Abstract
Massage therapy has a long history and has been widely believed effective in restoring tissue function, relieving pain and stress, and promoting overall well-being. However, the application of massage-like actions and the efficacy of massage are largely based on anecdotal experiences that are difficult to define and measure. This leads to a somewhat limited evidence-based interface of massage therapy with modern medicine. In this study, we introduce a mechatronic device that delivers highly reproducible massage-like mechanical loads to the hind limbs of small animals (rats and rabbits), where various massage-like actions are quantified by the loading parameters (magnitude, frequency and duration) of the compressive and transverse forces on the subject tissues. The effect of massage is measured by the difference in passive viscoelastic properties of the subject tissues before and after mechanical loading, both obtained by the same device. Results show that this device is useful in identifying the loading parameters that are most conducive to a change in tissue mechanical properties, and can determine the range of loading parameters that result in sustained changes in tissue mechanical properties and function. This device presents the first step in our effort for quantifying the application of massage-like actions used clinically and measurement of their efficacy that can readily be combined with various quantitative measures (e.g., active mechanical properties and physiological assays) for determining the therapeutic and mechanistic effects of massage therapies.
Keywords: Medical devices, Massage therapy, Mechanics, Viscoelasticity
INTRODUCTION
Massage therapies have been employed as a relaxing and enjoyable diversion for a long time, along with many therapeutic indications including reduction of pain and stress, improvement of tissue function, and the perception of “good-feeling”. It can be inferred from the natural impulse to apply pressure to and rub sore or injured body parts. Similar behavior is common among animals, in the form of rubbing the areas of physical discomfort, and being soothed both under normal circumstances and in cases of injury, hence in all likelihood predates human kinds.27 In prehistoric societies natural impulses were likely bolstered by and intertwined with shamanistic laying-on-of-hands and early attempts at modeling of pathology involving the magical commutation of spirits or energies through physical contact and anointing with herbs. This behavioral script is supported by practices found in places upon contact with anthropologically primitive cultures, and in remnants of such practices preserved in traditional medicines around the world.10–12,24,30,31,37
During the long history of existence and different origins, several massage techniques have evolved and been named either after their place of origin, applications, mechanism of efficacy, or the developers. The most prominent ones include Swedish/deep tissue, Chinese aka Acupressure, Japanese aka Shiatsu, sports massage, reflexology, neuromuscular, just to name a few. Each methodology consists of a combination of touch, gliding/effleurage, kneading, friction, percussion, and/or joint movements. In a given practice/ course of treatment, a hybrid of methodologies is often employed. Research has begun to verify the effects of massage on the nervous/endocrine, circulatory, immune, digestive and musculo-skeletal systems and in reproductive and psychological applications. The most surprising effects of massage are perhaps those on the immune system. Studies have positively correlated massage therapies with an increase in or maintenance of natural-killer and lymphocyte immune cells in immunocompromised cancer and HIV patients.6,15,26 Similar results were seen in the T lymphocyte proliferation of healthy subjects.35 It is speculated that this effect is related to stress reduction or lymphatic activation; however the underlying mechanisms have yet to be verified.6 The neurologic effects of massage range from psycho-chemical changes within the central nervous system to changes in autonomic and somatic nerve function. For example, analysis following social grooming behavior of monkeys showed a concomitant release of opiates in the central nervous system during the behavior.27 It is correlated to increased maturation in brain and ocular function, and increased levels of hormonal growth factors including insulin-like growth factor 1 (IGF-1).18 In addition, massage is believed to promote dopaminergic activity and hence slow motor-degeneration in Parkinson’s patients,44 although not verified. Short-term increases in digestive hormone production have been shown in animal models.3,25 Moreover, Swedish massage has been shown to reduce blood pressure both acutely and with some sustained effect over time.1 Studies have also demonstrated significant cumulative effects on cerebral blood flow before and after application of massage actions.8,33 Moreover, an automated massage device has shown efficacy in decreasing swelling in patients with lymphatic edema.39
The effects of massage on the musculo-skeletal system were among the first to be discovered,11 but only recently have mechanisms of action begun to be understood. Several clinical studies have conclusively correlated massage with improved athletic performance, accelerated recovery, and reduction in perceived pain.5 Recent research on animals following eccentric exercise and massage noted a significant decrease in swelling, leukocyte influx, torn muscle fibers, as well as accelerated recovery of function.43 Among musculo-skeletal pathologies treated with massage, the two that have been best studied are carpal-tunnel syndrome and back pain. Targeted massage of carpal-tunnel diagnosed patients found improvements in grip and pinch strength in those treated over a 6 weeks period.32 Similarly, among lower back pain sufferers massage has been found to have at least short-term benefits in improving range of motion.17
In spite of the above achievements, our understanding of the efficacy and mechanisms of action of massage, especially for musculo-skeletal problems, is still rather limited.13,38 Currently, the benefits of massage therapies are often based on dearly held beliefs of massage practitioners on what has been perceived to be common sense.41 Although much of this may be true, there are some contradictions in the scientific literature. For example, a popular claim is that massage can remove lactic acid from exercised skeletal muscle thereby benefiting recovery through reduced muscle weakness and soreness.36 This claim, however, has been proven incorrect by physiological measurements showing that lactic acid clearance is not enhanced by massage in post-exercise models.14,22 In addition, it has been frequently claimed that massage can increase blood flow to muscles,4,7 although this supposition has also been questioned by increasing scientific evidence showing no influence of massage on blood flow.23,34,40 These contradictory findings may be due in part to the fact that in many of the current animal and clinical studies, massage actions are delivered by the practitioner’s palms, fingers, or other parts of the body towards the subject tissues where the mechanical loads are largely not reproducibly controlled or measured. Although it is believed that an experienced massage practitioner may estimate tissue dysfunction through touch and feel and adjust the loads accordingly, such practice is often based largely on experience of the practitioner, making it difficult to definitively determine cause and effect. It is increasingly clear that in order to determine the mechanisms underlying massage therapies, evidence-based research using standardized and quantifiable techniques is needed. In addition, massage actions applied to subject tissues should ideally be quantified by the loading parameters including magnitude, duration and frequency of the contact forces or stresses in order to more accurately characterize the mechanotransduction effects and mechanisms of action.
Coupled with the advancement of engineering technologies, robotic devices, both hand-held and stationary, have become widely available to simulate massage without the aid of a practitioner. While most of these devices can adjust loading magnitude, frequency and duration, these parameters are often justified by anecdotal observations rather than quantified responses of the tissues. Moreover, only a few measure the actual mechanical loads applied to the subject tissues.2,43 In other words, current robotic devices only substitute hands or palms of the practitioners while the loading parameters are still determined by anecdotal experience and the perception of patients and practitioners.
