Abstract
Major regulators of long-term hematopoietic stem cell (LT-HSC) self-renewal and proliferation have been identified but knowledge of their in vivo interaction in a linear pathway is lacking. Here, we show a direct genetic link between the transcription factor E47 and the major cell cycle regulator p21 in controlling LT-HSC integrity in vivo under repopulation stress. Numerous studies have shown that E47 activates p21 transcription in hematopoietic subsets in vitro and we now reveal the in vivo relevance of the E47-p21 pathway by reducing the gene dose of each factor individually (E47het or p21het) versus in tandem (E47hetp21het). E47hetp21het LT-HSCs and downstream short-term HSCs (ST-HSCs) exhibit hyperproliferation and preferential susceptibility to mitotoxin compared to wild-type or single haploinsufficient controls. In serial adoptive transfers that rigorously challenge self-renewal, E47hetp21het LT-HSCs dramatically and progressively decline, indicating importance of cell-intrinsic E47-p21 in preserving LT-HSCs under stress. Transient numeric recovery of downstream ST-HSCs enabled the production of functionally competent myeloid but not lymphoid cells as common lymphoid progenitors (CLPs) were decreased and peripheral lymphocytes virtually ablated. Thus, we demonstrate developmental compartment-specific and lineage-specific requirement for the E47-p21 pathway in maintaining LT-HSC, B and T cells under hematopoietic repopulation stress in vivo.
INTRODUCTION
A limited pool of LT-HSCs constantly replenishes downstream lymphoid and myeloid lineages that have been depleted by normal turnover or consumed by infection stress (1). Self-renewing LT-HSCs differentiate to non-renewing multipotent progenitors (MPP) and then lymphoid-myeloid primed progenitors (LMPP), which subsequently produce common lymphoid progenitors (CLP) with B and T lymphoid potential (2, 3). The E47 transcription factor, in conjunction with transcriptional partners, regulates key aspects of hematopoiesis including HSC self-renewal, lymphoid lineage priming, B and T cell fate specification and antigen receptor repertoire formation (4, 5).
Recent progress has defined the E47-dependent genes that coordinate cell activity in both developmental stage-specific and lineage-specific manners (6–12). In striking contrast to the cohort of E47 target genes that are unique to individual developmental subsets, one recurrent target of E47 binding activity stands out: the major cyclin dependent kinase inhibitor p21 (cdkn1A/cip1/waf1). We and others have shown that E47 directly activates p21 transcription in primitive HSCs in vitro (7, 10, 12). In addition, E47 levels correlate with p21 expression in B lineage precursors, and loss of a single allele of E47 reduces p21 with concomitant hyperproliferation (13). Similarly, p21 is also a direct target of E47 transcriptional activity in T lineage cells (14). However, while these in vitro studies indicate that E47 activates p21 expression in HSCs and lymphoid precursors, the biological relevance of the E47-p21 pathway to HSC function has not yet been examined. Furthermore, in addition to E47, other transcription factors also expressed in HSCs such as ikaros and Notch are capable of regulating p21 expression (15, 16). Hence, the relative contribution of E47 in regulating p21 expression in HSCs in vivo remains unknown. Also unclear is whether the E47-mediated p21 activity is required in the multipotent stages between HSCs and lympho-myeloid segregation.
Here, we establish the biological relevance of genetic interactions between E47 and p21 in LT-HSCs and downstream compartments in vivo using mice with reduced gene dosage of each factor, E47het, p21het, and E47hetp21het. Defects specific to compound haploinsufficient animals, but not either haploinsufficiency alone, reveal combined effects of two interacting partners (17, 18). Moreover, the use of E47het mice permits an analysis of mature B and T cells that is not possible in E47 knockouts due to the severe immune deficiency. We directly track LT-HSC self-renewal integrity in compound heterozygotes and examine cumulative deficiencies in downstream B and T compartments. Since E47 is dispensable after the point of myeloid restriction, myeloid precursors serve as an internal reference population for comparing the magnitude of hematopoietic deficiencies incurred upstream versus downstream of lympho-myeloid specification. Together, this approach enables us to establish the biological importance of genetic interactions between E47 and p21 specifically within LT-HSCs, within developmental compartments upstream versus downstream of lympho-myeloid restriction, and the cumulative impact to B and T cells.
METHODS
Mice
Mice were bred in accordance with Institutional Animal Care and Use Committee (IACUC) policies at the University of Pittsburgh School of Medicine. E47het(C57BL/6) mice (12) were intercrossed with p21KO mice (129/Sv; purchased from The Jackson Laboratories), and backcrossed to the C57BL/6 background for 7–8 generations. E47 and p21 genotyping were done as described (19, 20).
Flow Cytometry
BM and spleen were harvested as previously described (6, 21). Cell staining was performed using antibodies from eBioscience, BD Pharmingen or Biolegend. Primary antibodies were AA4.1 APC (clone AA4.1), B220 APC, FITC or biotin (clone RA3–6B2), CD3 FITC or biotin (clone 2C11), CD4 biotin (clone), CD11b PE, biotin or FITC (clone M1/70), CD11c FITC, CD19 APC, biotin, FITC or Cy5PE or PerCPCy5.5 (clone MB19–1), CD43 PE (clone S7), CD45.2 PacBlue or APC (clone, CD48 APC (clone HM48–1), CD48 APC (clone), CD117 APCeFluor 780 (clone 2B8), CD127 PeCy5 (clone A7R34), CD135 PE (clone A2F10), CD150 PECy7 (clone 9D1), DX5 biotin, Gr-1 biotin or FITC (clone 8C5), IgM (clone 331) biotin or FITC, Ly6C biotin (clone HK1.4), Ly6D PE (clone), NK1.1 biotin or FITC (clone PK136), TER-119 biotin or FITC (clone TER-119), and Sca-1 FITC, Cy5PE or PerCPCy5.5 (clone D7). Secondary reagents were streptavidin-Cy7PE or streptavidin-eFluor 450. Flow cytometry was performed on a four-laser, twelve-detector LSR II and a three-laser, eleven-detector Aria (BD Biosciences). Data were analyzed using FlowJo software Version 9.9.1 (Tree Star).
