Abstract
OBJECTIVE
Thiazide diuretics have been associated with increased risk for new onset diabetes (NOD), but pharmacogenetic markers of thiazide-induced NOD are not well studied. Single nucleotide polymorphisms (SNPs) in the Transcription Factor 7-Like 2 gene (TCF7L2) represent the strongest and most reproducible genetic associations with diabetes. We investigated the association of tag SNPs in TCF7L2 with thiazide-induced NOD.
METHODS
We identified cases that developed NOD and age, gender, and race/ethnicity-matched controls from the INternational VErapamil SR Trandolapril STudy (INVEST). INVEST compared cardiovascular outcomes between two antihypertensive treatment strategies in ethnically diverse patients with hypertension and coronary artery disease. We genotyped 101 TCF7L2 tag SNPs and used logistic regression to test for pharmacogenetic (SNP*hydrochlorothiazide treatment) interactions. Permuted interaction p values were corrected with the PACT test and adjusted for diabetes-related variables.
RESULTS
In INVEST whites, we observed three TCF7L2 SNPs with significant SNP*treatment interactions for NOD. The strongest pharmacogenetic interaction was observed for rs7917983 (synergy index 3.37 [95%CI 1.72–6.59], p=5.0×10−4, PACT =0.03), which was associated with increased NOD risk in hydrochlorothiazide-treated patients (OR 1.53 [1.04–2.25], p=0.03) and decreased NOD risk in non hydrochlorothiazide-treated patients (OR 0.48 [0.27–0.86], p=0.02). The TCF7L2 SNP rs4506565, previously associated with diabetes, showed a similar, significant pharmacogenetic association.
CONCLUSIONS
Our results suggest that hydrochlorothiazide treatment is an environmental risk factor that increases diabetes risk beyond that attributed to TCF7L2 variation in white, hypertensive patients. Further study and replication of our results is needed to confirm pharmacogenetic influences of TCF7L2 SNPs on thiazide-induced NOD.
Keywords: pharmacogenetics, TCF7L2, diabetes mellitus, hydrochlorothiazide
INTRODUCTION
Hypertension is an independent risk factor for type 2 diabetes (T2D) and a patient with both diabetes and hypertension is at increased risk of an adverse cardiovascular (CV) outcome versus a patient with hypertension alone.(1, 2) Thiazide diuretics are considered first line agents for the treatment of hypertension, but consistent evidence from randomized antihypertensive trials supports that thiazide diuretics are associated with new onset diabetes (NOD).(3–5) Because thiazides contribute to NOD development, their benefit in a hypertensive patient, including reduction in CV risk, may be offset by this adverse metabolic effect.
Genetic influences on T2D are well established with heritability estimated at 50 percent.(6, 7) Single nucleotide polymorphisms (SNPs) have been reproducibly associated with T2D in genome wide association studies (GWAS) and variants in the Transcription Factor 7-Like 2 gene (TCF7L2) are the strongest and most reproducible signals identified to date.(8) Many environmental influences have also been consistently associated with T2D development, (9, 10) but interactions between genetic and environmental risk factors such as pharmacotherapy are not well studied with respect to T2D incidence. Gene environment interactions remain a potentially important component of the mileau that predicts T2D development.
Variants in TCF7L2 are well replicated genetic signals for diabetes, with an odds ratio (OR) of the rs7903146 T allele of 1.56 [95% confidence interval (95%CI) 1.29–1.89], meta-analysis p=1×10−140).(8, 11–13) TCF7L2 is a transcription factor involved in the WNT signaling pathway. TCF7L2 has been implicated in incretin signaling pathways since it has been shown to regulate transcription of the glucagon gene, which encodes glucagon-like peptide 1 (GLP1) in the L cells of the gut.(14) The rs7903146 T allele has been associated with increased TCF7L2 expression and has been implicated as a functional variant, being mapped to open chromatin sites in pancreatic islet cells.(15, 16) Functional impairment of TCF7L2 by SNPs could cause changes in gene expression and influence diabetes development.
The importance of identifying predictors of thiazide-induced dysglycemia was emphasized by a working group from the National Heart Lung and Blood Institute.(4) A priori identification of patients at risk for T2D during thiazide treatment could guide thiazide prescribing to reduce the risk of NOD. We hypothesize that thiazide diuretic treatment in a patient with T2D risk alleles might further increase diabetes risk, constituting a potential pharmacogenetic role for TCF7L2 SNPs. Although multiple SNPs have been associated with thiazide-induced NOD, (17–19) to our knowledge the pharmacogenetic interaction between TCF7L2 SNPs and thiazide treatment has not been studied with respect to NOD. Therefore, we investigated the interaction of TCF7L2 polymorphisms and hydrochlorothiazide (HCTZ) treatment on the development of NOD in hypertensive, coronary artery disease (CAD) patients from the INternational VErapamil SR-Trandolapril STudy (INVEST).
METHODS
Study design
INVEST evaluated adverse CV outcomes and NOD occurring during randomized treatment with either an atenolol-based or a verapamil sustained release (SR)-based antihypertensive strategy in patients with hypertension and CAD. The INVEST design, (20) primary outcome, (21) and NOD (9) results have been previously published in detail.(9, 20, 21) Briefly, patients were eligible if they were aged 50 years or older and had documented CAD, with essential hypertension as defined by the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI) requiring drug therapy.(22) Patients were excluded if they had class IV heart failure, significant renal insufficiency, were taking β-blockers within 2 weeks of randomization, or were taking β-blockers for a myocardial infarction that occurred in the previous 12 months. The verapamil-based strategy consisted of verapamil SR 240 mg daily (Step 1), addition of trandolapril 2 mg daily (Step 2), dose titration to verapamil SR 240 mg/trandolapril 2 mg twice daily (Step 3), and HCTZ 25 mg daily add-on treatment (Step 4) to achieve JNC VI blood pressure (BP) goals. The atenolol-based strategy consisted of atenolol 50 mg daily (Step 1), addition of HCTZ 25 mg daily (Step 2), titration to atenolol 50 mg/HCTZ 25 mg twice daily (Step 3), and trandolapril 2 mg daily add-on treatment (Step 4) for BP control as necessary. (Table S1 in Supplemental Materials, Supplemental Digital Content 1, http://links.lww.com/FPC/A651) Therefore, patients in the atenolol-based strategy were prescribed HCTZ at step 2 if BP control was not achieved and patients in the verapamil SR-based strategy were prescribed HCTZ at step 4 if BP control was not achieved. Both strategies were optimized to provide end organ protection with trandolapril in patients with diabetes and/or renal insufficiency. All patients enrolled in INVEST provided written informed consent and the institutional review boards of participating study centers approved the study protocol. INVEST is registered at clinicaltrials.gov (NCT00133692).
