Abstract
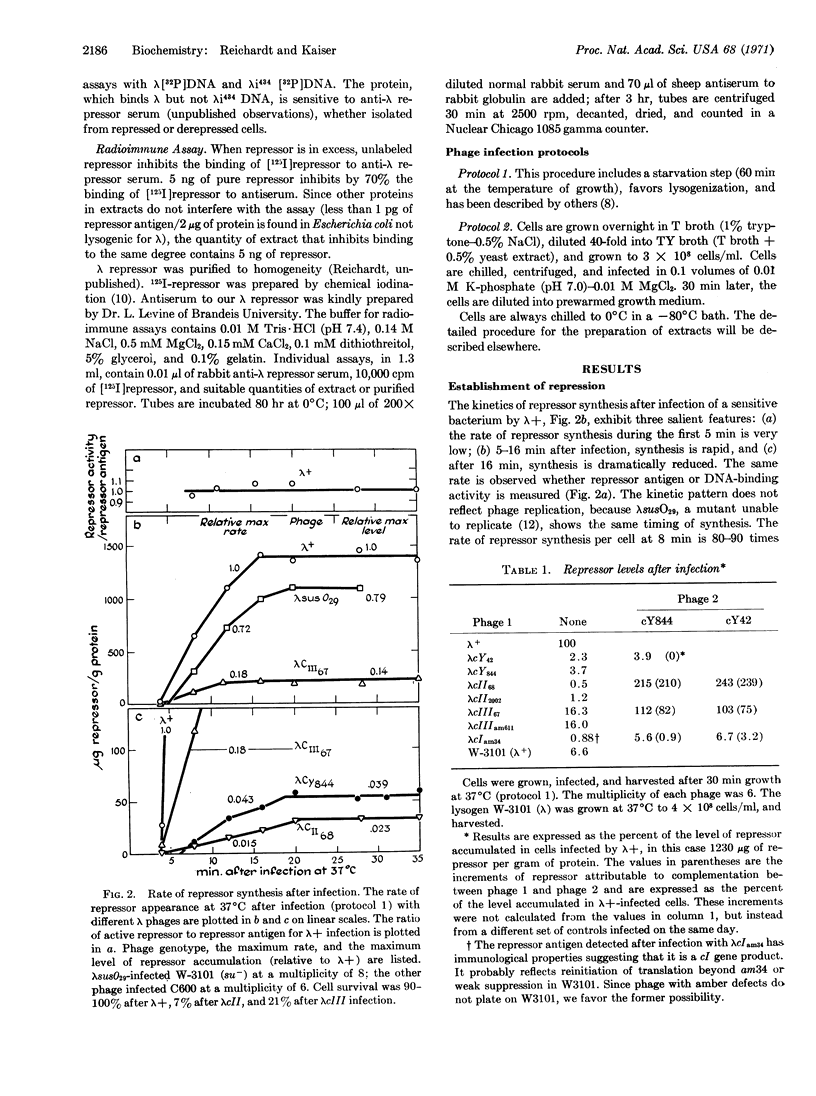
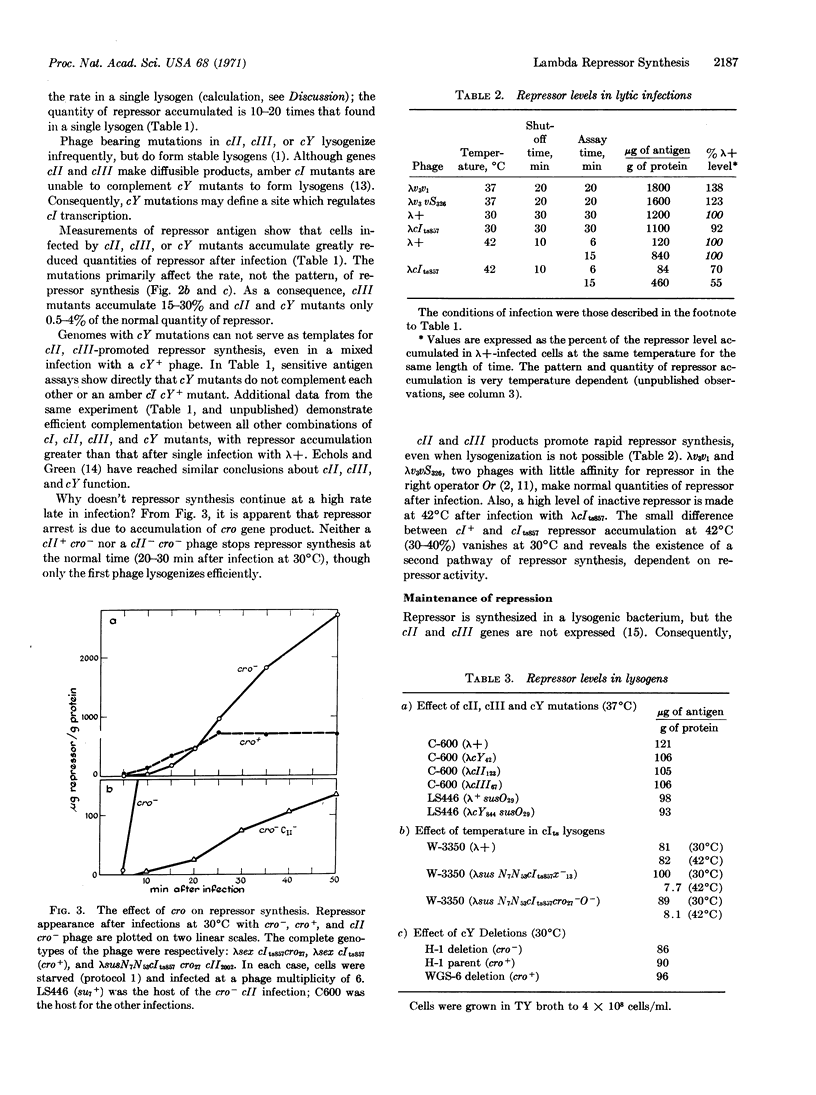
Direct measurements of the intracellular level of λ repressor have been made by a DNA-filter assay and a radioimmune assay. Transcription of cI, the structural gene for repressor, appears to initiate at two different promoters, prm and pre. Promoter pre is activated during the establishment of lysogeny by the action of cII and cIII proteins at the DNA site cY. Phage mutated in cII, cIII, or cY do not make a normal burst of repressor after infection and do not efficiently lysogenize the cell. Cro product stops repressor synthesis midway in the infective cycle.
