Abstract
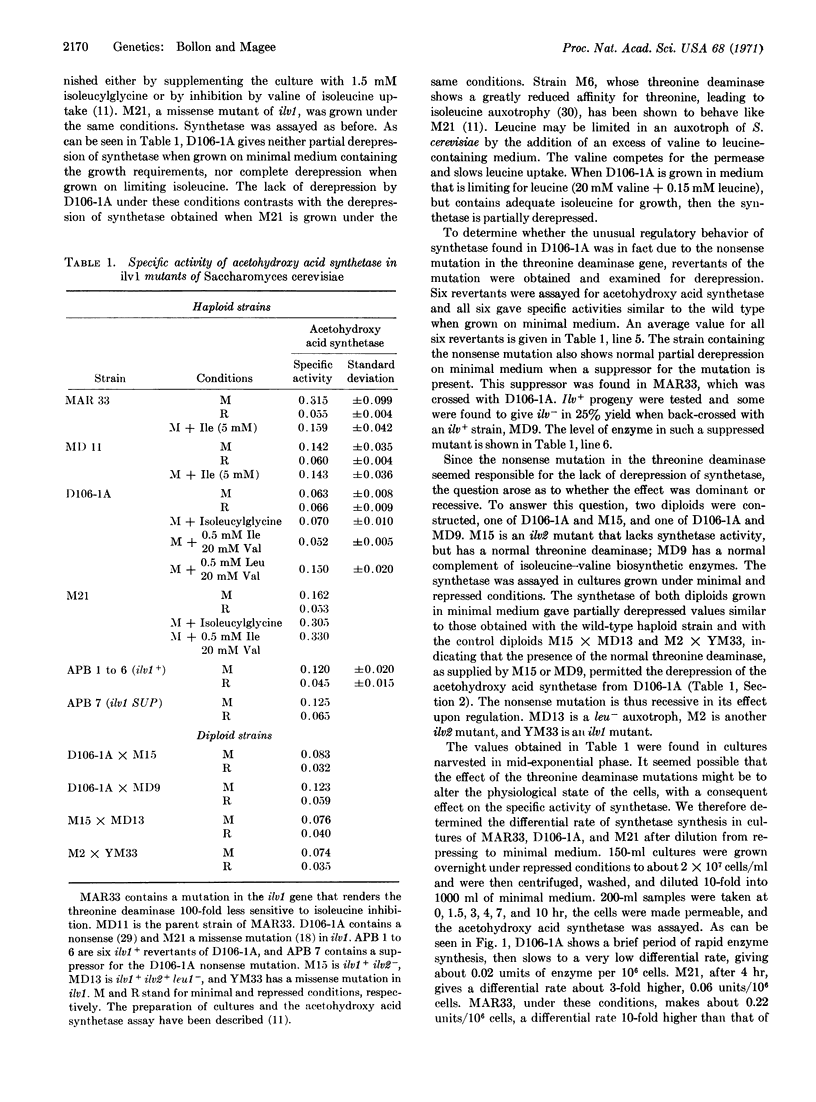
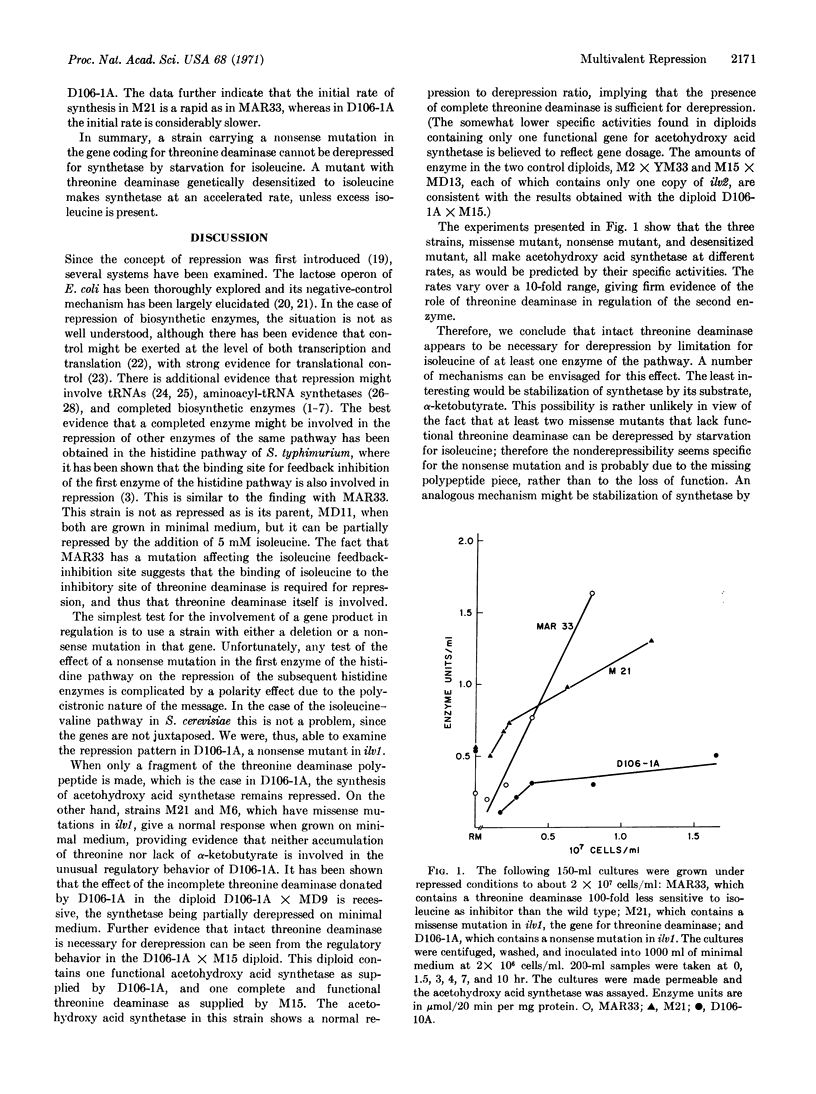
A strain (MAR33) of Saccharomyces cerevisiae containing a threonine deaminase [L-threonine hydrolyase (deaminating) EC 4.2.1.16] with decreased feedback sensitivity has been shown to have a specific activity of acetohydroxy acid synthetase higher than that of the parent strain (MD11) when both are grown on minimal medium. When strain MAR33 is grown on minimal medium supplemented only with isoleucine, the specific activity of the synthetase is reduced to that found in the parent strain. Another strain, D106-1A, contains a nonsense mutation in the middle of the gene for threonine deaminase. When this strain is grown on minimal medium containing appropriate supplements (which include a nonrepressing concentration of isoleucine), or on minimal medium supplemented with isoleucylglycine (which acts as a limiting source of isoleucine), acetohydroxy acid synthetase remains repressed. Leucine limitation causes partial derepression. With the reversion of the nonsense mutation, either intragenically or via a suppressor for the mutation, partial derepression of the synthetase returns. When D106-1A is diploidized with either M15, a mutant lacking the synthetase, or MD9, a strain containing the enzyme, normal, partially derepressed, values for this enzyme are found. This indicates that threonine deaminase is necessary for derepression, and that it possibly acts as an inducer.