The INVEST Genetic Substudy (INVEST-GENES) collected DNA samples from 5,979 INVEST patients at 187 sites in the United States and Puerto Rico, who provided additional written informed consent for genetic studies. We conducted a nested case control study including those who developed NOD during follow-up (cases) and age, race/ethnicity, and gender-matched participants who remained diabetes-free during follow-up (controls). NOD was determined by site investigators from a review of all available patient data, as well as new use of an anti-diabetic medication.(9) Information regarding diabetes status and use of anti-diabetic medications was collected at each follow-up visit, which occurred every 6 weeks for the first 6 months and then every six months until the study ended.(9) Patients with diabetes at baseline, defined by a history of diabetes mellitus and/or use of oral hypoglycemic medication or insulin, were excluded from this analysis.(23, 24)
Genotyping and quality control
Genotyping was accomplished using the HumanCVD BeadChip, (25) which contains approximately 50,000 cosmopolitan tag SNPs for 2,100 CV and metabolic- related genes.(25) The HumanCVD BeadChip includes 101 TCF7L2 tag SNPs, selected for r2>0.5 and minor allele frequency (MAF) >0.05. Genotypes were called using BeadStudio software and the GenTrain2 calling algorithm (Illumina, San Diego, CA). The INVEST-GENEs NOD case control cohort was genotyped as part of larger INVEST-GENEs cohort (n=2,239) and quality control procedures were performed on the entire sample set. Patients were excluded if sample genotype call rates were below 95% and SNPs were excluded if genotype call rates were below 90%. Monomorphic SNPs were removed and SNPs were excluded if GenTrain score was less than 0.3. A total of 87 blind duplicates were included in genotyping and had a concordance rate of 99.997%. Gender was confirmed from X chromosome genotype data, and those with discordant gender data were excluded (n=14). Cryptic relatedness was estimated by pairwise identity-by-descent (IBD) analysis implemented using PLINK.(14) Forty-one pairs of samples were identified as first degree relatives (pi-hat 0.30–0.57) and these individuals were maintained in the analysis. Heterozygosity was assessed using PLINK, by estimating the inbreeding coefficient, F. Six subjects had F values >4 standard deviations from the mean. One of these subjects also had a high missing genotype rate of > 4% and this subject was excluded. Outliers in the by race/ethnic group Principal Component Analysis were also removed (n=4). The final dataset consisted of 2,214 subjects, with 1,435 in the final NOD dataset.
Definition and treatment of race/ethnicity
INVEST race/ethnic groups were determined by patient report with interaction by the study investigator (26) and confirmed through principal components analysis (PCA) with linkage disequilibrium (LD)-pruned HumanCVD BeadChip data. PCA was performed using JMP Genomics version 5.0 (SAS, Cary, NC) in which the EIGENSTRAT method was implemented.(27) To minimize confounding by population stratification, pharmacogenetic analyses were performed separately by race/ethnicity and adjusted for principal components (PCs) one, two, and three. PCs were generated separately for each race/ethnic group. PCs one, two, and three were used since three PCs provided adequate separation of race/ethnic groups, fewer PCs did not provide adequate separation of race/ethnic groups, and additional PCs provided no further precision. LD analysis and pairwise LD (r2) were performed within race/ethnic groups using Haploview.(28)
Statistical analysis
Baseline characteristics for cases and controls were compared using t tests and chi square tests as appropriate. Multi-variable logistic regression models were used to estimate ORs for NOD, assess TCF7L2 SNP*HCTZ treatment interactions by race/ethnicity, and calculate synergy indices (SIs) for an estimate of multiplicative SNP*HCTZ interactions.(29) Relative risk estimates of interactions on an additive scale were also calculated using the Relative Excess Risk due to Interaction (RERI) for allele carriers with likelihood-based 95% confidence bounds as previously described.(30) In the absence of an interaction effect, the SI equals 1 and the RERI equals 0. Empirical p values were computed for all logistic regressions using Monte Carlo estimation of the exact p value with 10,000 permutations performed. Interaction p values were adjusted for correlated tests using PACT, which accounts for linkage disequilibrium of SNPs, attaining the accuracy of permutation or simulation-based tests in much less computation time.(31) Statistical significance was established at PACT=0.05. ORs per allele copy and 95%CIs were calculated in HCTZ-treated patients, non-HCTZ treated patients, and all patients using allelic trend tests adjusted for variables with potential impact on NOD. HCTZ treatment was defined as exposure to HCTZ with any dose or duration before diagnosis of NOD. Similar treatment definitions were used for atenolol and trandolapril, which belong to drug classes also known to affect NOD incidence.(5)
Sensitivity analyses were performed in patients treated with HCTZ ≥25 mg/day and in patients treated with continuous HCTZ for ≥6 months. All logistic regression models were adjusted for variables chosen based on potential impact on NOD, which include age, gender, body mass index (BMI), average on-treatment systolic BP, hypercholesterolemia, smoking history, PCs one, two, and three, treatment with trandolapril or atenolol, as well as trandolapril, atenolol, and HCTZ treatment duration. SNPs were fit as numeric variables to model effects and SNP*HCTZ interactions and HCTZ exposure is included in statistical models of interactions. Deviations from Hardy Weinberg Equilibrium (HWE) were assessed using exact statistics for Fisher’s Exact Test by race/ethnicity with alpha=0.01. Variants which deviated from HWE were flagged and are not presented in the results. All statistical analyses were performed using SAS version 9.2 and JMP Genomics version 5.0 (SAS, Cary, NC).