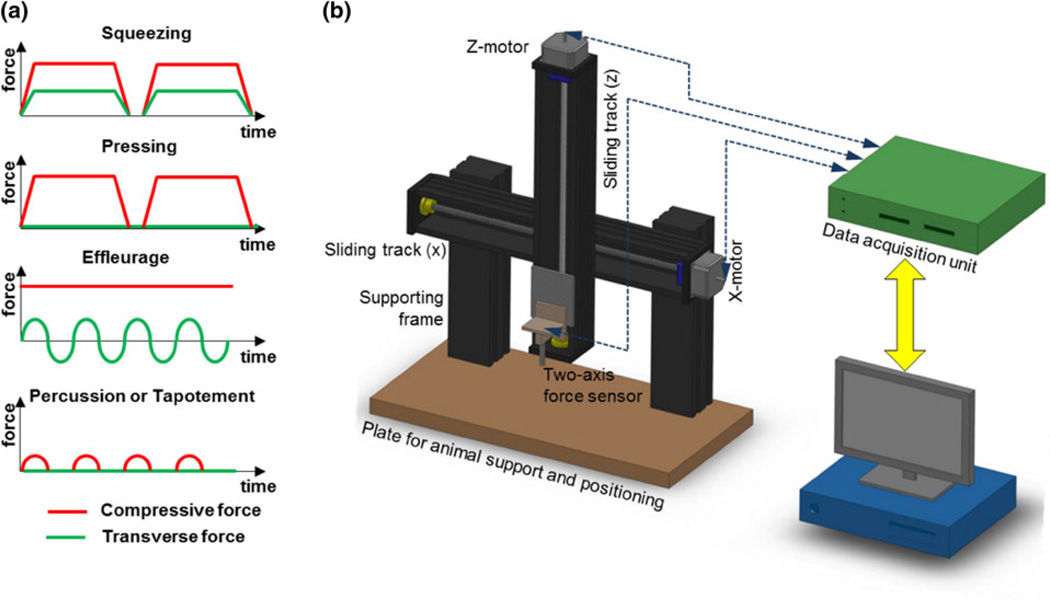
In an earlier study, we developed a pneumatic system for applying controllable length-wise mechanical loading to the hind limbs of small animals and showed that cyclic loading could enhance recovery of joint function following a controlled bout of exercise.43 In the current study, we introduce a mechatronic device that is capable of applying massage-like actions with real-time controllable loading parameters to the subject tissues of small animals as well as obtaining passive mechanical properties during tissue loading. This device is able to perform mechanical loading, with a high degree of reproducibility, and a profile similar to a variety of massage techniques. Specifically, the loading history of massage actions is represented by the profile of compressive forces normal to the skin and transverse forces parallel to the skin (Fig. 1a). Passive mechanical properties of the subject tissues before and after massage-like actions can be quantified by the device, similar to manual therapies in humans where the practitioner estimates tissue stiffness by touch. Such passive mechanical properties are believed an important measure of muscle function as evidenced by a few recent reports showing the effects of massage actions on changing passive mechanical properties of musculoskeletal tissues.13,29,42
FIGURE 1.
Mechatronic device that can generate massage-like actions to small animals: (a) Massage actions are defined by different profiles of compressive and transverse forces; (b) Schematic view of the mechatronic device that can generate different profiles of compressive and transverse forces towards small animals.
MATERIALS AND METHODS
Mechatronic Device for Mechanical Property Characterization and Massage Loading
The experimental setup (Fig. 1b) consists of a motion system for applying real-time mechanical loads with controllable parameters to the lower extremity muscles of small animals; a custom designed two-axis force sensor for measuring the mechanical forces, a solid framework for limb support and positioning, and a computer for motion control and data acquisition. The system is supplemented with a portable anesthesia machine for delivering 1.5% isoflurane to the subject animals during all experiments.
The motion system consists of two stepper motors (Oriental Motor, Co. Torrance, CA) that were positioned orthogonal to each other. Each motor was connected to a linear worm drive in a bi-directional sliding track. One sliding track was positioned horizontally and connected firmly to the ground. The other sliding track was positioned vertically and connected to a sliding block. The two tracks were assembled to generate two-axis motion along the vertical (z-axis) and horizontal (x-axis) directions. An aluminum plate was mounted on the linear worm drive of the vertical sliding track, and can move within two-dimensional plane with a resolution of 25 µm. The two stepper motors were controlled by the computer via RS-232 port using a data acquisition system (National Instruments PCI 6221, Austin, TX). A position limiter was attached to the vertical track to avoid unexpected movement of the plate to the extreme bottom.
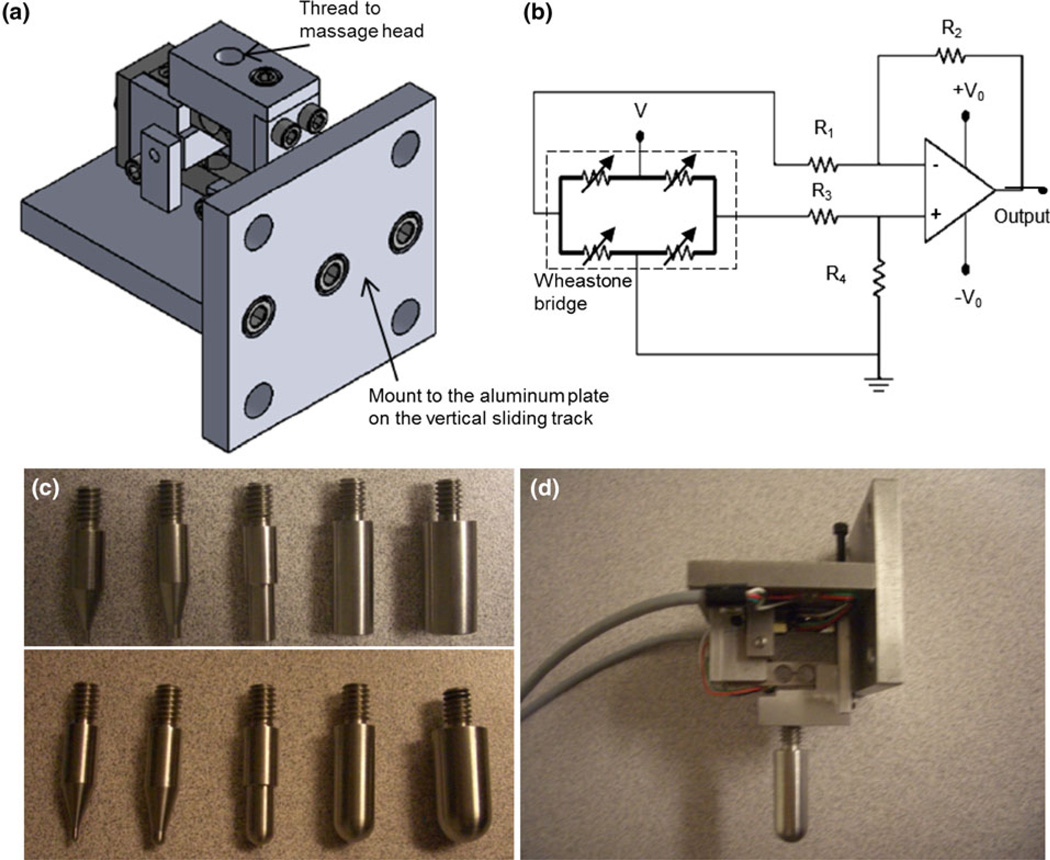
The two-axis force sensor (Fig. 2) consists of two strain gauges. Each gauge has four piezoresistors configured into a Wheatstone bridge to measure the normal mechanical strain (either compressive or tensile) exerted along each direction (x-axis or z-axis). An op amp circuit was used along each direction to amplify the strain signal. Stainless steel tips with diameters of 1/2 inch; 3/8 inch; 1/4 inch; 1/8 inch; and 1/16 inch were manufactured. The tip size was dictated by the size of the subject tissue. The tip was then mounted on the assembled two-axis force sensor for mechanical characterization or mechanical loading of the muscle tissues. Before experiments, a known gravitational force (along the compressive direction or along the transverse direction, and applied to the probing tip) of a standard weight was used to determine the relationship between the strain and the measured voltage, where the forces along the two axes were determined.
FIGURE 2.
The two-axis sensor for mechanical force measurement. (a) Schematic of the two-axis strain gauge configuration; (b) Electrical circuit for strain measurement in one direction; (c) The probing tips for mechanical loading and mechanical characterization. The tips in the top row have flat surfaces, while the tips in the bottom row have semi-spherical surfaces; and (d) The assembled sensor with a semi-spherical tip.
