vigorous activities/wk | 3.54 ± 0.16 | 3.81 ± 0.20 | 4.59 ± 0.21 | 4.68 ± 0.21 | <0.001 |
| BMI (kg/m2) | 26.2 ± 0.2 | 26.2 ± 0.2 | 26.1 ± 0.2 | 26.3 ± 0.2 | 0.7 |
| Current smoker (%) | 31.2 | 29.3 | 29.3 | 30.7 | 0.9 |
| Hypertension (%) | 19.6 | 17.6 | 16.0 | 15.8 | 0.02 |
| Microalbuminuria (%) | 8.9 | 8.4 | 7.4 | 6.4 | 0.005 |
| eGFR (mL · min−1 · 1.73 m−2) | 102.6 ± 0.7 | 101.6 ± 0.7 | 102.1 ± 0.6 | 104.4 ± 0.6 | 0.01 |
| Serum phosphorus (mg/dL) | 3.44 ± 0.02 | 3.43 ± 0.01 | 3.43 ± 0.02 | 3.44 ± 0.02 | 1.0 |
| Vitamin D (ng/dL) | 27.5 ± 0.7 | 28.8 ± 0.5 | 30.6 ± 0.6 | 32.6 ± 0.5 | <0.001 |
| Low vitamin D (%) | 17.2 | 13.4 | 8.4 | 5.8 | <0.001 |
Means and proportions of baseline characteristics were compared across quartiles by using logistic regression for categorical variables and linear regression for continuous variables. CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate.
Median; IQR in parentheses (all such values).
Age-standardized death rates.
Mean ± SE (all such values).
TABLE 2.
Baseline dietary characteristics by absolute phosphorus intake quartiles1
| Quartile 1 (n = 2424) | Quartile 2 (n = 2422) | Quartile 3 (n = 2421) | Quartile 4 (n = 2419) | P | |
| Absolute phosphorus intake (mg/d) | 629 (503–737)2 | 993 (905–1078) | 1356 (1259–1471) | 1992 (1769–2355) | |
| Phosphorus density (mg/kcal) | 0.48 (0.40–0.58) | 0.55 (0.47–0.67) | 0.61 (0.51–0.71) | 0.67 (0.57–0.79) | <0.001 |
| Total energy intake (kcal) | 1309 ± 113 | 1828 ± 17 | 2354 ± 17 | 3253 ± 28 | <0.001 |
| HEI4 score | 58.9 ± 0.4 | 65.2 ± 0.5 | 65.2 ± 0.5 | 63.7 ± 0.6 | <0.001 |
| Consumed any soda (%)5 | 56.9 | 58.8 | 59.2 | 61.2 | 0.07 |
| Consumed any fast food (%)6 | 14.9 | 18.1 | 19.4 | 17.4 | 0.2 |
| Nutrient and food group intakes standardized by total energy intake7 | |||||
| Sodium (mg/d) | 3149 ± 40 | 3269 ± 40 | 3291 ± 34 | 3364 ± 31 | <0.001 |
| Potassium (mg/d) | 2699 ± 46 | 2720 ± 25 | 2686 ± 25 | 2742 ± 33 | 0.5 |
| Calcium (mg/d) | 581 ± 9 | 705 ± 10 | 805 ± 13 | 944 ± 17 | <0.001 |
| Protein (g/d) | 67.4 ± 0.8 | 75.5 ± 0.7 | 77.6 ± 0.7 | 82.1 ± 0.7 | <0.001 |
| Total fat (g/d) | 70.0 ± 0.7 | 73.5 ± 0.8 | 76.1 ± 0.8 | 78.8 ± 0.9 | <0.001 |
| Saturated fat (g/d) | 22.4 ± 0.4 | 24.2 ± 0.3 | 25.7 ± 0.4 | 27.5 ± 0.4 | <0.001 |
| Fiber (g/d) | 15.4 ± 0.3 | 15.9 ± 0.2 | 15.9 ± 0.3 | 15.6 ± 0.2 | 0.9 |
| Grains (servings/d) | 6.69 ± 0.09 | 6.53 ± 0.11 | 6.41 ± 0.10 | 6.04 ± 0.10 | <0.001 |
| Fruit (servings/d) | 1.89 ± 0.09 | 1.54 ± 0.07 | 1.41 ± 0.05 | 1.23 ± 0.08 | <0.001 |
| Vegetables (servings/d) | 3.49 ± 0.10 | 3.44 ± 0.11 | 2.95 ± 0.07 | 2.86 ± 0.07 | <0.001 |
| Dairy (servings/d) | 1.14 ± 0.04 | 1.67 ± 0.05 | 2.02 ± 0.05 | 2.52 ± 0.06 | <0.001 |
| Meat (servings/d) | 1.95 ± 0.04 | 2.09 ± 0.04 | 2.08 ± 0.05 | 2.12 ± 0.04 | 0.01 |
| Legumes (servings/d) | 0.07 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.001 |
Means and proportions of baseline characteristics were compared across quartiles by using logistic regression for categorical variables and linear regression for continuous variables.
