Abstract
The mechanisms that enforce T cell quiescence are incompletely understood. Slfn2 has now been identified as another participant in this process, functioning as a critical regulator of T cell– and monocyte-mediated immunity.
The Marvel Comics heroine Elektra is a ninja assassin skilled in the martial arts. In contrast, elektra mice, described in this issue of Nature Immunology, are defenseless and succumb to normally harmless viral and bacterial infections1. Berger et al. report studies in which they screen N-ethyl-N-nitrosourea–exposed mice for their inability to survive infection with sublethal doses of mouse cytomegalovirus1. Using this approach, they identify the elektra mutation. Further investigation shows that elektra mice are also susceptible to lymphocytic choriomeningitis virus and Listeria monocytogenes, which shows them to be truly immunodeficient.
Initial analysis demonstrates that the serum of elektra homozygotes has normal concentrations of cytokines. Natural killer cell function, a critical component of anti-cytomegalovirus immunity, is also intact. In contrast, CD4+ and CD8+ T cell numbers are much lower in the periphery of elektra mice. Further analysis shows that there are fewer inflammatory monocytes in response to infection. Curiously, B cells seem to be unaffected in the mutant mice. Interestingly, T cells from elektra mice exist in a semiactivated state, expressing several activation markers on their surface. Although elektra T cells express CD44 on their surface, they lack memory markers such as CD122. Initially, T cells from elektra mice readily divide in response to diverse stimuli. However, activation leads to excessive cell death via the intrinsic apoptotic pathway. Those and other observations suggest that the immunodeficient phenotype is secondary to a defect in T cell quiescence. Ultimately, Berger et al. map the elektra phenotype to a mutation in Slfn2 (ref. 1). In support of that conclusion, transgenic expression of Slfn2 in elektra mice reverses the immunodeficiency. Thus, Slfn2 acts as a regulator of T cell quiescence.
Antigen-specific T cells have a vital role in the adaptive immune response. In the periphery, they stand guard, poised to respond to foreign invasion. However, peripheral lymphocytes must be kept in reserve in a state of suspended animation known as ‘quiescence’ to prevent nonspecific widespread immune activation and destruction. In addition, by ratcheting down the state of activation, quiescence prevents the unnecessary use of resources by the huge repertoire of lymphocytes. Thus, quiescence is a reversible state characterized by small, metabolically less active, apoptosis-resistant cells2. In contrast, senescence is an irreversible state characterized by large cells that have lost the ability to proliferate. Quiescent cells can further be distinguished from anergic T cells by their ability to proliferate after antigen stimulation, whereas anergic cells fail to respond to rechallenge. Although once thought to be a default state, cellular quiescence is now believed to be an actively maintained inhibitory state2,3. Quiescence is not due just to lack of antigen recognition but is due to well-orchestrated interactions among transcription factors and cell-cycle regulators. Microarray studies comparing activated and quiescent cells have demonstrated distinctly different patterns of ‘active’ versus ‘quiescent’ gene programs4,5. These negative pathways prevent inappropriate cellular activation and excessive metabolic activity while allowing continued cellular survival and responsiveness when needed. Additionally, it has been proposed that quiescence might have an important role in protecting cells from genetic damage resulting from repetitive replication. That is, reversibly arresting quiescent cells in G0 may help prevent the development of malignancy6. It is easy to imagine that in the immune system, constant replication of the enormous repertoire of lymphocytes (only a small fraction of which will ever be activated by specific antigens) might pose a threat for the development of leukemias and lymphomas. The active maintenance of quiescence helps to prevent this from occurring.
Quiescence seems to be a finely honed state that involves both the activation of ‘quiescent’ genes and the inhibition of ‘activation’ genes2,3,6. During thymic development, T cells are positively selected on the basis of their interaction with major histocompatibility complex and self peptide. It is thought that in the periphery this interaction signals the expression of not only proteins responsible for survival but also a set of negatively regulatory molecules responsible for inhibiting activation2. Although an increasing number of molecules, such as interleukin 15 and the transcription factors Klf2, NF-AT2c and Smad3 as well as those of the Foxo family and Tob, have been linked to the maintenance of quiescence, controversy exists regarding their exact roles2–7. This confusion stems from the ability of these molecules to regulate global cellular processes independently of quiescence, such as apoptosis and survival. For example, Tob, a member of the APRO (antiproliferative) family of proteins, can interact with the Smad proteins to regulate the transcription of genes involved in both the induction and the inhibition of cell growth6. Also, in T cells, Tob1 is downregulated by engagement of the T cell antigen receptor in the context of costimulation and has been suggested to be involved in T cell anergy.