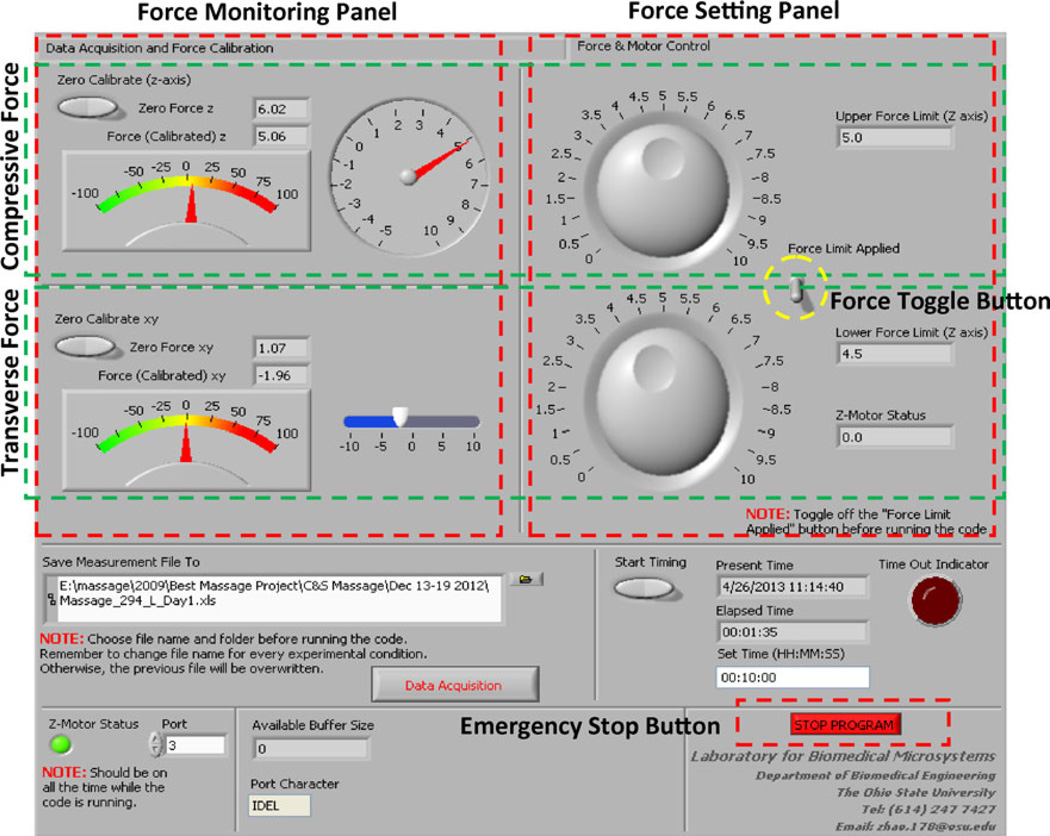
A graphic user interface (GUI) was developed using Labview software bundle to facilitate the operation (Fig. 3). The GUI has two modules: mechanical characterization and mechanical loading. The tip displacement and force profiles were set in the GUI before the operation, and their changes were monitored in real time during the muscle loading. In both modules, the measured displacements and/or the forces were fed back for appropriate control. As shown, a toggle button was provided to enable or disable the feedback mechanism. Once the feedback mechanism was turned on, the compressive force was maintained within a set range by feeding back this force to control the position of the vertical motor (Fig. 4). The transverse force was not involved in the feedback loop. The travelling stroke and frequency of the motor in the transverse direction were controlled separately using Velmex Cosmos 3.1.0. A “Stop Program” button was also provided for the operator to terminate the loading in case of an emergency such as excessive forces being applied for an extended period of time.
FIGURE 3.
Graphic user interface for mechanical characterization and mechanical loading.
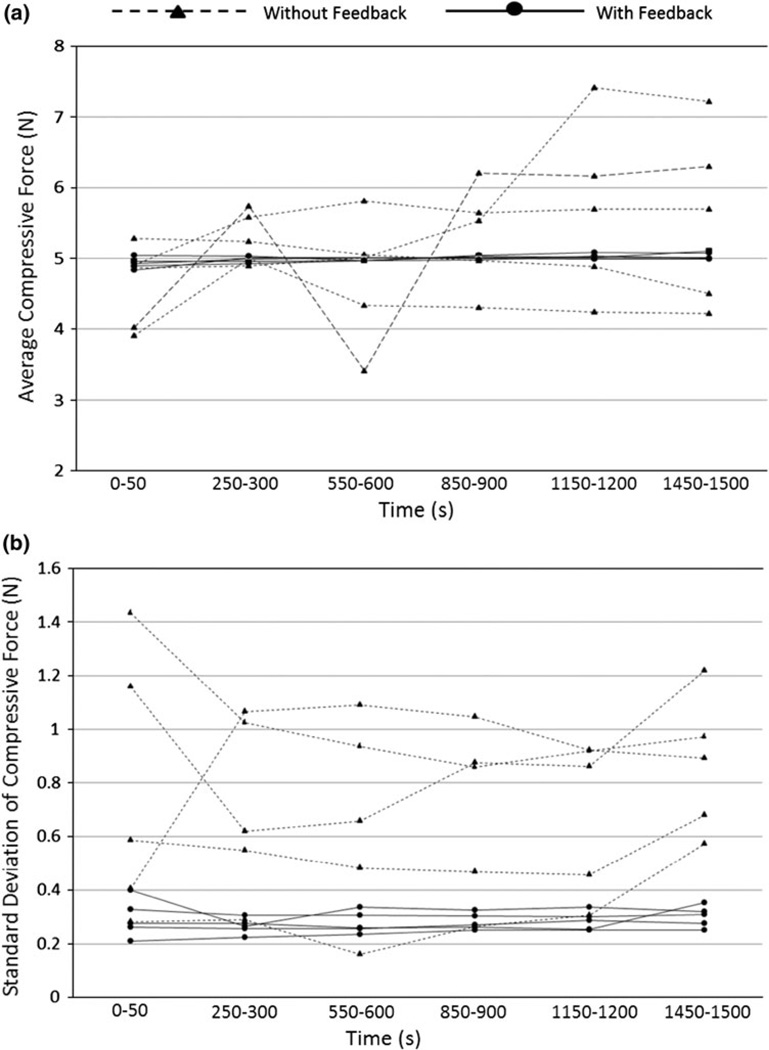
FIGURE 4.
Improvement of compressive force repeatability by the feedback mechanism can be seen from average values (a) and standard deviation (b) of the compressive forces during a 30 min loading period. The measurements were obtained from six time windows (0–50 s, 250–300 s, 550–600 s, 850–900 s, 1150–1200 s, and 1450–1500 s) from five rabbits while the feedback was enabled or disabled. It can be clearly seen that the feedback can maintain the compressive force around the set point with a smaller standard deviation comparing to the no feedback group. The compressive force was set at 5 N.
Animals
All animal experiments were approved by Institutional Laboratory of Animal Care & Use Committee (IACUC) at the Ohio State University. New Zealand White female rabbits (16–18 weeks old) and Sprague– Dawley female rats (12–14 weeks old) were used, which were anesthetized with 1.5% isoflourane and kept under anesthesia throughout all experiments. Once anesthetized, the animal’s hind limb was secured with a Velcro strap on a flat surface parallel to the traveling path of the kneading wheel described below.