Median; IQR in parentheses (all such values).
Mean ± SE (all such values).
HEI, Healthy Eating Index.
Includes regular and diet sodas.
Includes food consumed at fast-food, takeout, and delicatessen locations or menu items from major fast-food chains.
Values of nutrients and food groups presented are for a 2000-kcal/d diet.
Baseline characteristics of healthy NHANES participants by quartiles of phosphorus density
In quartiles of phosphorus density (mg/kcal), median values of absolute phosphorus intake were 827, 1104, 1313, and 1478 mg/d in lowest to highest quartiles (see Supplemental Table 1 under “Supplemental data” in the online issue). Racial and ethnic associations with phosphorus density were similar to those observed by using the absolute phosphorus intake. In contrast, individuals who consumed diets with a higher phosphorus density were older and appeared to lead healthier lifestyles [HEI score: quartile 1 (60.1) compared with quartile 4 (66.2), P < 0.001; moderate-vigorous physical activity per week: quartile 1 (3.7) compared with quartile 4 (4.7), P < 0.001; current smoking: quartile 1 (38.6%) compared with quartile 4 (23.9%), P < 0.001] (see Supplemental Table 2 under “Supplemental data” in the online issue). Adjustment for age, sex, and race and ethnicity did not alter these relations (data not presented).
Phosphorus intake and all-cause mortality
Higher absolute phosphorus consumption was associated with higher all-cause mortality at amounts >1400 mg/d [adjusted HR (95% CI): 2.23 (1.09, 4.55) per 1-unit increase in ln(phosphorus intake); P = 0.03] (Figure 1). There was no significant association between absolute phosphorus intake and mortality below this threshold (Table 3). In analyses that used phosphorus density as the exposure, higher phosphorus density was associated with increased mortality risk at amounts >0.35 mg/kcal [adjusted HR (95% CI): 2.27 (1.19, 4.33) per 0.1-mg/kcal increase; P = 0.01] (Figure 2). In contrast, at amounts <0.35 mg/kcal (approximately the fifth percentile), there was decreased mortality risk with increasing phosphorus density [adjusted HR (95% CI): 0.46 (0.24, 0.89); P = 0.02] (Table 4). When analyses were additionally adjusted for sodium and saturated fat intakes, there were no material differences in results (data not presented).
TABLE 3.
Adjusted HRs (95% CIs) of all-cause and cardiovascular mortality according to absolute phosphorus intake1
| Model 1 | P | Model 2 | P | |
| All-cause mortality | ||||
| Continuous (/1-unit increase in ln[phosphorus intake (mg/d)]) | ||||
| Less than ln(1400 mg/d) | 0.78 (0.51, 1.20) | 0.2 | 0.96 (0.64, 1.43) | 0.8 |
| Greater than or equal to ln(1400 mg/d) | 2.43 (1.12, 5.27) | 0.03 | 2.23 (1.09, 4.55) | 0.03 |
| CVD2 mortality | ||||
| Continuous (/1-unit increase in ln[phosphorus intake (mg/d)]) | ||||
| Less than ln(1400 mg/d) | 0.89 (0.42, 1.88) | 0.8 | 0.99 (0.46, 2.14) | 1.0 |
| Greater than or equal to ln(1400 mg/d) | 1.06 (0.25, 4.46) | 0.9 | 1.02 (0.25, 4.21) | 1.0 |
Cox proportional hazards regression was used to estimate HRs of mortality by absolute phosphorus intake. Absolute phosphorus intake was log-transformed to achieve a more normal distribution and modeled continuously by using linear splines with a knot at ln(1400 mg/d) on the basis of evidence of a nonlinear relation. Model 1 was adjusted for age, sex, race, ethnicity, poverty:income ratio, and total energy intake. Model 2 was adjusted as for model 1 and for BMI, systolic blood pressure, current and former smoking, physical activity, non–HDL cholesterol, log albumin:creatinine ratio, estimated glomerular filtration rate, and low vitamin D concentration.