RESULTS
For the NOD case control cohort, over a mean follow-up of 2.8 years, we identified 446 NOD cases who were age and race/ethnicity matched to 1,025 controls, in INVEST-GENES (Table 1). A total of 410 NOD cases were successfully genotyped on the HumanCVD BeadChip (Illumina, San Diego, CA). A total of 98 TCF7L2 SNPs passed QC and the average genotyping call rate was 99.75%. Three of 101 SNPs departed from HWE in at least one race/ethnic group, but none of these SNPs showed significant pharmacogenetic interactions. HWE p values and minor allele frequencies are presented in Supplementary Materials, Supplemental Digital Content 1, http://links.lww.com/FPC/A651. Characteristics for the NOD case control cohort are summarized in Table 1 and characteristics according to race/ethnicity are summarized in Table S2 in the Supplemental Materials, Supplemental Digital Content 1, http://links.lww.com/FPC/A651.
Table 1.
Characteristics of new onset diabetes cases and controls at baseline and during INVEST
| Characteristic1 | NOD Cases (n=446) | Controls (n=1,025) | p value2 |
|---|---|---|---|
| At baseline | |||
| Age (years) | 65 (10) | 65 (9) | 0.73 |
| Female, n (%) | 250 (56) | 573 (56) | 0.96 |
| BMI (kg/m2) | 31 (6) | 29 (5) | <0.0001 |
| Race/ethnicity, n (%) | 0.66 | ||
| White | 176 (40) | 409 (40) | |
| African American | 51 (11) | 121 (12) | |
| Hispanic | 217 (49) | 492 (48) | |
| Blood pressure (mm Hg) | |||
| Systolic | 149 (19) | 148 (18) | 0.26 |
| Diastolic | 87 (10) | 86 (10) | 0.04 |
| Verapamil SR strategy, n (%) | 208 (47) | 519 (51) | 0.16 |
| Hypercholesterolemia, n (%)3 | 242 (54) | 548 (54) | 0.78 |
| History of LVH, n (%) | 77 (17) | 128 (13) | 0.02 |
| History of MI, n (%) | 99 (22) | 194 (19) | 0.15 |
| History of smoking, n (%) | 184 (41) | 405 (40) | 0.53 |
| During INVEST | |||
| Blood pressure (mm Hg)4 | |||
| Systolic | 135 (11) | 134 (11) | 0.07 |
| Diastolic | 79 (6) | 79 (6) | 0.49 |
| HCTZ treatment, n (%) | 328 (74) | 640 (62) | <0.0001 |
| HCTZ (≥ 6 months), n (%) | 320 (72) | 627 (61) | <0.0001 |
| HCTZ dose (mg) | 28 (12) | 26 (12) | 0.17 |
| Atenolol treatment, n (%) | 229 (51) | 479 (47) | 0.10 |
| Atenolol dose (mg) | 72 (32) | 67 (31) | 0.02 |
| Trandolapril treatment, n (%) | 267 (60) | 664 (65) | 0.07 |
| Trandolapril dose (mg) | 3.3 (2.6) | 3.4 (2.6) | 0.49 |
| Verapamil SR treatment, n (%) | 208 (47) | 519 (51) | 0.16 |
| Verapamil SR dose (mg) | 234 (75) | 238 (75) | 0.71 |
INVEST indicates INternational VErapamil SR and Trandolapril Study; NOD, new onset diabetes; BMI body mass index; SR, sustained release; LVH, left ventricular hypertrophy; HCTZ, hydrochlorothiazide
Values are mean (standard deviation) unless otherwise noted.
P values represent t-tests and chi square tests where appropriate.
History of or currently taking lipid-lowering medications
Average of clinic blood pressure measurements during study
A higher mean atenolol dose was observed in NOD cases (72 mg versus 67 mg, p=0.02) than controls. Associations between model covariates and NOD are presented in Supplementary Table S3, Supplemental Digital Content 1, http://links.lww.com/FPC/A651. At baseline, NOD cases had higher BMI and diastolic BP, and a higher percentage with left ventricular hypertrophy. During follow-up, the proportion of patients who were treated with HCTZ was greater in cases than in controls resulting in a significant association between HCTZ treatment and NOD (OR 1.70 [95%CI 1.31–2.20], p<0.0001). The average duration of HCTZ treatment was 87.1 (standard deviation [SD] 73.6) weeks and duration of HCTZ was associated with NOD (OR 1.16 [95%CI 1.10–1.22] per 6 months HCTZ, p<0.0001). A total of 409 of the 713 patients (57%) assigned to the verapamil-based strategy were prescribed HCTZ and 532 of the 722 patients (74%) assigned to the atenolol-based strategy were prescribed HCTZ. Overall, HCTZ treated patients had higher metabolic risk compared to non-HCTZ treated patients, since HCTZ treated patients had higher average baseline systolic blood pressure (151 versus 144 mmHg [p<0.0001] respectively), higher average on-treatment systolic BP (137 versus 130 mmHg [p<0.0001] respectively), and higher BMI (30.1 versus 28.6 kg/m2 [p<0.0001] respectively). Characteristics for cases and controls by HCTZ treatment status are summarized in Table S4 of the Supplemental Materials, Supplemental Digital Content 1, http://links.lww.com/FPC/A651.
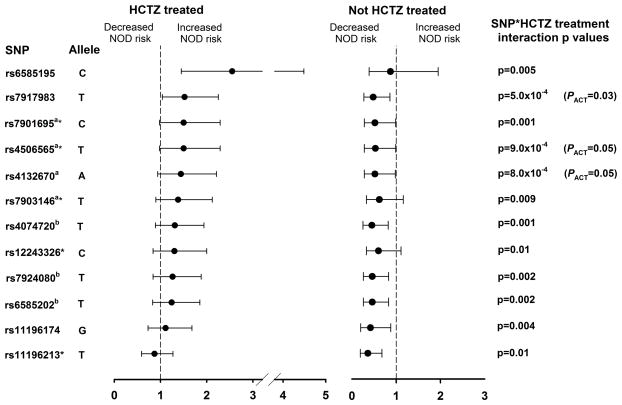
In INVEST whites, three TCF7L2 SNPs out of 101 tested showed a significant pharmacogenetic interaction with HCTZ treatment after PACT correction. SNPs with significant (n=3) or nominally significant (n=18) interactions are included in Table 2. All SNPs with significant pharmacogenetic interactions after PACT correction showed an increased point estimate for NOD in HCTZ treated patients, with ORs ranging from 1.11–2.55. The strongest pharmacogenetic interaction was observed for rs7917983 (pinx=5.0×10−4, PACT=0.03), which was associated with increased NOD risk in HCTZ-treated patients (OR 1.52 [95%CI 1.04–2.25], p=0.03) and decreased NOD risk in non HCTZ-treated patients (OR 0.48 [95%CI 0.27–0.86], p=0.02). The interaction resulted in a RERI of 0.99 (95%CI 0.43–1.50), indicating a 99 percent increase in NOD risk due to interaction of HCTZ and rs7917983 T allele carriage relative to the risk without either factor. This interaction resulted in a SI of 3.37 [95%CI 1.72–6.59], indicating a 3-fold risk of NOD per rs7917983 T allele for HCTZ exposure. In HCTZ treated patients, the strongest associations with NOD were for the SNPs rs6585195 (OR 2.55 [95%CI 1.45–4.49], p=8.0×10−4) and rs12573128 (OR 2.27 [95%CI 1.32–3.92], p=0.003), which were both identified as nominally significant associations in the primary analysis. (Table S5 in Supplemental Materials, Supplemental Digital Content 1, http://links.lww.com/FPC/A651)
Table 2.