Eccentric Exercise Model
For rabbits, a previously described surgical procedure was used,9 where the rabbits were surgically instrumented with bilateral peroneal nerve cuffs for stimulation of the tibialis anterior muscles. For the eccentric exercise, rabbits were secured supine in a sling with one foot attached to a footplate connected to a torque sensor on the cam of a servo-motor. Cyclic lengthening contractions were performed from a tibiotarsal angle of 95° to 145° of plantar flexion at 150°s−1, with activation preceding the muscle–tendon unit stretch by 100 ms. Previous studies have shown this set of parameters resulted in a reproducible magnitude of muscle damage, defined by the approximately 70% loss of torque production measured within 5 min of the exercise.9
Limb Positioning and Geometric Characterization
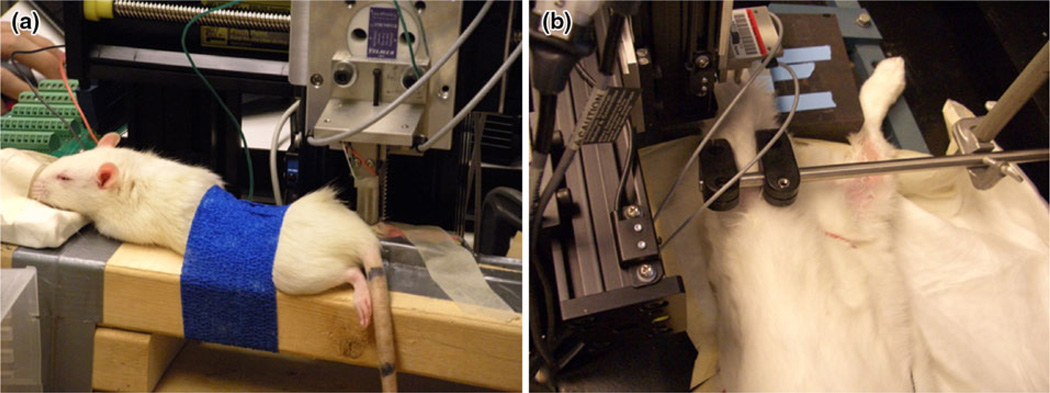
Mechanical testing was performed on the hind limbs of rats and rabbits (Fig. 5 and Supplementary Materials). For demonstration purposes, the subject animals in the rat test lay on the mounting plate in the prone position under anesthesia. The hind limb was positioned to expose the bicep femoris. The subject animals in the rabbit test lay on the mounting plate in the supine position. The hind limb was positioned to expose the tibialis anterior. For both tests, the mounting plate was rotated to align the x-axis of the sliding track with the longitudinal axis of the muscle.
FIGURE 5.
Mechanical loading on small animals using the massage device: (a) mechanical loading on the bicep femoris of a rat (a video of rat massaging is available in the supplementary material); (b) loading on the tibialis anterior muscle of a rabbit (a video of rabbit massaging is available in the supplementary material).
Estimation of tissue viscoelastic properties utilized an indentation test on the subject tissue with a small diameter probing tip.28 It was assumed that the radius of curvature of the subject tissue was greater than the size of the probing tip so that the surface of the tissue under compression was regarded planar. Given the geometries of the hind limbs of the small animals (tibialis anterior muscle of rabbits is 40–50 mm in length and 8–12 mm in width; bicep femoris of rats is 10–15 mm in length and 3–6 mm in width) and the size of the probing tips for mechanical characterization (1/8 inch flat tip for rabbits and 1/16 flat tip for rats), this assumption was valid.
Rabbit Massage Protocol
A proof-of-concept experiment was performed to show how the loading-induced mechanical property changes were dependent on the loading parameters. Muscle injury was induced to the rabbit tibialis anterior muscle of nine rabbits by a bout of intense eccentric exercise to one of the two legs.9 Following the exercise, massage-like loading that mimicked bidirectional kneading was performed on the injured muscle for 4 days. On each day, cyclic loading with a magnitude of 5 N (n = 3) or 10 N (n = 3) was applied for 30 min, with a loading frequency of 0.5 Hz. The force magnitude was determined through a pilot experiment where the probing tip was indented on the subject tissue. It was observed that when the force exceeded 15 N, intensive tissue swelling and bleeding were observed. Relaxation test was carried out on the subject muscles immediately after the exercise and after 4 days of the same massage actions. In addition, a control experiment (n = 3) was included where the animal was subject to exercise and pseudo-massage (the animal lay on the mounting plate with the same position for the same duration with those under massage but no mechanical loading was performed).
Quasilinear Viscoelastic (QLV) Model
A quasilinear model was used to qualify the viscoelastic change of the subject tissue, where the instantaneous elastic response and the viscous response are separated as:
| (1) |
where λ = ε + 1, ε is the engineering stain, G(t) was the reduced relaxation function, σe(λ) was the instantaneous elastic response, and the operator * denotes the convolution of these two factors. The instantaneous elastic response can be expressed as:
| (2) |
where AG0 was a linear parameter with the dimension of stress, and dimensionless B represents the nonlinearity of the elastic response. In this study, the reduced relaxation function G(t) was expressed using a second-order Prony series expansion:
| (3) |
where and were the 1st order and 2nd order Prony series parameters, and τ1 and τ2 were the corresponding relaxation time constants. Specifically, and τ1 corresponded to the fast relaxation; and and τ2 corresponded to the slow relaxation.
Biologic Response
Immediately after sacrifice, the tissue was harvested, weighed, flash frozen in liquid nitrogen, and carefully mounted on cork and oriented perpendicular to the long axis of the muscle. Samples were later sectioned at 8 µm thickness, and stained with hematoxylin and eosin to assess myofiber damage and cellular infiltration. Sections were viewed at 200× magnification (Nikon light microscope; Fryer Company, Inc) in order to count torn muscle fibers. In both longitudinal and cross sectional images, fibers were counted as damaged if there was any evidence of membrane discontinuity. Counts were performed by two blinded individuals for repeatability.
Statistical Analysis
Given the small sample size and the variation of data most likely due to difference in inter-animal biological responses and the degree of injury variation by ±6%, the Mann–Whitney U test was used for processing non-paired data and the Wilcoxon rank sum test was used for processing paired data. These non-parametric tests do not assume certain data distribution nor the normality condition. The alpha level was set to 0.05 for analysis. All statistics were based on the Mann–Whitney U test or the Wilcoxon rank sum test.
RESULTS
Performing Various Massage Actions on Small Animals
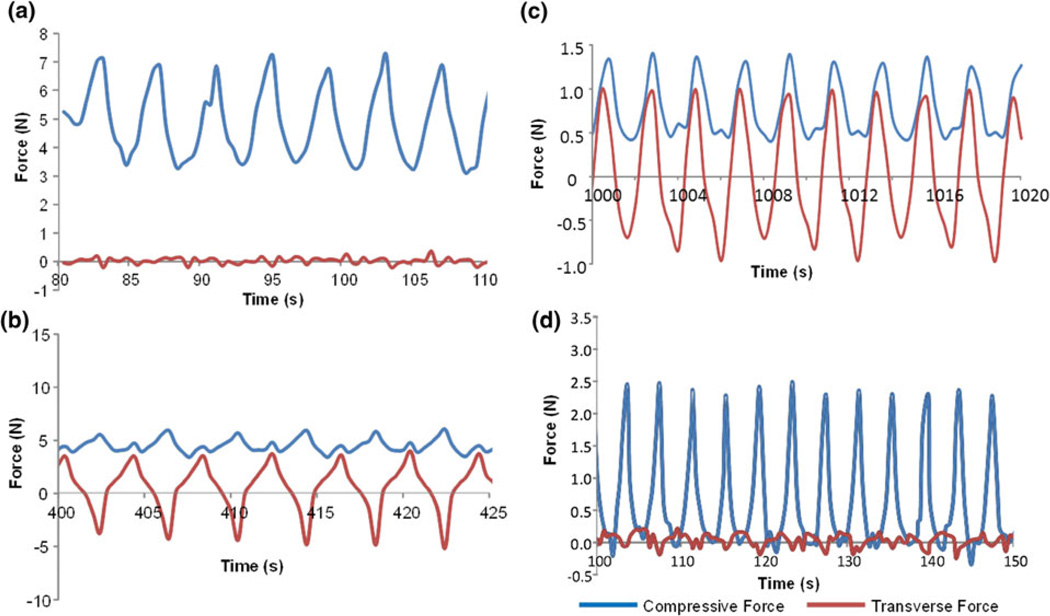
To demonstrate that the mechatronic device is able to perform various massage actions with controlled loading parameters, typical loading profiles were programmed and applied to the subject animals. For example, the action of cyclic normal pressing was characterized as periodic change of compressive force between a large positive value and a small positive value with no transverse force (Fig. 6a). Bi-directional kneading refers to a constant compressive force at a positive value, with the periodic change of transverse force between a positive value and a negative value of about the same magnitude (Figs. 6b, 6c). Tapping was characterized as the periodic change of a compressive force between a small positive value and zero with no transverse force, and with a relative high frequency (Fig. 6d).