Treatment of Mice with 5-FU or LPS
Mice were injected i.p or i.v with 150 mg/kg 5-FU (Sigma-Aldrich) (7, 12, 22) and sacrificed either 16 hours or 14 days later as described in figure legends. For LPS treatment, mice were injected i.p. with 15 μg LPS (Sigma-Aldrich) or PBS control once a day for two days and were sacrificed on the third day as described (23).
BrdU Incorporation and anti-BrdU Staining
Mice were injected i.p. with 200 μL of 3 mg/mL BrdU at 12-h intervals for 48 hours as described (7, 12). Two days after the first injection, mice were sacrificed and BM cells stained with antibodies to relevant surface markers. BM cells were fixed and permeabilized followed by staining with anti-BrdU FITC per manufacturer’s instructions (BD Pharmingen).
Serial Transplantation Assays
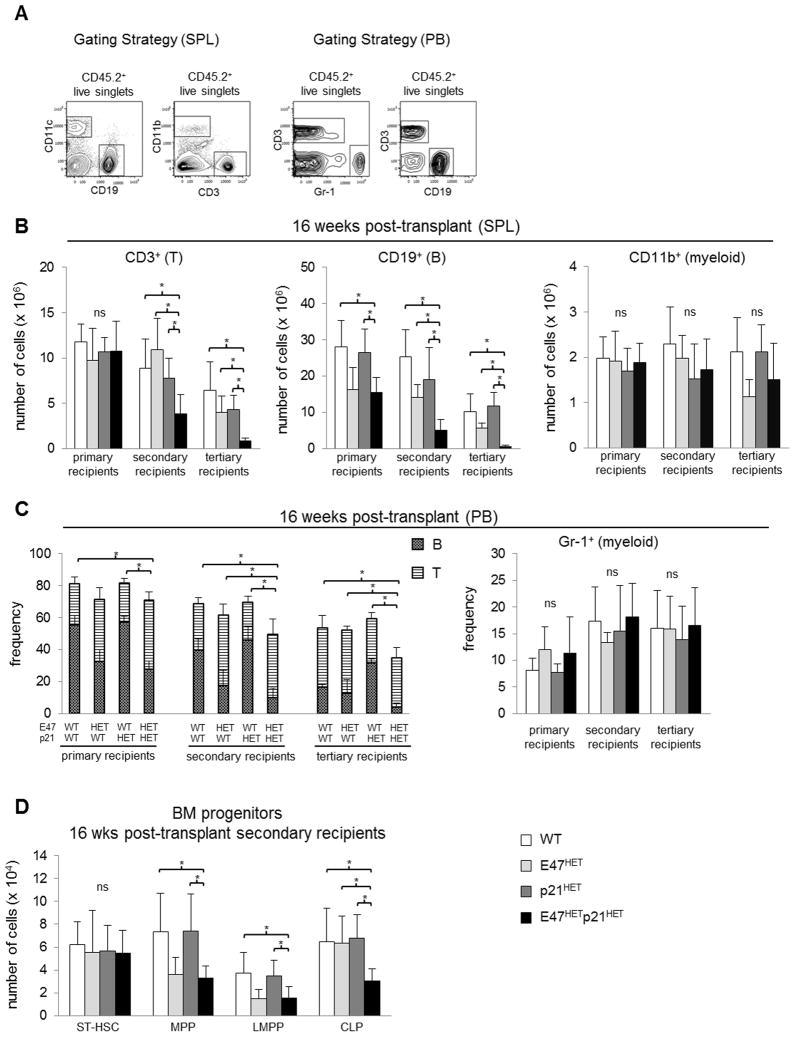
For serial transplantation assays, 2 × 106 BM cells from CD45.2+ donor mice were injected into the tail veins of sub-lethally irradiated (1000 rads) CD45.1+ C57BL/6 primary recipients. Sixteen weeks after transplantation, 2 × 106 BM cells from primary recipients were transferred into sub-lethally irradiated CD45.1+ secondary recipients. Eight weeks after transplant, 2 × 106 BM cells from secondary recipients were transferred into sub-lethally irradiated CD45.1+ tertiary recipients. Multi-lineage reconstitution was examined every 4 weeks in the peripheral blood and at 16 weeks post-transplant in spleen and BM of primary, secondary and tertiary recipients.
For homing and niche engraftment analysis, 2 × 106 CD45.2+ donor BM cells were injected into the tail veins of sub-lethally irradiated (1000 rads) CD45.1+ recipients. Recipient mice were sacrificed 2 weeks post-transplant, and donor-derived precursors enumerated.
Gene Expression Analysis
RNA was extracted using RNeasy Micro Kit (Qiagen) and reverse transcribed into cDNA with Superscript III Reverse Transcriptase (Invitrogen) using oligoDT primers (6). Quantitative real-time PCR (qPCR) reactions was performed in triplicates using Taqman probes (Invitrogen) and detected by StepOne Plus System (BioRad). Expression levels were calculated for each gene relative to actb and expressed as the fold difference relative to wild type.