Promoter prm maintains the repressor level in established lysogens. Delection mapping places it very near the right operator (Or). Prm is activated by repressor bound to the right operator. In the absence of cII or cIII protein, repressor synthesis requires active repressor and only proceeds on genomes able to bind repressor at Or.
Keywords: DNA binding, radioimmune assay, lysogeny versus lysis, E. coli
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODE V. C., KAISER A. D. REPRESSION OF THE C2 AND C3 CISTRONS OF PHAGE LAMBDA IN A LYSOGENIC BACTERIUM. Virology. 1965 Jan;25:111–121. doi: 10.1016/0042-6822(65)90258-8. [DOI] [PubMed] [Google Scholar]
- BROOKS K. STUDIES IN THE PHYSIOLOGICAL GENETICS OF SOME SUPPRESSOR-SENSITIVE MUTANTS OF BACTERIOPHAGE LAMBDA. Virology. 1965 Jul;26:489–499. doi: 10.1016/0042-6822(65)90011-5. [DOI] [PubMed] [Google Scholar]
- Brachet P., Thomas R. Mapping and functional analysis of y and CII mutants. Mutat Res. 1969 Mar-Apr;7(2):257–260. doi: 10.1016/0027-5107(69)90041-4. [DOI] [PubMed] [Google Scholar]
- Calef E., Neubauer Z. Active and inactive states of the CI gene in some lambda defective phages. Cold Spring Harb Symp Quant Biol. 1968;33:765–767. doi: 10.1101/sqb.1968.033.01.087. [DOI] [PubMed] [Google Scholar]
- Echols H., Green L. Establishment and maintenance of repression by bacteriophage lambda: the role of the cI, cII, and c3 proteins. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2190–2194. doi: 10.1073/pnas.68.9.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H., Brachet P., Pereira da Silva L., Jacob F. Regulation of repressor expression in lambda. Proc Natl Acad Sci U S A. 1970 Jul;66(3):855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H., Pereira da Silva L., Jacob F. Sur la régulation précoce du bactériophage lambda. C R Acad Sci Hebd Seances Acad Sci D. 1968 Mar 11;266(11):1176–1178. [PubMed] [Google Scholar]
- FRY B. A. Conditions for the infection of Escherichia coli with lambda phage and for the establishment of lysogeny. J Gen Microbiol. 1959 Dec;21:676–684. doi: 10.1099/00221287-21-3-676. [DOI] [PubMed] [Google Scholar]
- KAISER A. D., JACOB F. Recombination between related temperate bacteriophages and the genetic control of immunity and prophage localization. Virology. 1957 Dec;4(3):509–521. doi: 10.1016/0042-6822(57)90083-1. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- McMacken R., Mantei N., Butler B., Joyner A., Echols H. Effect of mutations in the c2 and c3 genes of bacteriophage lambda on macromolecular synthesis in infected cells. J Mol Biol. 1970 May 14;49(3):639–655. doi: 10.1016/0022-2836(70)90288-3. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., Bovre K., Szybalski W. Controls of rightward transcription in coliphage lambda. J Mol Biol. 1970 Dec 28;54(3):599–604. doi: 10.1016/0022-2836(70)90130-0. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., Bovre K., Szybalski W. Regulation of leftward transcription in the J-b2-att region of coliphage lambda. Mol Gen Genet. 1971;111(1):22–34. doi: 10.1007/BF00286551. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. Replication of bacteriophage DNA. I. Replication of DNA of lambda phage defective in early functions. J Mol Biol. 1968 Dec 14;38(2):217–225. doi: 10.1016/0022-2836(68)90407-5. [DOI] [PubMed] [Google Scholar]
- Pero J. Location of the phage lambda gene responsible for turning off lambda-exonuclease synthesis. Virology. 1970 Jan;40(1):65–71. doi: 10.1016/0042-6822(70)90379-x. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Signer E. R. Lysogeny: the integration problem. Annu Rev Microbiol. 1968;22:451–488. doi: 10.1146/annurev.mi.22.100168.002315. [DOI] [PubMed] [Google Scholar]
- Strack H. B., Cox J. H. Suppression of a mutant phenotype concerning head formation of bacteriophage lambda by the product of the lambda repressor gene. Virology. 1970 Jul;41(3):562–563. doi: 10.1016/0042-6822(70)90177-7. [DOI] [PubMed] [Google Scholar]
- Taylor K., Hradecna Z., Szybalski W. Asymmetric distribution of the transcribing regions on the complementary strands of coliphage lambda DNA. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1618–1625. doi: 10.1073/pnas.57.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. Control of development in temperate bacteriophages. 3. Which prophage genes are and which are not trans-activable in the presence of immunity? J Mol Biol. 1970 Apr 28;49(2):393–404. doi: 10.1016/0022-2836(70)90252-4. [DOI] [PubMed] [Google Scholar]