FIGURE 6.
Force profiles of mechanical loading that mimic typical massage actions: (a) cyclic normal pressing at 0.25 Hz; (b) and (c) bi-directional kneading at 0.25 Hz and 0.5 Hz and (d) tapotement at 0.25 Hz. (a), (b) and (d) were performed on the tibialis anterior muscle of rabbit subjects; (c) was performed on the bicep femoris of rat subjects.
Viscoelastic Property Characterization by Creep and Relaxation Tests
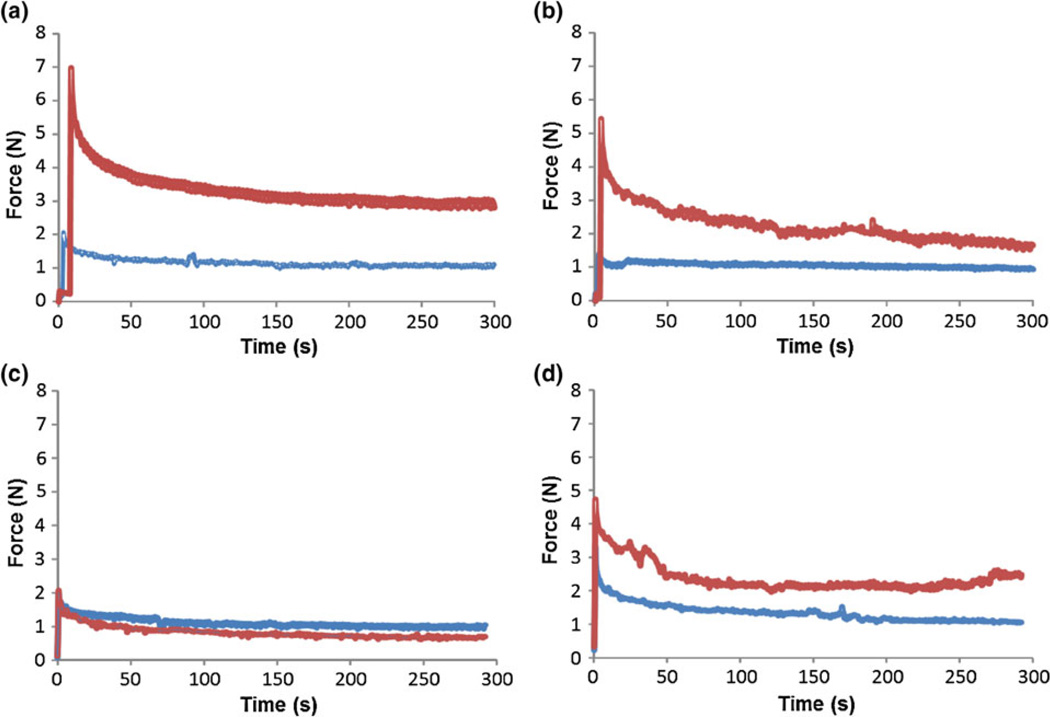
In the rabbit massage experiment, for 5 N compressive force, the tissue’s response after the massage action was distinctly different from that right after muscle exercise (Fig. 7a). This was similar to the control group (Fig. 7b), which indicated that the massage action with 5 N force amplitude did not have an obvious effect on tissue mechanical properties. The deviation of the response curve after 4 days massage action from that immediately after the exercise was attributed to the progressive tissue deterioration following the exercise. For 10 N massage, the tissue’s response curve after 4 days massage action was very close to that immediately after exercise (Fig. 7c). This was vastly different from the control group (Fig. 7d). This indicated that 10 N massage action may reduce the progressive tissue deterioration and help recovery. Collectively, the results suggested that the effect of massage action is dependent on the magnitude of compressive forces. In this study, although the change of mechanical properties can be clearly observed from the deviation of the response curve of relaxation (or creep) test after the massage action from that before the massage action, the effect was not well quantified. This was due to the fact that the actual loading profile cannot resemble the step function of an ideal creep or relaxation process due to the limited travelling velocity of the probing tip driven by the stepper motor. To this end, ramp-and-hold tests were performed to obtain the viscoelastic properties of the muscle tissue where the probing tip was driven at a finite travelling velocity in the first portion of the test and kept constant afterwards.
FIGURE 7.
Effect of mechanical loading parameters on muscle tissues. In the stress relaxation test, a step function of displacement is applied and the force evolution is measured. (a) under 5 N cyclic loading; (b) control (pseudo-massage) group for (a); (c) under 10 N cyclic loading; and (d) control (pseudo-massage) group for (c). All the tests are with 0.5 Hz loading frequency and loaded for 30 min each day for four consecutive days. The blue lines denote the measurements in the tissues post eccentric exercise, while the red lines denote the measurements in tissues post 4 days massage.
Viscoelastic Properties Characterization Using QLV Model
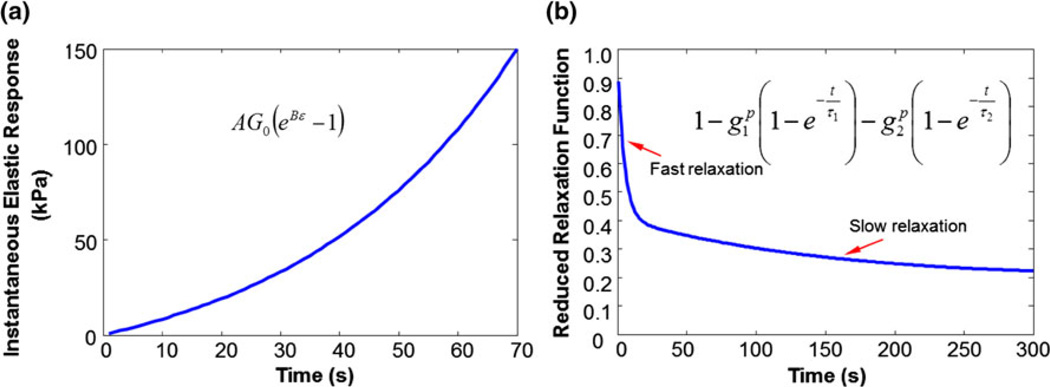
The parameters in Eqs. (2) and (3) can be determined from the experimental data. Figure 8 shows the curves of the instantaneous elastic response and the reduced relaxation function from a representative response curve to a ramp-and-hold test using the rabbit tibialis anterior.
FIGURE 8.
Quasilinear model for viscoelastic properties determination: (a) Instantaneous elastic response; and (b) reduced relaxation function.