Statistics
Statistical analysis was performed using one-way ANOVA with pairwise comparison. Asterisk (*) indicates p<0.05, ns indicates not statistically significant.
RESULTS
E47 and p21 functionally collaborate to regulate homeostatic LT-HSC proliferation in vivo
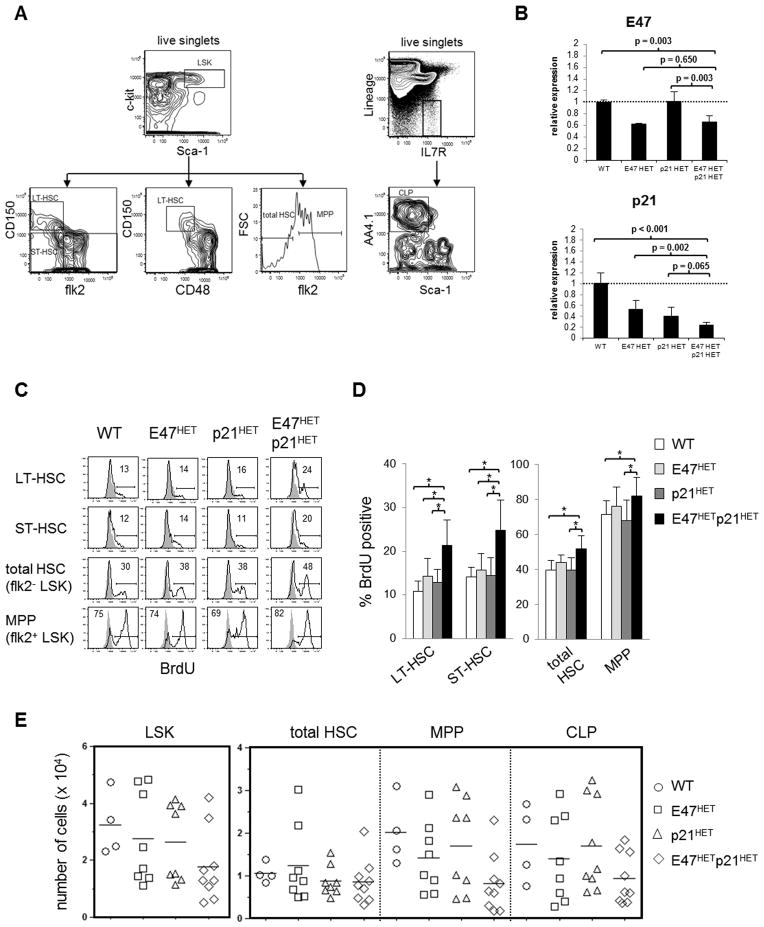
To better understand the biological importance of E47 and p21 collaboration during hematopoiesis, we developed a genetic model in which each gene is haploinsufficient individually, or in combination. We then tracked hematopoiesis during steady-state and under repopulation stress for visualization of defects following functional challenge. The genotypes are wild-type (WT), single haploinsufficiency in E47 or p21 (E47het, p21het) and compound haploinsufficiency (E47hetp21het). We directly examined E47 and p21 transcript levels in primitive, multipotent lineage negative c-kit+ Sca-1+ (LSKs) BM precursors (Figure 1A) using qPCR. E47 transcript levels were similar in WT and p21het LSKs, and were reduced by ~40% in both E47het and E47hetp21het LSKs (Figure 1B). Relative to WT, p21 transcript levels were reduced by 50–60% in both E47het LSKs and p21het LSKs and by ~75% in E47hetp21het LSKs (p<0.05). Thus, E47 activity appears to account for an appreciable proportion of p21 transcript expression. We then compared the impact of single E47 haploinsufficiency versus combined E47-p21 haploinsufficiency to hematopoietic stem and progenitor cell biology.
Figure 1. Increased homeostatic proliferation in E47hetp21hetLT-HSCs in vivo.
(A) BM from WT, E47HET, p21HET or E47HETp21HET mice was isolated and stained to resolve specific subsets of progenitor cells. Right panel, gating strategy used to identify LSKs (Lineage−c-kit+ Sca-1+), phenotypic LT-HSCs were identified as CD150+ flk2−LSK or CD150+ CD48−LSK, short-term HSCs (ST-HSC) as CD150−flk2− LSK, total HSCs as flk2− LSK and multipotent progenitors (MPP) as flk2+ LSK. Left panel, common lymphoid progenitors (CLP) were identified as Lin− IL7R+ AA4.1+ Sca-1low. (B) RNA extracted from sorted total LSKs was used to examine E47 and p21 transcript levels. Gene expression was normalized to β-actin and expression level of each gene is shown relative to WT LSK. Data are shown as mean ± SD of triplicates from two independent sorts. (C &D) WT, E47het, p21het or E47hetp21het mice were injected i.p. with 200 μL of 3 mg/mL BrdU twice a day for 2 days then sacrificed to examine proliferation status. (C) BM was either stained directly to identify total HSC (flk2−LSK) and MPP (flk2+ LSK); or was enriched for HSCs via depletion of Lineage+ cells followed by surface marker staining to identify LT-HSC (CD150+ flk2− LSK) and ST-HSC (CD150−flk2− LSK). Cells were then fixed and permeabilized followed by intracellular staining with anti-BrdU. Flow profiles shown are from one of 4–5 representative experiments. Grey histograms indicate PBS stained control. (D) Bar graph represents mean ± SD of data pooled from n=4–6 mice per genotype. (E) BM from WT, E47het, p21het or E47hetp21het mice was stained to enumerate LSK, total HSC, MPP or CLP numbers. Data represents 4–9 mice per genotype. *p<0.05
Focusing first on hematopoiesis during homeostasis in intact animals, we examined proliferation of BM progenitors in vivo. We pulsed mice with BrdU, a nucleotide analogue that is incorporated during S phase thereby reflecting de novo DNA synthesis, and resolved the sequential developmental subsets long-term renewing LT-HSCs, short-term renewing ST-HSCs, and non-renewing MPPs (Figure 1A). Bona fide LT-HSCs represent 1% of total LSKs, and complementary phenotypic schemes resolve LT-HSCs to increasing degrees of functional purity. Absence of the flk2 cytokine receptor marks total HSCs, of which 1 in 50 have long-term self-renewal capabilities (24). Self-renewal potential is enriched to ~1 in 3 using the CD150+CD48−LSK (25) or CD150+flk2−LSK definitions (10). There is no evidence that E47 transcriptionally regulates the expression of these phenotypic markers, and the mean fluorescence intensity of surface expression of CD150, CD48 and flk2 appears to be preserved in primitive precursors from E47 knockouts (7, 8, 10, 12). Downstream of LT-HSCs, ST-HSCs are CD150−flk2−LSKs, and MPPs are flk2+LSKs. Here, we explicitly validate our major findings by strategically exploiting all three phenotypic definitions of HSCs rather than relying on any single marker panel.