Significant and nominally significant pharmacogenetic interactions for TCF7L2 SNPs and hydrochlorothiazide treatment on new onset diabetes in INVEST whites
| SNP | Allele | Freq. | HWE1 | OR (95%CI) (HCTZ Treated, n=941)2 | OR (95%CI) (Not HCTZ Treated, n=494)2 | pinx3 | PACT4 |
|---|---|---|---|---|---|---|---|
| rs7917983 | T | 0.53 | 0.20 | 1.53 (1.04–2.25) | 0.48 (0.27–0.86) | 5.0×10−4 | 0.03 |
| rs4132670 | A | 0.32 | 0.73 | 1.44 (0.94–2.21) | 0.52 (0.28–0.98) | 8.0×10−4 | 0.05 |
| rs4506565* | T | 0.31 | 0.91 | 1.50 (0.98–2.29) | 0.53 (0.28–1.00) | 9.0×10−4 | 0.05 |
| rs4074720 | T | 0.54 | 0.49 | 1.31 (0.89–1.94) | 0.45 (0.25–0.82) | 0.001 | - |
| rs7901695* | C | 0.32 | 0.82 | 1.50 (0.98–2.29) | 0.52 (0.28–0.99) | 0.001 | - |
| rs7924080 | T | 0.53 | 0.62 | 1.26 (0.84–1.88) | 0.46 (0.26–0.83) | 0.002 | - |
| rs6585202 | T | 0.53 | 0.69 | 1.24 (0.83–1.85) | 0.46 (0.26–0.83) | 0.002 | - |
| rs11196174 | G | 0.26 | 1 | 1.11 (0.73–1.68) | 0.42 (0.20–0.88) | 0.004 | - |
| rs6585195 | C | 0.13 | 0.63 | 2.55 (1.45–4.49) | 0.87 (0.39–1.95) | 0.005 | - |
| rs10885399 | A | 0.21 | 0.16 | 1.87 (1.18–2.97) | 0.74 (0.37–1.51) | 0.009 | - |
| rs7903146* | T | 0.28 | 0.54 | 1.38 (0.90–2.12) | 0.62 (0.33–1.16) | 0.009 | - |
| rs4918789 | T | 0.55 | 0.01 | 0.87 (0.59–1.27) | 0.36 (0.19–0.68) | 0.01 | - |
| rs12243326* | C | 0.28 | 0.46 | 1.30 (0.84–2.00) | 0.60 (0.33–1.11) | 0.01 | - |
| rs7087006 | A | 0.55 | 0.02 | 0.86 (0.59–1.26) | 0.36 (0.19–0.68) | 0.01 | - |
| rs11196213 | T | 0.45 | 0.01 | 0.87 (0.59–1.27) | 0.36 (0.19–0.68) | 0.01 | - |
| rs12266632 | G | 0.06 | 0.62 | 2.24 (0.97–5.16) | 0.68 (0.21–2.24) | 0.02 | - |
| rs12573128 | G | 0.14 | 0.37 | 2.27 (1.32–3.92) | 1.01 (0.47–2.17) | 0.02 | - |
| rs7079711 | A | 0.20 | 1 | 1.11 (0.70–1.77) | 0.48 (0.21–1.09) | 0.03 | - |
| rs11594681 | A | 0.37 | 0.20 | 1.14 (0.78–1.68) | 0.58 (0.31–1.09) | 0.03 | - |
| rs17130192 | T | 0.08 | 0.26 | 1.39 (0.69–2.77) | 0.39 (0.11–1.38) | 0.04 | - |
| rs4081699 | G | 0.21 | 0.42 | 1.81 (1.15–2.84) | 0.84 (0.44–1.61) | 0.04 | - |
SNP indicates single nucleotide polymorphism; Freq, frequency of allele in INVEST whites; HWE, Hardy Weinberg Equilibrium p value; OR, odds ratio; 95%CI, 95% confidence interval; HCTZ, hydrochlorothiazide
Hardy Weinberg equilibrium p value using Fisher’s Exact test in whites
HCTZ treatment defined as exposure to HCTZ with any dose or duration before diagnosis of NOD. Odds ratios and 95% confidence intervals adjusted for age, gender, body mass index, average on treatment systolic blood pressure, hypercholesterolemia, history of smoking, principal components one, two, and three, trandolapril and atenolol treatment, and trandolapril, atenolol, and HCTZ treatment duration.
p value for interaction of HCTZ treatment and SNP after adjustment but before PACT correction
p value for interaction of HCTZ treatment and SNP after adjustment and correction for multiple testing using PACT
SNPs previously associated with type 2 diabetes or diabetes-related traits in genome wide association studies
The TCF7L2 SNP rs4506565, which has been previously associated with T2D in GWAS, showed a significant pharmacogenetic association in INVEST whites (RERI 1.08 [95%CI 0.36–1.87] and SI 3.58 [95%CI 1.67–7.66], pinx=9.0×10−4, PACT=0.05).(11, 32) An increased risk for NOD was observed with T2D risk alleles in HCTZ treated patients and decreasing risk for NOD was observed with T2D risk alleles in non-HCTZ treated patients. (Table 2) The most strongly associated SNP from GWAS literature, rs7903146, showed similar trends although with nominal significance (RERI 0.93 [95%CI 0.05–1.84] and SI 2.60 [95%CI 1.22–5.54], pinx=0.009). Another SNP associated with diabetes from GWAS, rs7901695, showed a nominally significant pharmacogenetic interaction (RERI 1.11 [95%CI 0.42–1.88] and SI 3.67 [95%CI 1.71–7.89], pinx=0.001). The SNPs rs12243326 and rs11196213, which have been previously associated with two hour post oral glucose tolerance test glucose and T2D respectively, (33, 34) also showed nominally significant pharmacogenetic interactions. Figure 1 shows ORs, 95%CIs, and p values for significant SNPs and nominally significant SNPs that have been previously associated with T2D or T2D-related traits.