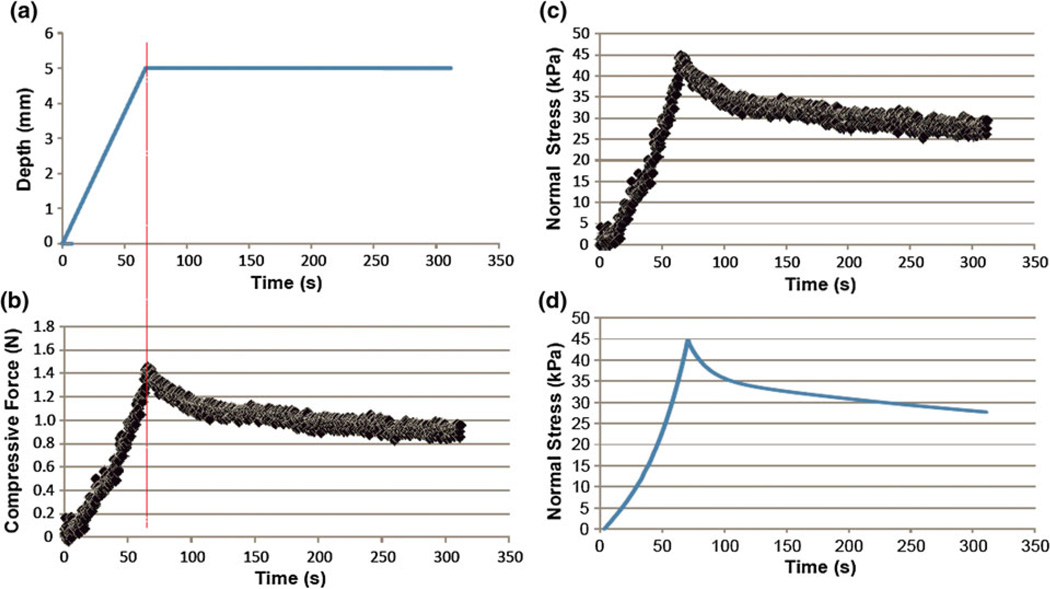
In the ramp-and-hold test, the probing tip approached the tissue at a constant traveling velocity (75 µm/s) until the indentation depth reached 5 mm. The displacement was then kept at 5 mm. The total time of the ramp-and-hold test (Fig. 9a) was 300 s. The evolution of the compressive stress (Fig. 9b) in response to the ramp-and-hold was derived from the measured compressive force (Fig. 9c). The fitted curve of the stress profile using the QLV model was used to determine the loading parameters for both the instantaneous elastic response and the reduced relaxation function in Eqs. (2) and (3). The stress profile was used to fit the QLV model.
FIGURE 9.
Ramp-and-hold test for mechanical characterization (stress relaxation) of subject tissues on a rabbit: (a) depth vs. time; (b) compressive force vs. time; (c) normal stress vs. time; and (d) fitted curved by the QLV model.s
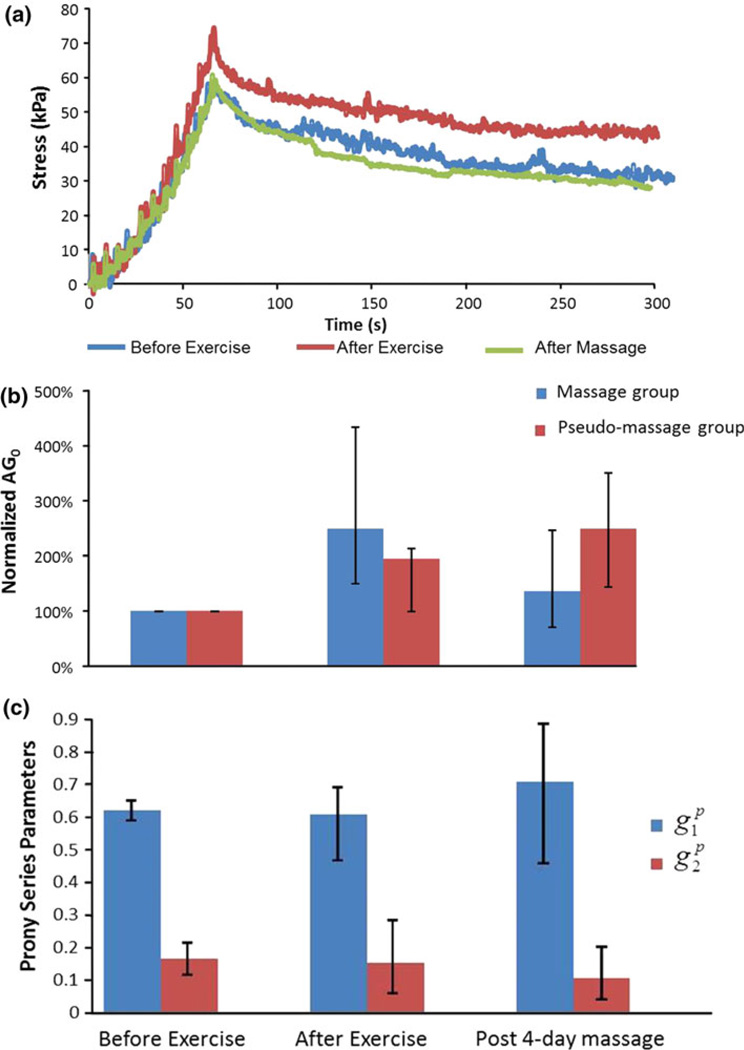
Using the QLV model, the quantitative effects of eccentric exercise and massage actions on the viscoelastic properties were determined. Figure 10a shows the response curves of the subject tissues before eccentric exercise, immediately following exercise, and after four consecutive days of massage. It was clearly shown that the massage action can facilitate recovery of passive properties of the injured tissue to a level similar to that prior to the exercise. In order to quantify such effects, the evolution of the parameters presenting the instantaneous elastic response AG0 and the viscous parameters and were investigated. To simplify the analysis, the AG0 values were normalized using the values before eccentric exercise as the reference (Fig. 10b). After exercise, the average AG0 value of the massage and pseudo-massage groups increased to 248.50 and 194.28%, respectively. Both were significantly different from the pre exercise values (p < 0.05). After 4 days, the AG0 value of the massaged group (the maximal magnitude of 10 N at the loading frequency of 0.5 Hz 15 min per day for four consecutive days) decreased to 136.17%, showing a significant recovery of elastic properties (p < 0.05). The average AG0 value of the pseudo-massage group continues to increase to 248.02%. These findings suggest that without mechanical intervention, the instantaneous elastic properties of the subject muscle would continuous to deteriorate (i.e., muscle becomes stiffer) during the 4 days period.
FIGURE 10.
Massage-like loading has a notable effect in changing the viscoelastic properties of muscle tissue: (a) the change of force profiles under ramp-and-hold test; (b) the evolution of instantaneous elastic parameter AG0 and (c) the evolution of Prony series parameters that represent viscosity changes.
The Prony series parameters of the fast relaxation and the slow relaxation were also investigated (Fig. 10c). As shown, the average and values did not exhibit significant change after exercise and after massage (p > 0.05), which suggested that under the mechanical loading used in this specific study, the Prony series parameters did not have a notable correlation with the massage actions. A more in-depth study of the effect of massage in the post eccentric exercise rabbit model can be found in a separate report.19
Biologic Response
Average TA muscle wet weight for pseudo-massage rabbits (n = 3) was 3.92 ± 0.22 g, 5 N massage (n = 3) was 3.32 ± 0.18 g, and 10 N massage (n = 3) was 3.41 ± 0.13 g. Statistical tests showed that both groups were significantly different from the pseudo-massage control (p < 0.05 for both tests). Similarly, there were significant differences in torn muscle fibers between the pseudo-massage group and the two massage groups (p < 0.05 for both tests): pseudo-massage (6.78 ± 0.41), 5 N massage (2.23 ± 0.42), and 10 N massage (2.78 ± 0.29) respectively.
DISCUSSION
Our mechatronic system can deliver compressive and transverse forces to subject tissues of small animals that mimic various types of massage-based therapies. Different from manual therapies delivered by humans, the loading parameters (magnitude, frequency and duration) can be controlled with high fidelity and adjusted within the desired ranges during the entire procedure. Moreover, the device can apply a variety of loading patterns intended to simulate a number of different types of massage. Changes in passive mechanical properties of the subject tissues, which are typically estimated clinically by the hands/palms of the practitioner, can be quantified by characterization of viscoelastic parameters utilizing the same mechatronic system. To our knowledge, these capabilities make the device the first engineering tool of its type for correlating the efficacy of massage actions with the mechanical properties of subject tissues in small animals. We demonstrated in rabbit skeletal muscle that our device can apply both reproducible compressible loads mimicking massage with high precision following an intense bout of eccentric exercise, as well as obtain passive mechanical properties of the exercised and massaged muscles. The results showed that these massage-like loading patterns alter the passive mechanical properties of the muscle following the eccentric exercise. Our system permits in vivo loading of the muscles and therefore further integration with histological and biomolecular assays to investigate biologic mechanisms underlying the efficacy of massage actions. It is also recognized however that the use of small animals and the experimental conditions in this study are different from that of manual therapy in humans, which may affect data analysis and extrapolation to the human condition. Further studies are needed to identify and clarify these potential differences as noted below.