In intact animals, the percentage of BrdU+ proliferating E47hetp21het LT-HSCs (CD150+flk2−LSK; 21 ± 5%), was increased two-fold relative to WT or single E47het or p21het mice (11 ± 2%, 14 ± 4%, and 13 ± 3%, respectively, p<0.05, n=4–6 mice/group; Figure 1C&D). The percentage of BrdU+ E47hetp21het ST-HSCs (CD150−flk2−LSK; 25 ± 7%) was also increased relative to controls (14 ± 1%, 16 ± 4%, and 14 ± 4%, respectively, p<0.05, n=4–6 mice/group; Figure 1C&D). Proliferation in E47hetp21het MPPs was elevated relative to WT and p21het, but was not statistically different than single E47het controls. Under these steady-state conditions, absolute numbers of LSKs, total HSC, MPP, and lymphoid-restricted CLP (lineage−ScaloIL7R+AA4.1+) subsets were comparable across all four genotypes (Figure 1E). These data suggest that E47 and p21 interactions may be particularly important within the self-renewing LT-HSC subset.
Preferential susceptibility of E47hetp21het HSCs to a cellular mitotoxin
Hematopoiesis under stress may reveal biological defects not detectable under steady-state conditions in intact animals. Therefore, we examined the biological responses of E47hetp21het mice to three different stresses that test hematopoietic function: challenge with the mitotoxic drug 5-fluorouracil (5-FU), induction of emergency myelopoiesis by lipopolysaccharide (LPS), and long-term serial adoptive transfer. Emergency myelopoiesis is a short-term acute stress while repeated serial transfer is a long-term chronic stress. Serial adoptive transfer studies additionally provide a strategic opportunity to distinguish the biological importance of E47-p21 interactions within hematopoietic tissue (i.e. cell-intrinsic) versus within the cells of the microenvironment (i.e. cell-extrinsic).
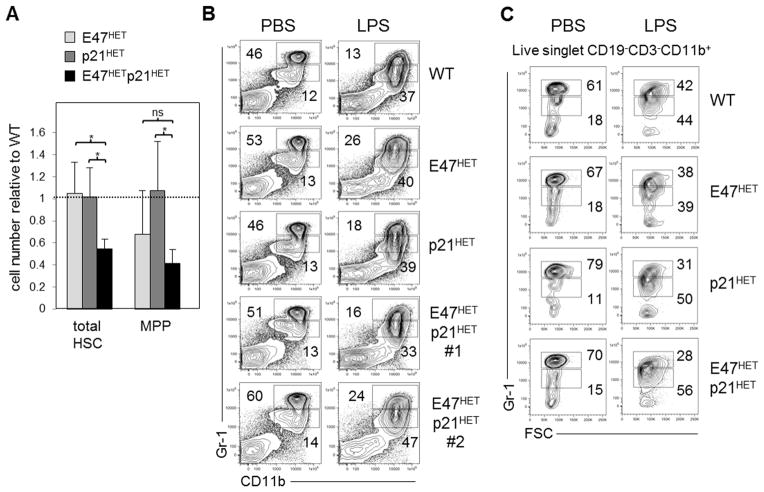
The chemotherapeutic 5-FU is selectively toxic to actively dividing cells, providing an independent experimental measure of loss of LT-HSC quiescence in vivo (26). Following two rounds of 5-FU treatment, total HSCs from E47hetp21het mice were reduced ~50% compared to any of the control groups (n=4–8 mice/group, p<0.05) (Figure 2A). E47hetp21het MPPs were also reduced by 40–60% relative to WT but were not statistically different than the single haploinsufficient controls. Together, these data reinforce findings in Figure 1 that the E47-p21 pathway regulates LT-HSC proliferation during homeostasis and is important in maintaining HSC numbers under mitotoxic stress.
Figure 2. Response of E47hetp21het progenitors to two different hematopoietic stressors.