CVD, cardiovascular disease.
FIGURE 2.
Distribution and adjusted HRs (95% CIs) of death by phosphorus density. Cox proportional hazards regression was used to estimate HRs of mortality by phosphorus density by using linear splines with a knot at 0.35 mg/kcal adjusted for age, sex, race, ethnicity, poverty:income ratio, total energy intake, BMI, systolic blood pressure, current and former smoking, physical activity, non–HDL cholesterol, albumin:creatinine ratio, estimated glomerular filtration rate, and low vitamin D concentration. Values were centered at 0.35 mg/kcal, which corresponded to 700 mg/d for a 2000-kcal/d diet, and the graph is truncated at 0.25 and 1.5 mg/kcal for ease of presentation.
TABLE 4.
Adjusted HRs (95% CIs) of all-cause and cardiovascular mortality according to phosphorus density1
| Model 1 | P | Model 2 | P | |
| All-cause mortality | ||||
| Continuous [/0.1-unit increase in phosphorus density (mg/kcal)] | ||||
| <0.35 mg/kcal | 0.36 (0.20, 0.66) | 0.001 | 0.46 (0.24, 0.89) | 0.02 |
| ≥0.35 mg/kcal | 2.88 (1.59, 5.23) | 0.001 | 2.27 (1.19, 4.33) | 0.01 |
| CVD2 mortality | ||||
| Continuous [/0.1-unit increase in phosphorus density (mg/kcal)] | ||||
| <0.35 mg/kcal | 0.22 (0.10, 0.48) | <0.001 | 0.30 (0.13, 0.73) | 0.01 |
| ≥0.35 mg/kcal | 4.74 (2.12, 10.6) | <0.001 | 3.39 (1.43, 8.02) | 0.01 |
Cox proportional hazards regression was used to estimate HRs of mortality by phosphorus density. Phosphorus density was modeled as a continuous variable by using linear splines (knot at 0.35 mg/kcal, which corresponded to 700 mg for a 2000-kcal/d diet) on the basis of a visual inspection of locally weighted smoothing plots. Model 1 was adjusted for age, sex, race, ethnicity, poverty:income ratio, and total energy intake. Model 2 was adjusted as for model 1 and for BMI, systolic blood pressure, current and former smoking, physical activity, non–HDL cholesterol, log albumin:creatinine ratio, estimated glomerular filtration rate, and low vitamin D concentration.
CVD, cardiovascular disease.
Phosphorus intake and cardiovascular mortality
Similar to associations seen with all-cause mortality, higher phosphorus density was associated with increased cardiovascular mortality risk at amounts >0.35 mg/kcal [adjusted HR (95% CI): 3.39 (1.43, 8.02) per 0.1-mg/kcal increase in phosphorus density; P = 0.01] (Table 4). At amounts <0.35 mg/kcal, higher phosphorus density was associated with lower cardiovascular mortality risk [adjusted HR (95% CI): 0.30 (0.13, 0.73); P = 0.01]. There was no significant association between the higher absolute intake of phosphorus and cardiovascular mortality.
Consistency of effect across subgroups of diet quality
In subgroup analyses, risk of all-cause mortality associated with the highest quartile of absolute phosphorus consumption (≥1611 compared with <1611 mg/d) did not differ by HEI score, soda consumption, and fast-food consumption (P-interaction > 0.05 for each).
Associations after the addition of serum phosphorus as a covariate
When serum phosphorus was added as a covariate to analyses, the association between absolute phosphorus intake and all-cause mortality was essentially unchanged [>1400 mg/d: adjusted HR (95% CI): 2.24 (1.09, 4.62); P = 0.03; no relation observed <1400 mg/d]. Similarly, the association between phosphorus density and all-cause mortality was unchanged after the addition of serum phosphorus [>0.35 mg/kcal, adjusted HR (95% CI): 2.26 (1.16, 4.43) (P = 0.02); <0.35 mg/kcal, adjusted HR (95% CI): 0.46 (0.24, 0.91) (P = 0.03)]. Serum phosphorus was associated with all-cause mortality in both analyses (absolute phosphorus intake analysis, adjusted HR (95% CI): 1.37 (1.13, 1.67) per 1-mg/dL increase in serum phosphorus (P = 0.002); phosphorus density analysis, adjusted HR (95% CI): 1.37 (1.13, 1.66) per 1-mg/dL increase in serum phosphorus; (P = 0.002)].