Keywords: leucine, feedback, nonsense, missense
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz J. L., Hereford L. M., Magee P. T. Threonine deaminases from Saccharomyces cerevisiae mutationally altered in regulatory properties. Biochemistry. 1971 May 11;10(10):1818–1824. doi: 10.1021/bi00786a014. [DOI] [PubMed] [Google Scholar]
- Brunner A., Devillers-Mire A., Robichon-Szulmajster H. Regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. Altered threonine deaminase in an is-1 mutant responding to threonine. Eur J Biochem. 1969 Aug;10(1):172–183. [PubMed] [Google Scholar]
- Bussey H., Umbarger H. E. Biosynthesis of branched-chain amino acids in yeast: regulation of synthesis of the enzymes of isoleucine and valine biosynthesis. J Bacteriol. 1969 May;98(2):623–628. doi: 10.1128/jb.98.2.623-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. J., Pateman J. A. Autoregulation of the synthesis of nitrate reductase in Aspergillus nidulans. J Bacteriol. 1969 Mar;97(3):1374–1378. doi: 10.1128/jb.97.3.1374-1378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda E., Staub M., Venetianer P., Dénes G. Interaction between phenylalanine-tRNA and the allosteric first enzyme of the aromatic amino acid biosynthetic pathway. Biochem Biophys Res Commun. 1968 Sep 30;32(6):992–997. doi: 10.1016/0006-291x(68)90126-5. [DOI] [PubMed] [Google Scholar]
- Dwyer S. B., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XVI. Pattern of multivalent repression in strain K-12. J Bacteriol. 1968 May;95(5):1680–1684. doi: 10.1128/jb.95.5.1680-1684.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- KAKAR S. N., WAGNER R. P. GENETIC AND BIOCHEMICAL ANALYSIS OF ISOLEUCINE-VALINE MUTANTS OF YEAST. Genetics. 1964 Feb;49:213–222. doi: 10.1093/genetics/49.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach J. S., Phang J. M., Ference M., Goldberger R. F. Studies on repression of the histidine operon. II. The role of the first enzyme in control of the histidine system. Proc Natl Acad Sci U S A. 1969 Jun;63(2):481–488. doi: 10.1073/pnas.63.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisinger T., Vogel R. H., Vogel H. J. Repression-dependent alteration of an arginine enzyme in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Oct;64(2):686–692. doi: 10.1073/pnas.64.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax C. A., Woods R. A. Prototrophic regulatory mutants of adenylosuccinate synthetase in yeast. Nat New Biol. 1971 Jan 27;229(4):116–116. doi: 10.1038/newbio229116a0. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Hereford L. M. Multivalent repression of isoleucine- valine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jun;98(3):857–862. doi: 10.1128/jb.98.3.857-862.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 2. Identification and characterization of mutants lacking the acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):502–506. doi: 10.1111/j.1432-1033.1967.tb19559.x. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 3. Properties and regulation of the activity of acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):507–511. doi: 10.1111/j.1432-1033.1967.tb19560.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. S., Magee P. T., Hartwell L. H. Role of isoleucyl-transfer ribonucleic acid synthetase in ribonucleic acid synthesis and enzyme repression in yeast. J Bacteriol. 1969 Nov;100(2):579–584. doi: 10.1128/jb.100.2.579-584.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan W. L., Vogel H. J. Translational repression in the arginine system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1703–1709. doi: 10.1073/pnas.67.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. I. GENETIC DEREPRESSION OF ENZYME FORMATION. J Bacteriol. 1964 Mar;87:566–573. doi: 10.1128/jb.87.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Magee P. T. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. I. Threonine deaminase. Eur J Biochem. 1968 Feb;3(4):492–501. doi: 10.1111/j.1432-1033.1967.tb19558.x. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Fink G. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium 3. A class of regulatory mutants deficient in tRNA for histidine. J Mol Biol. 1966 Dec 28;22(2):335–347. doi: 10.1016/0022-2836(66)90136-7. [DOI] [PubMed] [Google Scholar]