(A) WT, E47het, p21het or E47hetp21het mice were injected i.p. with 150 mg/kg 5-FU and sacrificed after 16 hours. BM cells were stained to determine total HSC (flk2−LSK) or MPP (flk2+ LSK) numbers. Data is shown as mean ± SD of cell number relative to WT, n=4–8 mice per group. (B & C) Mice were treated with either PBS or 30 μg total LPS over 2 days and sacrificed 24 hours after last treatment. BM (B) or PB (C) cells were stained to identify mature (CD11b+ Gr-1hi) or immature granulocytes (CD11b+ Gr-1int). Data shown is representative of two independent experiments. *p<0.05
E47 and p21 are dispensable for rapid myeloid differentiation under emergency hematopoiesis
The careful balance between self-renewal and differentiation replenishes HSC numbers and sustains the replacement of mature cells (27). In addition to compromising proliferation and cellularity of LT-HSCs and uncommitted progenitors, we hypothesized that compound haploinsufficiency could impair functional integrity of immediate downstream progeny derived from these primitive subsets during hematopoiesis under stress conditions.
Exposure to bacterial LPS elicits rapid granulopoiesis and concomitant mobilization to peripheral blood. In reactive neutrophilia, multipotent HSCs and MPPs as well as bi-potent granulocyte-monocyte progenitors (GMPs) contribute to accelerated granulopoiesis (23, 28, 29). We challenged mice with two rounds of LPS exposure and examined immature (CD11b+Gr-1int) and mature (CD11b+Gr-1hi) granulocytes in the BM and peripheral blood (PB), respectively. Following LPS exposure, CD11b+Gr-1int immature BM granulocytes were comparably increased 3-fold (Figure 2B). Newly differentiated granulocytes also appeared competent to emigrate to PB, regardless of genotype (Figure 2C). Thus, despite the characteristic hyperproliferation, the immediate progeny of E47hetp21het LT-HSCs and MPPs displayed grossly normal responses to acute LPS challenge.
Compromised E47hetp21het LT-HSC persistence in long-term serial transfer assays
Serial repopulation remains the gold standard assay for studying LT-HSC function (26). We examined self-renewal and functional integrity of E47hetp21het LT-HSCs in serial transfer through primary, secondary and tertiary WT recipients (Figure 3A). CD45 congenic markers permit the tracking of cells of donor origin. Following engraftment, we examined donor-derived leukocytes every 4 weeks in the PB, and at 16 weeks in BM and spleen. Reconstitution at 16 weeks or longer ensures that hematopoiesis is derived from engrafted LT-HSCs and not by shorter-lived non-renewing progenitors co-transferred during transplantation (26).
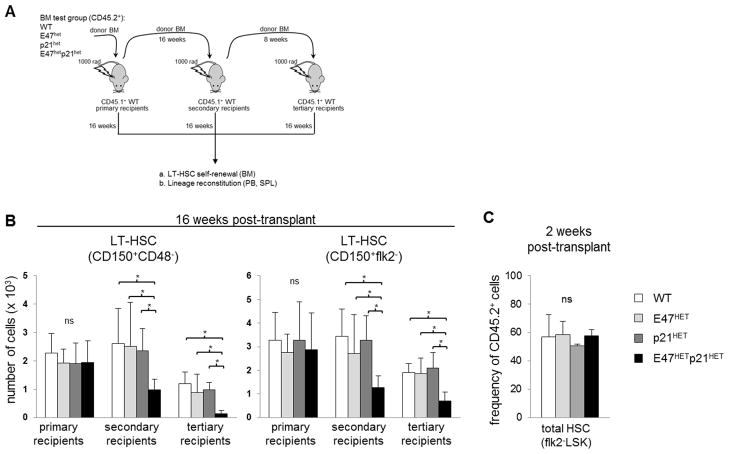
Figure 3. E47hetp21het LT-HSCs exhibit decreased self-renewal and persistence in vivo.
(A) Serial transplantation was performed by transferring 2 × 106 BM cells from CD45.2+ WT, E47het, p21het or E47hetp21het mice into sub-lethally irradiated CD45.1+ C57BL/6 recipient mice to examine LT-HSC self-renewal and persistence through three rounds of serial transfer. (B) Mice were sacrificed at sixteen weeks post-transplant and the number of donor-derived BM LT-HSCs (CD45.2+ CD150+ CD48− LSK or CD45.2+ CD150+ flk2− LSK) was enumerated in primary, secondary or tertiary recipients. Graph is shown as mean ± SD of data pooled from n= 4–8 recipient mice for each genotype. (C) Mice were sacrificed two weeks after primary transplantation and the frequency of CD45.2+ total HSCs was examined. Data is shown as mean ± SD from n= 2–3 recipient mice per group. *p<0.05
In contrast to controls, E47hetp21het LT-HSCs were profoundly depleted by serial transfer. While primary recipients had comparable numbers of LT-HSCs regardless of donor genotype, secondary recipients and tertiary recipients of E47hetp21het donor BM had a striking ~40% and 90% reduction in LT-HSCs, respectively (p<0.05, n=4–8 mice/group) (Figure 3B). By contrast, the progressive loss in LT-HSCs from WT as well as the single heterozygous control groups in secondary recipients was mild. Further, BM LT-HSCs from tertiary recipients in any of the control groups was reduced to ~50%, consistent with previous findings (20). The magnitude of the dramatic reduction in E47hetp21het donor-derived LT-HSCs was identical using both the stringent CD150+CD48−LSK and CD150+flk2−LSK LT-HSC definitions (Figure 3B) suggesting that the findings were not specific to one particular gating scheme (see Figure 1A for gating strategy).
The striking reduction of donor-derived E47hetp21het LT-HSCs in serial transfer could not be explained by poor homing/engraftment. Two weeks after transfer, the frequency of donor-derived HSCs (CD45.2+flk2−LSKs) was similar across all primary recipients regardless of donor genotype, suggesting comparable engraftment (Figure 3C).