DISCUSSION
In this nationally representative sample of healthy US adults with normal kidney function, we showed that high phosphorus consumption was associated with increased all-cause mortality. The relation between increasing absolute phosphorus intake and mortality appeared flat until a threshold of ∼1400 mg/d, which is an amount of phosphorus consumption that is twice the adult US RDA. A similar continuous relation was seen with increasing phosphorus density and mortality at amounts >0.35 mg/d, which corresponded to 700 mg/d in subjects who consumed a 2000-kcal diet. Findings were also robust by the subgroup of dietary quality. These findings may have important public health implications because more than one-third of Americans reported phosphorus consumption that exceeded 1400 mg/d.
An accurate determination of associations between nutrient intake and adverse outcomes is complex because of a high correlation with and confounding by the total energy intake. To address this difficulty, several different methods have been proposed (16). We modeled phosphorus intake as the absolute intake with adjustment for the total energy intake as one method given the ease of interpretation. However, some researchers believe that modeling intake as nutrient density (nutrient intake standardized by total energy intake) can reduce bias because of underreporting or overreporting because nutrient and total calorie intakes tend to be strongly correlated (16). It is reassuring that analyses that used absolute phosphorus intake and phosphorus density were similar except in the range of very-low phosphorus density (lower than the fifth percentile), where lower phosphorus density was associated with increased mortality risk. It is plausible that this could signify increased risk from insufficient phosphorus consumption; in contrast, this finding might be driven by latent confounding because individuals who consumed the least phosphorus-dense diets exercised less, ate unhealthier diets, and were more likely to smoke.
Few previous studies have examined the association of dietary phosphorus intake and mortality (22). In contrast to our findings, urinary phosphorus excretion (a proxy for dietary phosphorus consumption) was not related to mortality in a cohort of elderly men and was inversely associated with mortality in persons with coronary artery disease (23, 24). However, total energy and phosphorus intakes may change with aging or poor health and, thus, inaccurately represent the average premorbid phosphorus consumption. Moreover, 24-h urine phosphorus may reflect abnormalities in phosphorus metabolism, particularly in sicker populations (25). Because of the concern about reverse causality (ie, chronic diseases may lead to decreased energy and nutrient intakes), which is a concern echoed in recent debates regarding sodium and mortality (26), differences observed between our findings and those in previous studies may have reflected our choice to restrict the sample to a healthy population free of diabetes, cardiovascular disease, and chronic kidney disease.
Excessive dietary phosphorus could lead to death by increasing mean serum phosphorus concentrations throughout the course of the day (27), although there was no evidence of a mediation by serum phosphorus in our study. It is possible that repeated measurements of serum phosphorus are needed to accurately capture the effect of phosphorus intake. Previous investigators, by using repeated measurements of serum phosphorus, have shown that changes in phosphorus intake have little effect on random or fasting serum phosphorus concentrations but do result in changes in 24-h mean serum phosphorus concentrations (27, 28). Elevated serum phosphorus concentrations are associated with endothelial dysfunction, vascular calcification, and low-grade albuminuria (29–32). In addition, changes in phosphorus intake are directly associated with changes in urinary albumin excretion (33). Unfortunately, few data exist on long-term effects of restricting phosphorus intake. Previous randomized trials either have been of short duration (9, 34, 35), focused primarily on protein intake (36), or used phosphorus-binding medications, which may have independent effects on vascular calcification (37). In addition, phosphorus may have a role in regulating cell growth, proliferation (38), and protein translation (39). In a mouse model of skin carcinogenesis, elevated serum phosphorus concentrations promoted cell transformation and tumorigenesis (40), and epidemiologic studies have suggested a link between dietary (41) and serum phosphorus (42) with certain forms of cancer. Alternatively, high phosphorus intake could lead to increased mortality by stimulating higher concentrations of FGF-23 (8, 9), which may lead to left ventricular hypertrophy (11), congestive heart failure, and mortality (7, 12, 13).