Figure 1.
CF7L2 SNP*HCTZ treatment interactions in INVEST whites, including significant SNPs after PACT correction and nominally significant SNPs that have been previously associated with T2D or T2D-related traits. Odds ratios, 95% confidence intervals, and interaction p values adjusted for age, gender, body mass index, average on treatment systolic blood pressure, hypercholesterolemia, history of smoking, principal components one, two, and three, trandolapril and atenolol treatment, and trandolapril, atenolol, and HCTZ treatment duration. Superscript letters indicate that SNPs are in linkage disequilibrium (r2>0.80); *Allele previously associated with T2D or T2D-related traits. HCTZ indicates hydrochlorothiazide; NOD, new onset diabetes; SNP, single nucleotide polymorphism.
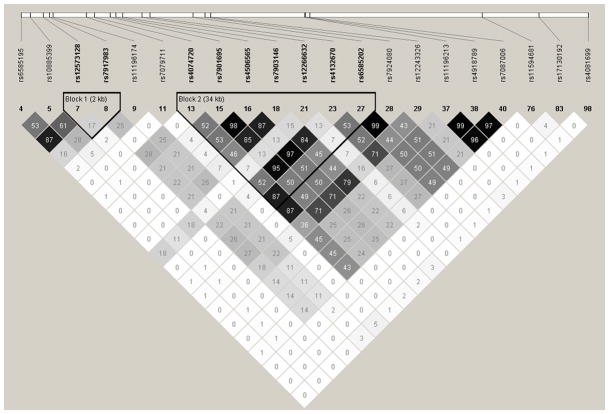
Multiple significant and nominally significant associations were observed for SNPs in high linkage disequilibrium (LD) in INVEST whites. (Figure 2) The SNP rs4074720, for which a nominally significant SNP*HCTZ treatment interaction was observed (RERI 0.91 [95%CI 0.24–1.47] and SI 3.22 [95%CI 1.60–6.48], pinx=0.001), was in high LD with rs6585202 (r2=0.87) and rs7924080 (r2=0.87), which also showed significant interactions. The nominally significant SNP rs7901695 was also in high LD with rs4506565 (r2=0.98), rs4132670 (r2=0.95), and rs7903146 (r2=0.85). The SNP rs7917983, for which the strongest pharmacogenetic interaction was observed, was not in high LD with any other significantly or nominally associated SNP.
Figure 2.
Haploview-generated linkage disequilibrium (LD) plot of TCF7L2 SNPs, including significant SNPs after PACT correction and nominally significant SNPs in INVEST whites. Regions of higher LD are shaded darker according to higher r2 values. The number within each box indicates the r2 value.
In sensitivity analyses, results were similar when the definition of HCTZ treatment was restricted to 6 months or greater and daily HCTZ dose of 25mg or greater. (Table S6 in Supplemental Materials, Supplemental Digital Content 1, http://links.lww.com/FPC/A651) For the SNP rs7917983 with the strongest pharmacogenetic interaction, the association strengthened although the SI was similar in patients taking HCTZ for 6 months or more (OR 1.88 [95%CI 1.16–3.04] in HCTZ treated patients and OR 0.49 [95%CI 0.27–0.87] in non-HCTZ treated patients, RERI 1.01 [95%CI 0.43–1.54] and SI 3.34 [95%CI 1.71–6.52], pinx=2.0×10−4). When HCTZ treatment was restricted to 25 mg/day or more, the interaction remained significant (OR 1.89 [95%CI 1.14–3.16] in HCTZ treated patients and OR 0.64 [95%CI 0.38–1.06] in non-HCTZ treated patients, RERI 1.19 [95%CI 0.59–1.87] and SI 3.21 [95%CI 1.61–6.42], pinx=0.008).
No significant pharmacogenetic associations were observed in Hispanics or blacks after PACT correction. In blacks, a trend toward a significant SNP*HCTZ treatment interaction was observed for the rs290490 G allele (pinx=0.04), with an increased risk for NOD in HCTZ treated patients (OR 1.49 [95%CI 1.06–2.10], p=0.02).
DISCUSSION
To our knowledge, the present study is the first to investigate TCF7L2 SNPs for pharmacogenetic influences on thiazide-induced NOD and the first to observe significant pharmacogenetic interactions between TCF7L2 SNPs and HCTZ treatment on NOD risk. We have shown that a TCF7L2 SNP previously associated with T2D in GWAS may also have a pharmacogenetic interaction in whites treated with HCTZ. TCF7L2 pharmacogenetic associations were confirmed when HCTZ treatment was limited to higher HCTZ doses and extended treatment duration. The present study is strengthened by our ability to assess TCF7L2 risk allele point estimates in HCTZ-treated and non HCTZ-treated patients. Our study provides evidence for TCF7L2 as a candidate gene for the pharmacogenetics of thiazide-induced NOD and suggests the importance of assessing SNP*HCTZ treatment interactions in GWAS of T2D.
Since the initial T2D GWAS in 2006, (35) many T2D GWAS have been published that establish TCF7L2 SNPs as the strongest and most reproducible genetic risk factors for T2D.(8, 11, 12, 32, 36, 37) We found significant pharmacogenetic associations in whites, which is the race group in which the strongest and most reproducible TCF7L2 disease associations are found. We observed pharmacogenetic associations with a TCF7L2 SNP that was previously associated with T2D, namely rs4506565 (OR 1.36 [95%CI 1.20–1.54]).(11, 32) Among the SNPs with nominally significant pharmacogenetic interactions were rs7903146, the strongest SNP from T2D GWAS (OR 1.56 [95%CI 1.29–1.89]), (8, 12), rs7901695 (OR 1.37 [95%CI 1.25–1.49]), (11, 32) and rs12243326, previously associated with two hour glucose after glucose challenge.(33) The strongest pharmacogenetic interaction observed was for the intronic SNP rs7917983, which has not been previously associated with T2D to our knowledge. Although rs7917983 was not in LD with any other significantly or nominally associated SNP, this SNP may be in LD with other TCF7L2 SNPs not included in this study.