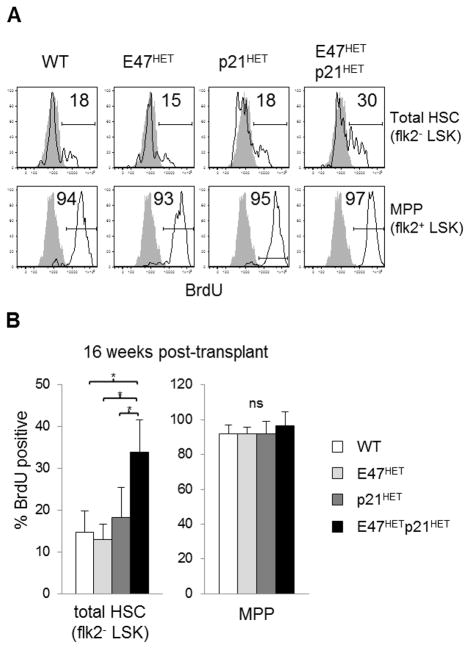
While the frequency of donor-derived E47hetp21het LT-HSC was similar relative to controls, the pattern of proliferation was not. The frequency of BrdU+ donor-derived LT-HSC from E47hetp21het mice, ~34%, was increased ~2-fold compared to WT, E47het or p21het in WT secondary recipients (respectively, p<0.05, n=3–5 mice/group; Figure 4A & B). As expected under transplantation stress, MPP subsets from all groups were actively cycling (p>0.05, n=3–5 mice/group), and no subtle differences were revealed in this BrdU pulsing regimen. Taken together, E47 and p21 collaborate to restrain LT-HSC proliferation, thereby preserving LT-HSC numbers in the context of long-term repopulation stress. Moreover, the requirement for E47-p21 interactions is cell-intrinsic, since E47hetp21het LT-HSCs fail to persist when engrafted into WT recipient hosts.
Figure 4. E47hetp21het LT-HSCs display hyperproliferation following transplantation stress.
Serial transplantation was performed as described in Figure 3. Secondary recipient mice were transplanted with 2 × 106 BM cells using primary recipient mice as donors. Sixteen weeks after transplantation, mice were sacrificed and BM cells stained to identify donor-derived total HSCs (CD45.2+ flk2− LSK) and MPPs (CD45.2+ flk2+ LSK) followed by anti-BrdU staining to examine proliferation status (A) Flow cytometry profiles from one experiment representative of 3 experiments are shown. Shaded grey histograms indicate PBS stained control. (B) Bar graph represents mean ± SD of data from n=3–5 recipient mice per donor genotype. *p<0.05
Cumulative versus developmental-compartment specific burden of combined E47-p21 haploinsufficiency
Lymphopoiesis and myelopoiesis differ in the requirement for E47, providing a unique experimental opportunity to evaluate the biological impact of E47-p21 collaboration to progenitor compartments upstream versus downstream of lympho-myeloid restriction. E47 is essential for multiple aspects of B and T cell development (6, 9, 19, 30–32) including p21-mediated proliferation (7, 12–14). By contrast, once uncommitted precursors have restricted to the myeloid lineage, E47 activity and, by extrapolation, E47-dependent p21 activity becomes dispensable (33). Thus, defects in lymphoid output from E47hetp21het LT-HSCs should reflect the aggregate burden of haploinsufficiency at the multipotent and lymphoid specified stages of development while defects in myeloid output should reflect the burden of haploinsufficiency incurred exclusively at the multipotent stages.
The poor persistence of E47hetp21het LT-HSCs in serial transfer through WT hosts was accompanied by marked divergence in peripheral lymphoid and myeloid reconstitution. Specifically, while the failure of B and T lymphoid production closely tracked the numeric loss of LT-HSCs through serial transfer, myeloid production remained intact in these same animals. At 16 weeks post-transplant, the absolute number and frequency of donor-derived E47hetp21het CD19+ B and CD3+ T cells was dramatically decreased by ~60% and ~90% in secondary and tertiary recipients, respectively (p<0.05, n=2–9 mice/group), (Figure 5A–C), proportional to reductions in E47hetp21het LT-HSCs (Figure 3B). By contrast, numbers and frequency of myeloid cells (Gr-1+ or CD11b+) derived from E47hetp21het donor BM were normal across all three serial transfers (Figure 5B & C). Moreover, that E47hetp21het mice had largely normal neutrophil responsiveness to LPS challenge (Figures 2A & B), suggests that myeloid lineage cells are grossly intact both phenotypically and functionally.
Figure 5. E47hetp21het LT-HSCs display progressive in vivo decrease in lymphoid lineage reconstitution accompanied by normal myeloid lineage reconstitution.