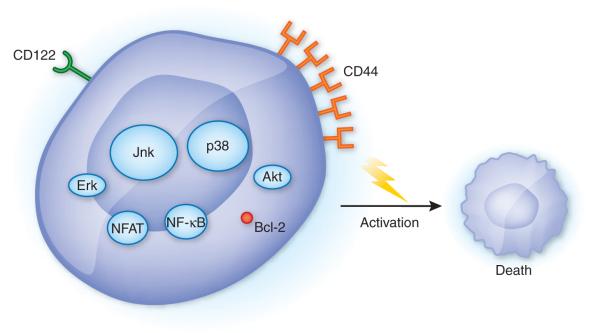
The elektra mutation identifies another participant in the maintenance of quiescence1. Slfn2 is one of nine members of the Schlafen family of genes clustered on mouse chromosome 11. These genes were first described in mice as being transcribed during thymocyte development and have been linked to the regulation of cell growth and differentiation8. Subsequently, they have been shown to be variably expressed in effector and regulatory T cells, as well as macrophages9,10. Although it is clear that these genes are expressed differently in cells of hematopoietic origin, their precise function has yet to be determined. Adding to the mystery of this gene family is the fact that many orthopoxviruses contain homologs of Schlafen genes, which suggests that these viruses have learned to manipulate Schlafen function for their own purposes11. The electra mutation turns out to be a thymidine-to-adenosine transversion in Slfn2 that leads to an isoleucine-to-asparagine substitution at position 135 of Slfn2. At this time, the region in which the substitution is located has no known or predicted function. Indeed, the precise mechanism by which Slfn2 mediates its inhibitory effect has yet to be determined; however, some clues exist. First, although elektra mice show normal activation of the signaling pathways of the transcription factors NFAT and NF-κB and the kinases Erk and Akt, the mitogen-activate protein kinases p38 and Jnk are constitutively phosphorylated, even under basal conditions (Fig. 1). Second, expression of the antiapoptotic factor Bcl-2 is specifically lower in T cells from the elektra mice. Transgenic expression of Bcl-2 prevents the apoptosis seen in CD4+ and CD8+ T cells from elektra mice. However, Bcl-2 expression is not able to prevent the semiactivated state of these cells.
Figure 1.
Role of Slfn2 in maintaining T cell quiescence. T cells from elektra mice show normal activation of NFAT, NF-κB, Erk and Akt but enhanced basal activation of Jnk and p38 (oval size indicates degree of activation) relative to that of wild-type cells. Furthermore, elektra T cells demonstrate an activated phenotype, with the expression of CD44, but lack memory markers such as CD122. After being activated, the cells die by the intrinsic apoptotic pathway and show lower expression of Bcl-2.
The study of Berger et al.1 demonstrates the potential power of N-ethyl-N-nitrosourea–induced mutational screening. The finding that the immunodeficiency seen in the elektra mice is due to a mutation in a gene that is a member of a gene family with as-yet-unclearly defined function opens many new avenues in terms of exploring regulation of immune responses. Indeed, the finding that the Slfn2 mutation disrupts quiescence, which in turn has such a profound and specific effect on immunocompetence, is somewhat surprising. It might have been expected that the elimination of a regulator of quiescence would have led to hyperactive immune responses and even autoimmunity. Likewise, the relative specificity of the Slfn2 mutation is intriguing. It affects T cells and monocytes but not natural killer cells or B cells. It affects specific T cell antigen receptor–induced signaling pathways while having no effect on others. In this context, it will be of great interest to decipher the downstream targets responsible for mediating Slfn2 function.
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Contributor Information
Maureen R Horton, Department of Medicine, Johns Hopkins University school of Medicine, Baltimore, Maryland, USA..
Jonathan D Powell, Department of Oncology, Johns Hopkins University school of Medicine, Baltimore, Maryland, USA..
References
- 1.Berger M, et al. Nat. Immunol. 2010;11:335–343. doi: 10.1038/ni.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modiano JF, Johnson LD, Bellgrau D. Immunol. Res. 2008;41:137–153. doi: 10.1007/s12026-008-8017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzachanis D, Lafuente EM, Li L, Boussiotis VA. Leuk. Lymphoma. 2004;45:1959–1967. doi: 10.1080/1042819042000219494. [DOI] [PubMed] [Google Scholar]
- 4.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Nat. Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 5.Glynne R, Ghandour G, Rayner J, Mack DH, Goodnow CC. Immunol. Rev. 2000;176:216–246. doi: 10.1034/j.1600-065x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 6.Tzachanis D, Boussiotis VA. Cell Cycle. 2009;8:1019–1025. doi: 10.4161/cc.8.7.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo CT, Veselits ML, Leiden JM. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz DA, Katayama CD, Hedrick SM. Immunity. 1998;9:657–668. doi: 10.1016/s1074-7613(00)80663-9. [DOI] [PubMed] [Google Scholar]
- 9.Condamine T, et al. J. Leukoc. Biol. 2009 Dec 8; published online, doi:10.1189/jlb.0609410. [Google Scholar]
- 10.Sohn WJ, et al. Mol. Immunol. 2007;44:3273–3282. doi: 10.1016/j.molimm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Gubser C, et al. J. Gen. Virol. 2007;88:1667–1676. doi: 10.1099/vir.0.82748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]