Impact of Limb Position
During the operation, the hind limb of the subject animal was stretched to have its longitudinal axis aligned with the x-axis sliding track. Such stretching may cause tension in the hind limb. In order to compensate for the effects of limb stretching, animals under pseudo-massage actions can serve as a control group, where the animals were fixed on the mounting plate and the limb was stretched similar to those in the massage groups. Since the body position of animals in the massage and the pseudo-massage groups were kept the same, the effect of stretching induced tension can be minimized.
Impact of the Transverse Force
In the current study, the change of the muscle’s passive mechanical properties in response to massage with controlled compressive force loading was demonstrated. However, in most cases of human therapy, a transverse force in the resident tissue is also produced. As shown in Figs. 6b, 6c, transverse forces were produced during the cyclic kneading which also produced a mechanotransduction effect. Although there are active debates, there has been a speculation that the beneficial effects of specific massage actions (e.g., kneading) may be attributed to the enhancement of local circulation,7 which is highly dependent on the transverse forces parallel to the underlying blood vessels. While the quantification of the effects of these transverse forces was not demonstrated in this proof-of-concept study, future efforts will alter the feedback mechanism of the device to separate the effects of transverse and compressive forces.
Impact of Massage Induced Temperature Changes in the Tissues
The kneading action of the probing tip on the subject tissue may generate heat and elevate temperature of the animal’s skin and underlying tissues. This effect is likely similar to what occurs in human manual therapies and device assisted massage. A previous study showed that massage by roller, mechanical, and pneumatic massage units may raise skin temperature significantly.16 Heat is believed to facilitate local circulation by dilating blood vessels and increasing tissue metabolism. Although the mechanism is not clear, the heat generated by massage actions is thought to lead to an analgesic effect and fatigue reduction. The impact of massage induced heat could be better understood in future studies by incorporating a thermal couple on the probing tip or an infrared camera to measure temperature of the subject skin.
Necessity of Anesthesia
Although massage actions are usually performed in a non-anesthetic state for human subjects, the animals in this study were put under anesthesia for all massage and mechanical testing. The measurement of mechanical properties is likely affected by the level of muscle tension which would be affected by the depth of anesthesia. The exact effects of anesthesia in our model and its relevance to human massage warrant further attention.
Method of Nerve Stimulation
In order to achieve repeatable and reproducible nerve stimulation during the bout of exercise, we used a method well described and developed in our laboratory.20,21 We recognize that this is a supramaximal stimulation of the nerve and therefore not entirely clinically relevant, nevertheless it permits for a reproducible injury.
Beyond Mechanical Characterization
In addition to investigating the passive mechanical properties of skeletal muscle in response to massage, active muscle function can also been examined, e.g., by measuring the isometric torque of the ankle. A study of the time course for recovery of muscle and joint function has been reported separately.20,21 It is clear from these studies that under proper loading conditions, massage delivered by this mechatronic device can facilitate recovery of muscle and joint function. Moreover, we have also shown that massage can reduce muscle edema associated with a bout of intense eccentric exercise. Histological analysis of our tissues has shown that massage can decrease muscle fiber damage and inflammatory cell infiltration following a bout of intense eccentric exercise.20,21 Collectively, these findings further support the benefits of massage and the efficacy of our device to deliver quantifiable and repeatable bouts of massage that can facilitate recovery of mechanical properties as well as recovery of muscle and joint function and reduced tissue damage.
CONCLUSION
A mechatronic device that applies massage-like mechanical loads with controlled loading parameters in small animals and measures passive mechanical properties of the subject tissues is described. Massage-like actions were delivered to the hind limbs of rats and rabbits. Changes in passive mechanical properties can also be determined and correlated with the applied mechanical loads. By providing a quantitative measure of both the applied loads and their effects on muscle mechanical properties, this device provides a useful tool for studying the effect of therapeutic massage for interfacing this ancient alternative therapy with modern medicine.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in the publication was supported by Department of Biomedical Engineering at the Ohio State University and the National Center for Complementary and Alternative Medicine of the National Institutes of Health under Award Number RO1AT004922. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
ELECTRONIC SUPPLEMENTARY MATERIAL
The online version of this article (doi:10.1007/s 10439-013-0886-3) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Aourell M, Skoog M, Carleson J. Effects of Swedish massage on blood pressure. Complement Ther. Clin. Pract. 2005;11(4):242–246. doi: 10.1016/j.ctcp.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Ariji Y, Katsumata A, Ogi N, Izumi M, Sakuma S, Iida Y, Hiraiwa Y, Kurita K, Igarashi C, Kobayashi K, Ishii H, Takanishi A, Ariji E. An oral rehabilitation robot for massaging the masseter and temporal muscles: a preliminary report. Oral Radiol. 2009;25(1):53–59. [Google Scholar]
- 3.Ayas S, Leblebici B, Sozay S, Bayramoglu M, Niron EA. The effect of abdominal massage on bowel function in patients with spinal cord injury. Am. J. Phys. Med. Rehabil. 2006;85(12):951–955. doi: 10.1097/01.phm.0000247649.00219.c0. [DOI] [PubMed] [Google Scholar]
- 4.Beard G, Wood E. Massage: Principles and Techniques. Philadelphia, PA: Saunders; 1964. [Google Scholar]
- 5.Best TM, Hunter R, Wilcox A, Haq F. Effectiveness of sports massage for recovery of skeletal muscle from strenuous exercise. Clin. J. Sport Med. 2008;18(5):446–460. doi: 10.1097/JSM.0b013e31818837a1. [DOI] [PubMed] [Google Scholar]
- 6.Billhult A, Lindholm C, Gunnarsson R, Stener-Victorin E. The effect of massage on immune function and stress in women with breast cancer—a randomized controlled trial. Auton Neurosci. 2009;150(1–2):111–115. doi: 10.1016/j.autneu.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Brunton TL, Tunnicliffe FW. On the effects of the kneading of muscles upon the circulation, local and general. J Physiol. 1894;17(5):364–377. doi: 10.1113/jphysiol.1894.sp000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckle J, Newberg A, Wintering N, Hutton E, Lido C, Farrar JT. Measurement of regional cerebral blood flow associated with the M technique-light massage therapy: a case series and longitudinal study using SPECT. J. Altern. Complement. Med. 2008;14(8):903–910. doi: 10.1089/acm.2007.0613. [DOI] [PubMed] [Google Scholar]
- 9.Butterfield T, Zhao Y, Agarwal S, Haq F, Best T. Cyclic compressive loading facilitates recovery after eccentric exercise. Med. Sci. Sports Exerc. 2008;40(7):1289–1296. doi: 10.1249/MSS.0b013e31816c4e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvert RN. The Greek and Roman Baths. Massage Magazine. 2005 [Google Scholar]