Serial transplantation was performed in Figure 3. Sixteen weeks after transplantation donor-derived cells were identified from spleen or peripheral blood (PB). (A) Left panel, gating strategy used to identify donor-derived B (CD45.2+ CD19+), T (CD45.2+ CD3+) and myeloid lineage (CD45.2+CD11b+) cells in spleen. Right panel, gating strategy used to identify donor-derived B (CD45.2+ CD19+), T (CD45.2+ CD3+) and myeloid lineage (CD45.2+ Gr-1+) cells in PB. Donor-derived reconstitution of lymphoid and myeloid lineage was examined in (B) spleen and (C) peripheral blood in BM recipients sixteen weeks after each round of transplantation. Graphs are shown as mean ± SD of data from n= 2–9 mice per recipient for each donor genotype. (D) Sixteen weeks after transplant, presence of donor-derived ST-HSC, MPP, LMPP and CLP in BM of secondary recipients was examined. Data are shown as mean ± SD from n= 5–9 mice per donor genotype. *p<0.05
A recent study showed that significant reductions in CD150+ LT-HSCs following deletion of the CXCL12 chemokine from BM endothelial niches could be counterbalanced by homeostatic mechanisms that restore downstream MPPs and LMPPs to normal frequencies (34). We examined hematopoietic subsets derived from LT-HSCs to establish the consequences of the E47hetp21het defect to immediate downstream progenitors, 16 weeks post-transfer. Despite a 50% reduction in LT-HSCs, secondary recipients of E47hetp21het donor BM had largely normal numbers of ST-HSCs, suggesting that the requirement for the E47-p21 pathway at the LT-HSC stage could be overcome in downstream compartments. Donor-derived MPPs and LMPPs (using CD150−flk2+LSK and flk2high LSK definitions, respectively (10, 34)) were reduced relative to WT and p21het mice, but were identical to that of single E47het mice (Figure 5D). By contrast, E47hetp21het CLPs were reduced 2-fold compared to WT or single heterozygous controls, and downstream B and T cells were severely depleted (Figure 5D & B). Thus, while the striking numerical depletion of E47hetp21het LT-HSCs can be transiently compensated by expansion of downstream multipotent subsets competent to produce a functional myeloid compartment, the lymphoid compartment could not be replenished. Together, our findings indicate a cell-intrinsic role for genetic interactions between E47 and p21 in the selective maintenance of LT-HSC, B cell and T cell compartments under long-term hematopoietic repopulation stress in vivo.
Gene expression analysis in E47hetp21het total HSCs
To obtain a broader perspective on the impact of combined E47 and p21 heterozygosity to the expression profile of other known regulators of HSC pool size, we examined transcript levels of known E47-regulated target genes as well as other cell cycle associated genes (14, 35–37). (14, 35–37). Here, we used 5-FU treatment to deplete cycling HSCs and examined the gene expression profile of residual HSCs forced to undergo dramatic repopulation stress (22, 26). E47hetp21het total HSCs undergoing repopulation stress exhibit a ~1.5–3-fold increase in cdk6, p18, and ikaros transcripts as assessed by QPCR (Figure 6). There were no detectable changes in p27 and Notch1 transcript levels (data not shown), two factors that regulate the precursor pool size but not HSC cell cycle activity per se (38, 39). While both ikaros and Notch can activate p21 expression, neither factor was able to compensate for E47 haploinsufficiency. We saw no evidence of differences in γ-ph2AX levels or caspase-3 protein in E47hetp21het total HSCs undergoing repopulation stress (data not shown), suggesting that loss of LT-HSCs could not be readily explained by impaired DNA damage repair response or increased apoptosis, even though rapid clearance of dying cells in vivo may slightly underestimate their detection via flow cytometry. Together, these data demonstrate that E47 mechanistically regulates LT-HSC self-renewal by controlling p21-dependent cell cycle activity.
Figure 6. Gene expression analysis in E47hetp21het HSCs.
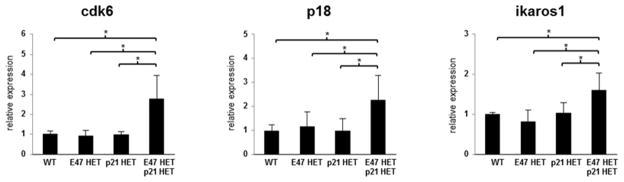
At day 14 after a single 5-FU i.v. injection, total HSCs (flk2− LSK) were sorted from WT, E47het, p21het or E47hetp21het mice. RNA was extracted and cDNA was generated via RT-PCR and used to examine expression levels of cdk6, p18, and ikaros1 using quantitative real-time PCR (qPCR). Gene expression was normalized to β-actin. Data are shown as mean ± SD of triplicates from at least two independent sorts. *p<0.05
DISCUSSION
E47 is a key component of gene regulatory networks that establish B and T cell fate, and participates in HSC maintenance (7, 9, 10, 12, 14, 40). While our in vitro evidence showed that E47 directly regulates p21 in HSCs, establishing the biological contribution of E47-p21 pathway to hematopoiesis has been elusive due to the difficulty of analyzing rare HSCs in vivo, the challenge of overcoming early lymphoid arrest in E47-deficient mice, and the knowledge that both factors are broadly expressed in cells other than leukocytes. To determine the extent of functional collaboration by E47 and p21 during hematopoiesis, we engineered mice with reduced gene dosage of each factor and transplanted BM from E47hetp21het mice into WT hosts. Our findings reveal a severe progressive deficiency in E47hetp21het LT-HSC self-renewal following long-term serial repopulation. A transient recovery of cellularity in donor-derived ST-HSC/MPP stages enabled normal myeloid production but lymphoid production was unrecoverable. The gross severity of the lymphoid defect in compound E47hetp21het mice compared to single haploinsufficiency of either gene alone was striking, an important finding given the increasingly frequent linkage of E47 loss of heterozygosity with lymphoid malignancies and immune deficiency.