Our results suggest that TCF7L2 variation affects the influence of HCTZ on risk for NOD. If our results are supported by replication and functional studies, TCF7L2 SNPs might be used to guide HCTZ treatment to avoid new onset diabetes. In addition, these results suggest the importance of considering antihypertensive treatment when identifying and utilizing genetic predictors for T2D. HCTZ might affect expression of TCF7L2 or the ability of the transcription factor to bind to promoters, further increasing an individual’s risk for T2D. The majority of TCF7L2 SNPs associated with NOD during HCTZ treatment were primarily intronic with few predicted functional consequences. Furthermore, rs7903146 did not show the strongest pharmacogenetic interaction, suggesting that other SNPs may be responsible for the observed pharmacogenetic association.
We observed differences in associations between race/ethnic groups, which might suggest differences in LD and the need to identify functional variants. Such differences in LD between race/ethnic groups are observable in the INVEST population in LD analyses. Our observation of SNP effects only in whites might also suggest that TCF7L2 SNPs have pharmacogenetic influences only in individuals with European ancestry. Although GWAS have identified SNPs in TCF7L2 as consistent and reliable risk factors for T2D in European populations, TCF7L2 SNPs identified in whites may not be genetic risk factors for T2D in black and Hispanic populations.(35, 36, 38) A relatively small number of studies in populations with African descent and Hispanic ethnicity have inconsistently observed genetic associations.(12, 37, 39–45) These differences in observed associations between TCF7L2 SNPs and diabetes might explain the differences in results observed between race/ethnic groups in the present study.
Although we observed several TCF7L2 SNPs with an increased risk of NOD in HCTZ treated patients and a decreased NOD risk in non HCTZ treated patients, many of the ORs for NOD in HCTZ treated patients were not significant. In sensitivity analyses, many of these ORs were significant when HCTZ treatment was more stringently defined (e.g. longer duration or higher dose). In addition, a number of the observed interactions appeared to be driven largely by decreased odds ratios in non HCTZ treated patients. These inverse associations might suggest that lack of HCTZ treatment is protective for diabetes when TCF7L2 risk SNPs are present. The possibility also exists that HCTZ treatment is collinear with another unknown factor affecting diabetes risk. These results are difficult to interpret since GWAS studies do not generally consider HCTZ treatment as a covariate. These data underscore the need for replication and additional study of these associations and might suggest the importance of considering antihypertensive treatment when assessing TCF7L2 SNPs as predictors of diabetes risk.
We observed a significant difference in baseline systolic BP, average on-treatment systolic BP, and BMI between HCTZ treated and non-HCTZ treated patients, suggesting that HCTZ treated patients were at higher metabolic risk. Because HCTZ was not randomly assigned and was part of stepped care as an add-on treatment to achieve BP goals, patients receiving HCTZ during INVEST were more likely to have uncontrolled hypertension. Since increased BP and BMI have been identified as risk factors for diabetes, (9) BP control and an increased BMI may be partially responsible for the strength of the observed association between TCF7L2 SNPs and NOD in HCTZ treated patients and the inverse association in non-HCTZ-treated patients. In addition, concomitant antihypertensive treatments used in INVEST treatment strategies, which can affect NOD development, may have contributed to the inverse NOD association in non HCTZ treated patients. HCTZ treatment was not uniform within INVEST treatment strategies and our results may be applicable only when HCTZ is used in antihypertensive strategies similar to INVEST. We attempted to reduce confounding of these variables by including on-treatment systolic BP, BMI, and additional drug exposure and duration as covariates in all models.
Since the pathophysiologic role TCF7L2 plays in T2D development remains unclear, speculation regarding the physiology of a pharmacogenetic interaction is difficult. TCF7L2 is primarily thought of as a T2D genetic risk factor. However, HCTZ treatment may represent an environmental risk factor that precipitates T2D in a patient who is otherwise at risk for T2D development based on their TCF7L2 genotype. Our observation of multiple significant SNP*HCTZ interactions and significant interaction point estimates using both RERIs and SIs supports this hypothesis. In a different population, we have shown that SNPs identified in GWAS studies of hypertension also affect variability in response to antihypertensive drugs.(46) If such associations are replicated and prospectively evaluated, SNPs from GWAS might be used to tailor antihypertensive treatment.
Our study has several limitations. First, our sample size by race/ethnicity is small relative to most T2D disease genetics studies. However, TCF7L2 is the strongest known T2D risk factor and we observed a number of significant pharmacogenetic interactions, suggesting that we had sufficient power to detect associations in whites. In INVEST, NOD diagnosis was based on investigator reports or new use of anti-diabetes drugs, rather than collection of glucose data. However, accuracy of investigator-reported diabetes compared to laboratory data has been verified by others, (47) and has been used in other trials.(48–50) Furthermore, we have shown previously that NOD and baseline diabetes in INVEST are associated with clinical characteristics indicative of type 2 diabetes, including increased BMI, Hispanic ethnicity, left ventricular hypertrophy, and hypercholesterolemia, increasing our confidence in the validity of the NOD outcome used in INVEST.(9, 21, 23, 24) Although antihypertensive treatment during INVEST might have confounded our associations, statistical models were adjusted for exposure to drugs associated with diabetes. We also did not observe consistent TCF7L2 pharmacogenetic associations in Hispanic and African race/ethnic groups. Our observations may reflect differences in LD structure or may represent type 2 error. In whites, significant pharmacogenetic associations after PACT correction, multiple pharmacogenetic associations, and consistency of these associations with different HCTZ treatment definitions lend credence to the validity of pharmacogenetic findings. We do recognize the potential for false positive results in pharmacogenetic analyses and our observations need to be independently replicated.