Several limitations of our study merit consideration. First, we were unable to differentiate between organic and inorganic sources of phosphorus. Inorganic phosphate additives are commonly used to enhance flavor and preserve food and are as ubiquitous in our food supply as sodium (43). Because inorganic phosphate additives are more bioavailable (90–100%) than organic sources of phosphorus (40–60%) (44, 45), it is plausible that dietary phosphorus derived from inorganic sources could have a greater effect on serum phosphorus, FGF-23, and parathyroid hormone. There may also have been a measurement error because the phosphorus content in food products rich with inorganic phosphate additives may be higher than estimates by using nutrient databases (46). Because of the high prevalence of processed foods in the American diet, phosphorus intake may be systematically underestimated in nutrient content databases (43). Thus, thresholds in our study should not be interpreted as exact values above which risk increases. We relied on a single measurement of dietary recall to assess phosphorus intake and did not have 24-h urine measurements of phosphorus; however, the reported phosphorus intake was fairly consistent in the subsample with a repeated dietary recall. As in many studies that used cardiovascular mortality as an outcome, the cause of death was not independently adjudicated, which may explain the observed null association with absolute phosphorus intake. Last, there remains the possibility of residual confounding. It is possible that high phosphorus intake may represent other aspects of an unhealthy diet although analyses were unchanged after adjustment for sodium and saturated fat intakes.
Strengths of our study included the use of a nationally representative sample of a healthy US adult population, long duration of follow-up (median: 14.7 y), and restriction of our sample to a healthy population to minimize confounding by reverse causation. We used 2 different methods to account for exposure to dietary phosphorus intake (absolute phosphorus intake and phosphorus density), carefully adjusted for a number of potential confounders, and conducted subgroup analyses by dietary quality. All methods showed consistent associations between high dietary phosphorus intake and increased mortality risk.
In conclusion, high phosphorus intake was associated with increased mortality in a nationally representative, healthy US population. Because of prevalence of high phosphorus intake in healthy adults and the widespread use of inorganic phosphorus additives in processed food, our findings may have far-reaching public health implications. Additional studies are needed to determine whether this relation is causal.
Supplementary Material
Acknowledgments
An abstract of this work was presented at the Nutrition, Physical Activity and Metabolism Young Investigator Award oral abstract competition at the American Heart Association's Scientific Session in Dallas, TX, on 17 November 2013.
The authors’ responsibilities were as follows—ARC, ML, MEG, and OMG: designed the study; ARC: analyzed the data; MEG and ML: assisted with data analysis; and all authors: wrote the manuscript. OMG has served as a consultant to Vifor Pharma. ARC, ML, MEG, and LJA had no conflicts of interest.
Footnotes
Abbreviations used: ACR, albumin:creatinine ratio; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; FGF-23, fibroblast growth factor 23; HEI, Healthy Eating Index; PIR, poverty income ratio; RDA, Recommended Dietary Allowance.
REFERENCES
- 1.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Food, Nutrition Board IoM. Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: The National Academies Press, 1997. [PubMed]
- 2.Bijvoet OL. Relation of plasma phosphate concentration to renal tubular reabsorption of phosphate. Clin Sci 1969;37:23–36. [PubMed] [Google Scholar]
- 3.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 2011;305:1119–27. [DOI] [PubMed] [Google Scholar]
- 4.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RBS, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007;167:879–85. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005;112:2627–33. [DOI] [PubMed] [Google Scholar]
- 6.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (cardiovascular health study). J Am Coll Cardiol 2012;60:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med 2010;152:640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 2006;21:1187–96. [DOI] [PubMed] [Google Scholar]
- 9.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 2006;91:3144–9. [DOI] [PubMed] [Google Scholar]
- 10.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 2012;82:737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121:4393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009;119:2545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 2013;24:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1 1994;32:1–407. [PubMed]
- 15.Stevens PE, Levin A. Kidney disease: improving global outcomes chronic kidney disease guideline development work group members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(4 suppl):1220S–8S; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J. Calibration of serum creatinine in the national health and nutrition examination surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis 2007;50:918–26. [DOI] [PubMed] [Google Scholar]
- 19.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol 2002;13:1034–9. [DOI] [PubMed] [Google Scholar]
- 20.NHANES III linked mortality file. Atlanta, GA: Centers for Disease Control and Prevention, 2009.. Updated September 25. Available from: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm#analytic (cited 13 August 2012).