- 11.Calvert RN. The History of Massage : An Illustrated Survey from Around the World. Rochester, VT: Healing Arts Press; 2002. [Google Scholar]
- 12.Christopoulou-Aletra H, Papavramidou N. Methods used by the hippocratic physicians for weight reduction. World J. Surg. 2004;28(5):513–517. doi: 10.1007/s00268-004-7373-9. [DOI] [PubMed] [Google Scholar]
- 13.Chunco R. The effects of massage on pain, stiffness, and fatigue levels associated with ankylosing spondylitis: a case study. Int J Ther Massage Bodywork. 2011;4(1):12–17. [PMC free article] [PubMed] [Google Scholar]
- 14.Clarkson PM, Sayers SP. Etiology of exercise-induced muscle damage. Can. J. Appl. Physiol. 1999;24(3):234–248. doi: 10.1139/h99-020. [DOI] [PubMed] [Google Scholar]
- 15.Diego MA, Field T, Hernandez-Reif M, Shaw K, Friedman L, Ironson G. HIV adolescents show improved immune function following massage therapy. Int. J. Neurosci. 2001;106(1–2):35–45. doi: 10.3109/00207450109149736. [DOI] [PubMed] [Google Scholar]
- 16.Durkin JL, Harvey A, Hughson RL, Callaghan JP. The effects of lumbar massage on muscle fatigue, muscle oxygenation, low back discomfort, and driver performance during prolonged driving. Ergonomics. 2006;49(1):28–44. doi: 10.1080/00140130500356882. [DOI] [PubMed] [Google Scholar]
- 17.Field TM. Massage therapy effects. Am. Psychol. 1998;53(12):1270–1281. doi: 10.1037//0003-066x.53.12.1270. [DOI] [PubMed] [Google Scholar]
- 18.Guzzetta A, Baldini S, Bancale A, Baroncelli L, Ciucci F, Ghirri P, Putignano E, Sale A, Viegi A, Berardi N, Boldrini A, Cioni G, Maffei L. Massage accelerates brain development and the maturation of visual function. J. Neurosci. 2009;29(18):6042–6051. doi: 10.1523/JNEUROSCI.5548-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas C, Best TM, Wang Q, Butterfield TA, Zhao Y. In vivo passive mechanical properties of skeletal muscle improve with massage-like loading following eccentric exercise. J. Biomech. 2012;45(15):2630–2636. doi: 10.1016/j.jbiomech.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas C, Butterfield TA, Zhao Y, Zhang X, Jarjoura D, Best TM. Dose-dependency of massage-like compressive loading on recovery of active muscle properties following eccentric exercise: rabbit study with clinical relevance. Br. J. Sports Med. 2013;47(2):83–88. doi: 10.1136/bjsports-2012-091211. [DOI] [PubMed] [Google Scholar]
- 21.Haas C, Butterfield TA, Abshire S, Zhao Y, Zhang X, Jarjoura D, Best TM. Massage timing affects post-exercise muscle recovery and inflammation in a rabbit model. Med. Sci. Sports Exerc. 2012;45(6):1105–1112. doi: 10.1249/MSS.0b013e31827fdf18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmings B, Smith M, Graydon J, Dyson R. Effects of massage on physiological restoration, perceived recovery, and repeated sports performance. Br. J. Sports Med. 2000;34(2):109–114. doi: 10.1136/bjsm.34.2.109. discussion 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinds T, McEwan I, Perkes J, Dawson E, Ball D, George K. Effects of massage on limb and skin blood flow after quadriceps exercise. Med. Sci. Sports Exerc. 2004;36(8):1308–1313. doi: 10.1249/01.mss.0000135789.47716.db. [DOI] [PubMed] [Google Scholar]
- 24.Hippocrates AF. The Genuine Works of Hippocrates. New York: W. Wood and Company; 1886. [Google Scholar]
- 25.Holst S, Lund I, Petersson M, Uvnas-Moberg K. Massage-like stroking influences plasma levels of gastrointestinal hormones, including insulin, and increases weight gain in male rats. Auton Neurosci. 2005;120(1–2):73–79. doi: 10.1016/j.autneu.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Ironson G, Field T, Scafidi F, Hashimoto M, Kumar M, Kumar A, Price A, Goncalves A, Burman I, Tetenman C, Patarca R, Fletcher MA. Massage therapy is associated with enhancement of the immune system’s cytotoxic capacity. Int. J. Neurosci. 1996;84(1–4):205–217. doi: 10.3109/00207459608987266. [DOI] [PubMed] [Google Scholar]
- 27.Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroen-docrinology. 1989;14(1–2):155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 28.Loerakker S, Stekelenburg A, Strijkers GJ, Rijpkema JJM, Baaijens FPT, Bader DL, Nicolay K, Oomens CWJ. Temporal effects of mechanical loading on deformation-induced damage in skeletal muscle tissue. Ann. Biomed. Eng. 2010;38(8):2577–2587. doi: 10.1007/s10439-010-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macgregor R, Campbell R, Gladden MH, Tennant N, Young D. Effects of massage on the mechanical behaviour of muscles in adolescents with spastic diplegia: a pilot study. Dev. Med. Child Neurol. 2007;49(3):187–191. doi: 10.1111/j.1469-8749.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 30.Marti-Ibanez F. The epic of medicine. Rev. Cubana Med. 1962;1:99–116. contd. [PubMed] [Google Scholar]
- 31.Massage and Bodywork. Greek Medicine. 2010 http://www.greekmedicine.net/therapies/Massage_and_Bodywork.html.
- 32.Moraska A, Chandler C, Edmiston-Schaetzel A, Franklin G, Calenda EL, Enebo B. Comparison of a targeted and general massage protocol on strength, function, and symptoms associated with carpal tunnel syndrome: a randomized pilot study. J. Altern. Complement. Med. 2008;14(3):259–267. doi: 10.1089/acm.2007.0647. [DOI] [PubMed] [Google Scholar]
- 33.Ouchi Y, Kanno T, Okada H, Yoshikawa E, Shinke T, Nagasawa S, Minoda K, Doi H. Changes in cerebral blood flow under the prone condition with and without massage. Neurosci Lett. 2006;407(2):131–135. doi: 10.1016/j.neulet.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 34.Shoemaker JK, Tiidus PM, Mader R. Failure of manual massage to alter limb blood flow: measures by Doppler ultrasound. Med. Sci. Sports Exerc. 1997;29(5):610–614. doi: 10.1097/00005768-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 35.So CS, Sarath JV, Giolli RA, Gollapudi S. The effect of thermal massage on human T-Lymphocyte and natural killer cell function. Internet J. Altern. Med. 2008;6(1) doi:10.5580/317/ [Google Scholar]
- 36.Tiidus PM. Massage and ultrasound as therapeutic modalities in exercise-induced muscle damage. Can. J. Appl. Physiol. 1999;24(3):267–278. doi: 10.1139/h99-022. [DOI] [PubMed] [Google Scholar]
- 37.Veith I. Huang Ti nei ching su wên. The Yellow Emperor’s classic of internal medicine. Berkeley: University of California Press; 1966. New ed. [Google Scholar]
- 38.Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005;35(3):235–256. doi: 10.2165/00007256-200535030-00004. [DOI] [PubMed] [Google Scholar]
- 39.Wilburn O, Wilburn P, Rockson SG. A pilot, prospective evaluation of a novel alternative for maintenance therapy of breast cancer-associated lymphedema [ISRCTN76522412] BMC Cancer. 2006;6:84. doi: 10.1186/1471-2407-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiltshire EV, Poitras V, Pak M, Hong T, Rayner J, Tschakovsky ME. Massage impairs postexercise muscle blood flow and “lactic acid” removal. Med. Sci. Sports Exerc. 2010;42(6):1062–1071. doi: 10.1249/MSS.0b013e3181c9214f. [DOI] [PubMed] [Google Scholar]
- 41. http://www.foreveractivemed.com/cms/page/whos-at-rist
- 42. http://www.chirocommunity.com/muscletightness.htm
- 43.Zeng H, Butterfield T, Agarwal S, Haq F, Best T, Zhao Y. An engineering approach for quantitative analysis of the lengthwise strokes in massage therapies. J. Med. Devices Trans. ASME. 2008;2(4):041003. [Google Scholar]
- 44.Zesiewicz TA, Evatt ML. Potential influences of complementary therapy on motor and non-motor complications in Parkinson’s disease. CNS Drugs. 2009;23(10):817–835. doi: 10.2165/11310860-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.