In summary, our results suggest that genetic variation in TCF7L2, including SNPs associated with T2D from GWAS, may influence the risk of HCTZ-associated NOD. Our observations suggest that an environmental risk factor such as HCTZ treatment further increases diabetes risk in TCF7L2 risk allele carriers. Our observation of SNP effects only in whites suggests differences in LD, or that TCF7L2 SNPs have pharmacogenetic influences only in individuals with European ancestry. Functional studies and replication of pharmacogenetic associations are needed to confirm our observed association and define the potential role of TCF7L2 SNPs in predicting NOD during HCTZ treatment.
Supplementary Material
Acknowledgments
This work is supported by a grant from the National Institutes of Health (Bethesda, MD), grant U01 GM074492, funded as part of the Pharmacogenetics Research Network. Additional support for this work includes: National Institutes of Health grants R01 HL74730 and HL086558 (R.C.D.); and grants from Abbott Laboratories and the University of Florida Opportunity Fund. The project described was supported by Award Number TL1RR029888 from the National Center for Research Resources (J.H.K.). We thank Ben Burkley, Lynda Stauffer, and Cheryl Galloway for processing and genotyping samples and the patients who participated in INVEST-GENES.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflicts of interest.
References
- 1.Verdecchia P, Reboldi G, Angeli F, Borgioni C, Gattobigio R, Filippucci L, et al. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension. 2004 May;43(5):963–9. doi: 10.1161/01.HYP.0000125726.92964.ab. [DOI] [PubMed] [Google Scholar]
- 2.Alderman MH, Cohen H, Madhavan S. Diabetes and cardiovascular events in hypertensive patients. Hypertension. 1999 May;33(5):1130–4. doi: 10.1161/01.hyp.33.5.1130. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Carter BL, Einhorn PT, Brands M, He J, Cutler JA, Whelton PK, et al. Thiazide-induced dysglycemia: call for research from a working group from the national heart, lung, and blood institute. Hypertension. 2008 Jul;52(1):30–6. doi: 10.1161/HYPERTENSIONAHA.108.114389. [DOI] [PubMed] [Google Scholar]
- 5.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007 Jan 20;369(9557):201–7. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 6.Barroso I. Genetics of Type 2 diabetes. Diabet Med. 2005 May;22(5):517–35. doi: 10.1111/j.1464-5491.2005.01550.x. [DOI] [PubMed] [Google Scholar]
- 7.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000 Dec;49(12):2201–7. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 8.Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010 Jul;42(7):579–89. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper-Dehoff R, Cohen JD, Bakris GL, Messerli FH, Erdine S, Hewkin AC, et al. Predictors of development of diabetes mellitus in patients with coronary artery disease taking antihypertensive medications (findings from the INternational VErapamil SR-Trandolapril STudy [INVEST]) Am J Cardiol. 2006 Oct 1;98(7):890–4. doi: 10.1016/j.amjcard.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 10.de Miguel-Yanes JM, Shrader P, Pencina MJ, Fox CS, Manning AK, Grant RW, et al. Genetic risk reclassification for type 2 diabetes by age below or above 50 years using 40 type 2 diabetes risk single nucleotide polymorphisms. Diabetes Care. 2011 Jan;34(1):121–5. doi: 10.2337/dc10-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007 Jun 7;447(7145):661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cauchi S, El Achhab Y, Choquet H, Dina C, Krempler F, Weitgasser R, et al. TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: a global meta-analysis. J Mol Med. 2007 Jul;85(7):777–82. doi: 10.1007/s00109-007-0203-4. [DOI] [PubMed] [Google Scholar]
- 13.Tong Y, Lin Y, Zhang Y, Yang J, Liu H, Zhang B. Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: a large Human Genome Epidemiology (HuGE) review and meta-analysis. BMC Med Genet. 2009;10:15. doi: 10.1186/1471-2350-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007 Sep;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009 Feb;32(2):287–94. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muendlein A, Saely CH, Geller-Rhomberg S, Sonderegger G, Rein P, Winder T, et al. Single nucleotide polymorphisms of TCF7L2 are linked to diabetic coronary atherosclerosis. PLoS One. 2011;6(3):e17978. doi: 10.1371/journal.pone.0017978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bozkurt O, de Boer A, Grobbee DE, de Leeuw PW, Kroon AA, Schiffers P, et al. Variation in Renin-Angiotensin system and salt-sensitivity genes and the risk of diabetes mellitus associated with the use of thiazide diuretics. Am J Hypertens. 2009 May;22(5):545–51. doi: 10.1038/ajh.2009.38. [DOI] [PubMed] [Google Scholar]
- 18.Irvin MR, Lynch AI, Kabagambe EK, Tiwari HK, Barzilay JI, Eckfeldt JH, et al. Pharmacogenetic association of hypertension candidate genes with fasting glucose in the GenHAT Study. J Hypertens. 2010 Oct;28(10):2076–83. doi: 10.1097/HJH.0b013e32833c7a4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karnes JH, McDonough CW, Gong Y, Vo TT, Langaee TY, Chapman AB, et al. Association of KCNJ1 variation with change in fasting glucose and new onset diabetes during HCTZ treatment. Pharmacogenomics J. 2012 Aug 21; doi: 10.1038/tpj.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepine CJ, Handberg-Thurmond E, Marks RG, Conlon M, Cooper-DeHoff R, Volkers P, et al. Rationale and design of the International Verapamil SR/Trandolapril Study (INVEST): an Internet-based randomized trial in coronary artery disease patients with hypertension. J Am Coll Cardiol. 1998 Nov;32(5):1228–37. doi: 10.1016/s0735-1097(98)00423-9. [DOI] [PubMed] [Google Scholar]
- 21.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003 Dec 3;290(21):2805–16. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 22.The sixth report of the Joint National Committee on prevention, detection evaluation, and treatment of high blood pressure. Arch Intern Med. 1997 Nov 24;157(21):2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 23.Bakris GL, Gaxiola E, Messerli FH, Mancia G, Erdine S, Cooper-DeHoff R, et al. Clinical outcomes in the diabetes cohort of the INternational VErapamil SR-Trandolapril study. Hypertension. 2004 Nov;44(5):637–42. doi: 10.1161/01.HYP.0000143851.23721.26. [DOI] [PubMed] [Google Scholar]