- 21.World Health Organization. International statistical classification of diseases and related health problems. tenth revision. (ICD-10-CM). Geneva, Switzerland: World Health Organization, 1992. [Google Scholar]
- 22.Murtaugh MA, Filipowicz R, Baird BC, Wei G, Greene T, Beddhu S. Dietary phosphorus intake and mortality in moderate chronic kidney disease: NHANES III. Nephrol Dial Transplant 2012;27:990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominguez JR, Kestenbaum B, Chonchol M, Block G, Laughlin GA, Lewis CE, Katz R, Barrett-Connor E, Cummings S, Orwoll ES, et al. Relationships between serum and urine phosphorus with all-cause and cardiovascular mortality: the osteoporotic fractures in men (MrOS) study. Am J Kidney Dis 2013;61:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palomino HL, Rifkin DE, Anderson C, Criqui MH, Whooley MA, Ix JH. 24-hour urine phosphorus excretion and mortality and cardiovascular events. Clin J Am Soc Nephrol 2013;8:1202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez JR, Shlipak MG, Whooley MA, Ix JH. Fractional excretion of phosphorus modifies the association between fibroblast growth factor-23 and outcomes. J Am Soc Nephrol 2013;24:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M, et al. Sodium, blood pressure, and cardiovascular disease: Further evidence supporting the american heart association sodium reduction recommendations. Circulation 2012;126:2880–9. [DOI] [PubMed] [Google Scholar]
- 27.Portale AA, Halloran BP, Morris RC., Jr Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest 1987;80:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third national health and nutrition examination survey (NHANES III). Am J Kidney Dis 2009;53:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int 2006;70:2141–7. [DOI] [PubMed] [Google Scholar]
- 30.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 2009;20:1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H, Oh SW, Heo NJ, Chin HJ, Na KY, Kim S, Chae DW. Serum phosphorus as a predictor of low-grade albuminuria in a general population without evidence of chronic kidney disease. Nephrol Dial Transplant 2012;27:2799–806. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle KR, Short RA. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin J Am Soc Nephrol 2009;4:1968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang A, Batch BC, McGuire HL, Vollmer WM, Svetkey LP, Tyson CC, Sanguankeo A, Anderson C, Houston J, Appel LJ. Association of a reduction in central obesity and phosphorus intake with changes in urinary albumin excretion: The PREMIER study. Am J Kidney Dis (Epub ahead of print 27 June 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011;6:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Juppner H, Wolf M. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant 2011;26:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Adler S, Caggiula AW, England BK, Greene T, Hunsicker LG, Kusek JW, Rogers NL, Teschan PE. Effects of dietary protein restriction on the progression of advanced renal disease in the modification of diet in renal disease study. Am J Kidney Dis 1996;27:652–63. [DOI] [PubMed] [Google Scholar]
- 37.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 2012;23:1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang SH, Yu KN, Lee YS, An GH, Beck GR, Jr, Colburn NH, Lee KH, Cho MH. Elevated inorganic phosphate stimulates akt-ERK1/2-Mnk1 signaling in human lung cells. Am J Respir Cell Mol Biol 2006;35:528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin H, Chang SH, Xu CX, Shin JY, Chung YS, Park SJ, Lee YS, An GH, Lee KH, Cho MH. High dietary inorganic phosphate affects lung through altering protein translation, cell cycle, and angiogenesis in developing mice. Toxicol Sci 2007;100:215–23. [DOI] [PubMed] [Google Scholar]
- 40.Camalier CE, Young MR, Bobe G, Perella CM, Colburn NH, Beck GR., Jr Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev Res (Phila) 2010;3:359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinkman MT, Buntinx F, Kellen E, Dagnelie PC, Van Dongen MC, Muls E, Zeegers MP. Dietary intake of micronutrients and the risk of developing bladder cancer: results from the belgian case-control study on bladder cancer risk. Cancer Causes Control 2011;22:469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wulaningsih W, Michaelsson K, Garmo H, Hammar N, Jungner I, Walldius G, Holmberg L, Van Hemelrijck M. Inorganic phosphate and the risk of cancer in the Swedish AMORIS study. BMC Cancer 2013;13:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calvo MS, Uribarri J. Contributions to total phosphorus intake: all sources considered. Semin Dial 2013;26:54–61. [DOI] [PubMed] [Google Scholar]
- 44.Bell RR, Draper HH, Tzeng DY, Shin HK, Schmidt GR. Physiological responses of human adults to foods containing phosphate additives. J Nutr 1977;107:42–50. [DOI] [PubMed] [Google Scholar]
- 45.Karp HJ, Vaihia KP, Karkkainen MU, Niemisto MJ, Lamberg-Allardt CJ. Acute effects of different phosphorus sources on calcium and bone metabolism in young women: a whole-foods approach. Calcif Tissue Int 2007;80:251–8. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr 2007;17:350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.