- 24.Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010 Jul 7;304(1):61–8. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keating BJ, Tischfield S, Murray SS, Bhangale T, Price TS, Glessner JT, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS One. 2008;3(10):e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper-DeHoff RM, Aranda JM, Jr, Gaxiola E, Cangiano JL, Garcia-Barreto D, Conti CR, et al. Blood pressure control and cardiovascular outcomes in high-risk Hispanic patients--findings from the International Verapamil SR/Trandolapril Study (INVEST) Am Heart J. 2006 May;151(5):1072–9. doi: 10.1016/j.ahj.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006 Aug;38(8):904–9. doi: 10.1038/ng1847. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 28.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005 Jan 15;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 29.Khoury MJ, Flanders WD. Nontraditional epidemiologic approaches in the analysis of gene-environment interaction: case-control studies with no controls! Am J Epidemiol. 1996 Aug 1;144(3):207–13. doi: 10.1093/oxfordjournals.aje.a008915. [DOI] [PubMed] [Google Scholar]
- 30.Richardson DB, Kaufman JS. Estimation of the relative excess risk due to interaction and associated confidence bounds. Am J Epidemiol. 2009 Mar 15;169(6):756–60. doi: 10.1093/aje/kwn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conneely KN, Boehnke M. So many correlated tests, so little time! Rapid adjustment of P values for multiple correlated tests. Am J Hum Genet. 2007 Dec;81(6):1158–68. doi: 10.1086/522036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007 Jun 1;316(5829):1336–41. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010 Feb;42(2):142–8. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott LJ, Bonnycastle LL, Willer CJ, Sprau AG, Jackson AU, Narisu N, et al. Association of transcription factor 7-like 2 (TCF7L2) variants with type 2 diabetes in a Finnish sample. Diabetes. 2006 Sep;55(9):2649–53. doi: 10.2337/db06-0341. [DOI] [PubMed] [Google Scholar]
- 35.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006 Mar;38(3):320–3. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 36.Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, Shuldiner AR, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006 Jul 20;355(3):241–50. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humphries SE, Gable D, Cooper JA, Ireland H, Stephens JW, Hurel SJ, et al. Common variants in the TCF7L2 gene and predisposition to type 2 diabetes in UK European Whites, Indian Asians and Afro-Caribbean men and women. J Mol Med. 2006 Dec;84(12):1005–14. doi: 10.1007/s00109-006-0108-7. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg NA, Huang L, Jewett EM, Szpiech ZA, Jankovic I, Boehnke M. Genome-wide association studies in diverse populations. Nat Rev Genet. 2010 May;11(5):356–66. doi: 10.1038/nrg2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbein SC, Chu WS, Das SK, Yao-Borengasser A, Hasstedt SJ, Wang H, et al. Transcription factor 7-like 2 polymorphisms and type 2 diabetes, glucose homeostasis traits and gene expression in US participants of European and African descent. Diabetologia. 2007 Aug;50(8):1621–30. doi: 10.1007/s00125-007-0717-x. [DOI] [PubMed] [Google Scholar]
- 40.Yan Y, North KE, Heiss G, Klein R, Girman CJ, Lange EM, et al. Transcription factor 7-like 2 (TCF7L2) polymorphism and context-specific risk of impaired fasting glucose in African American and Caucasian adults: the atherosclerosis risk in communities (ARIC) study. Diabetes Metab Res Rev. 2010 Jul;26(5):371–7. doi: 10.1002/dmrr.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parra EJ, Cameron E, Simmonds L, Valladares A, McKeigue P, Shriver M, et al. Association of TCF7L2 polymorphisms with type 2 diabetes in Mexico City. Clin Genet. 2007 Apr;71(4):359–66. doi: 10.1111/j.1399-0004.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 42.Lehman DM, Hunt KJ, Leach RJ, Hamlington J, Arya R, Abboud HE, et al. Haplotypes of transcription factor 7-like 2 (TCF7L2) gene and its upstream region are associated with type 2 diabetes and age of onset in Mexican Americans. Diabetes. 2007 Feb;56(2):389–93. doi: 10.2337/db06-0860. [DOI] [PubMed] [Google Scholar]
- 43.Sale MM, Smith SG, Mychaleckyj JC, Keene KL, Langefeld CD, Leak TS, et al. Variants of the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in an African-American population enriched for nephropathy. Diabetes. 2007 Oct;56(10):2638–42. doi: 10.2337/db07-0012. [DOI] [PubMed] [Google Scholar]
- 44.Palmer ND, McDonough CW, Hicks PJ, Roh BH, Wing MR, An SS, et al. A genome-wide association search for type 2 diabetes genes in African Americans. PLoS One. 2012;7(1):e29202. doi: 10.1371/journal.pone.0029202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waters KM, Stram DO, Hassanein MT, Le Marchand L, Wilkens LR, Maskarinec G, et al. Consistent association of type 2 diabetes risk variants found in europeans in diverse racial and ethnic groups. PLoS Genet. 2010 Aug;6(8) doi: 10.1371/journal.pgen.1001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong Y, McDonough CW, Wang Z, Hou W, Cooper-DeHoff RM, Langaee TY, et al. Hypertension susceptibility loci and blood pressure response to antihypertensives: results from the pharmacogenomic evaluation of antihypertensive responses study. Circ Cardiovasc Genet. 2012 Dec;5(6):686–91. doi: 10.1161/CIRCGENETICS.112.964080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol. 2003 Feb;56(2):148–54. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- 48.Aguilar D, Solomon SD, Kober L, Rouleau JL, Skali H, McMurray JJ, et al. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation. 2004 Sep 21;110(12):1572–8. doi: 10.1161/01.CIR.0000142047.28024.F2. [DOI] [PubMed] [Google Scholar]
- 49.Bosch J, Lonn E, Pogue J, Arnold JM, Dagenais GR, Yusuf S. Long-term effects of ramipril on cardiovascular events and on diabetes: results of the HOPE study extension. Circulation. 2005 Aug 30;112(9):1339–46. doi: 10.1161/CIRCULATIONAHA.105.548461. [DOI] [PubMed] [Google Scholar]
- 50.Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH, et al. Ramipril and the development of diabetes. JAMA. 2001 Oct 17;286(15):1882–5. doi: 10.1001/jama.286.15.1882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.