While several lines of evidence hint at a relationship between E47 and p21 in each HSCs and lymphoid-committed precursors, the biological impact has been unclear (7, 10, 12). Indeed, the severe defect in the earliest stages of hematopoiesis in p21 null mice has precluded analysis of p21 contributions in compartments downstream of lympho-myeloid divergence. Here, we exploited the fact that the E47 pathway is dispensable in myeloid lineage cells to establish the biological importance of the E47-p21 pathway within HSCs upstream of lympho-myeloid divergence. Our results demonstrate a role for the E47-p21 pathway in LT-HSC maintenance under activation stress but not steady state, and reveal a stringent requirement for E47-p21 activity in the lymphoid lineages following forced repopulation. In E47hetp21het HSCs, reductions in p21 were accompanied by dysregulation of cdk6, p18 and ikaros, all of which directly regulate cell cycling and likely contribute to the overall phenotype. Since none of these genes are altered in HSCs from mice singly haploinsufficient in either E47 or p21, their dysregulation in E47hetp21het HSCs may be a consequence of observed hyperproliferation rather than the cause. While E47 is known to directly regulate expression of these genes under homeostatic conditions (14), our observations may reflect the contribution by other cell cycle regulators activated in HSCs undergoing repopulation stress. p21 null mice on either the original 129 background or the pure B6 background exhibit poor HSC persistence under certain repopulation stress paradigms but changes in companion cell cycle regulators have not been examined (20, 41). Our findings reveal a skewing of B/T ratios that emerges in tertiary recipients of p21 haploinsufficient BM, demonstrating a negative biological consequence of even relatively minor reductions in p21. Indeed, a striking feature of our findings is that the combination of two relatively subtle lesions, haploinsufficiency in each E47 and p21, curtails lymphopoiesis to a severe degree not observed in the context of either single lesion alone.
Several studies indicate that reduced dosage of E47 predisposes individuals to cancer (4). For example, recurrent E2A gene deletions are detectable in acute lymphoblastic leukemia (ALL) patients (42) as well as Sezary syndrome patients (43). Ectopic expression of the E47 antagonist Id2 in classical Hodgkin’s lymphoma is mechanistically linked to B-cell derived lymphomas (44). The sensitive requirement for E47 activity in hematopoiesis can exacerbate the impact of additional mutations. Our studies demonstrate that loss of a single allele of p21 exacerbates the phenotype associated with simple E47 haploinsufficiency. Specifically, compound heterozygosity of E47 and p21 magnified the defects in hyperproliferation, LT-HSC self-renewal and lymphoid reconstitution observed in E47het mice. It is possible that a reduction in E47 gene dosage, combined with a second heterozygous mutation in a different gene, can predispose patients afflicted with loss of heterozygosity mutations in E2A to hematologic malignancies.
In summary, our results define the differential contribution of cell-intrinsic E47-p21 within discrete developmental hematopoietic compartments in vivo. E47hetp21het LT-HSCs exhibit a profound loss of self-renewal in serial repopulation across mice accompanied by ablation of the lymphoid lineages. The E47-p21 pathway appeared to be dispensable in the ST-HSC/MPP stages of hematopoiesis, and downstream myeloid activity remained intact. That the combination of two subtle lesions, loss of a single allele of a transcription factor and a cell cycle regulator factor, amplifies the magnitude of hematopoietic disruption beyond either defect alone, has important clinical implications. E47 loss of heterozygosity is linked to multiple leukemias, and our findings provide an opportunity screening at-risk individuals for cooperating lesions.
Acknowledgments
The authors would like to thank Christine Milcarek for helpful advice and Dewayne Falkner for flow cytometry expertise.
Abbreviations used in this article
- HSC
hematopoietic stem cell
- LT-HSC
long-term hematopoietic stem cell
- ST-HSC
short-term hematopoietic stem cell
- MPP
multipotent progenitors
- LMPP
lymphoid-myeloid primed progenitors
- CLP
common lymphoid progenitors
Footnotes
CONFLICTS OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
References
- 1.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 4.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 5.Santos PM, Borghesi L. Molecular resolution of the B cell landscape. Curr Opin Immunol. 2011;23:163–170. doi: 10.1016/j.coi.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghesi L, Aites J, Nelson S, Lefterov P, James P, Gerstein R. E47 is required for V(D)J recombinase activity in common lymphoid progenitors. J Exp Med. 2005;202:1669–1677. doi: 10.1084/jem.20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, Kardava L, St Leger A, Martincic K, Varnum-Finney B, Bernstein ID, Milcarek C, Borghesi L. E47 controls the developmental integrity and cell cycle quiescence of multipotential hematopoietic progenitors. J Immunol. 2008;181:5885–5894. doi: 10.4049/jimmunol.181.9.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dias S, Mansson R, Gurbuxani S, Sigvardsson M, Kee BL. E2A Proteins Promote Development of Lymphoid-Primed Multipotent Progenitors. Immunity. 2008;29:217–227. doi: 10.1016/j.immuni.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin YC, Jhunjhunwala S, Benner C, Heinz S, Welinder E, Mansson R, Sigvardsson M, Hagman J, Espinoza CA, Dutkowski J, Ideker T, Glass CK, Murre C. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol. 2010;11:635–643. doi: 10.1038/ni.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semerad CL, Mercer EM, Inlay MA, Weissman IL, Murre C. E2A proteins maintain the hematopoietic stem cell pool and promote the maturation of myelolymphoid and myeloerythroid progenitors. Proc Natl Acad Sci U S A. 2009;106:1930–1935. doi: 10.1073/pnas.0808866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazaki M, Rivera RR, Miyazaki K, Lin YC, Agata Y, Murre C. The opposing roles of the transcription factor E2A and its antagonist Id3 that orchestrate and enforce the naive fate of T cells. Nat Immunol. 2011;12:992–1001. doi: 10.1038/ni.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Esplin B, Borghesi L. E47 regulates hematopoietic stem cell proliferation and energetics but not myeloid lineage restriction. Blood. 2011;117:3529–3538. doi: 10.1182/blood-2010-07-297689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herblot S, Aplan PD, Hoang T. Gradient of E2A activity in B-cell development. Mol Cell Biol. 2002;22:886–900. doi: 10.1128/MCB.22.3.886-900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz R, Engel I, Fallahi-Sichani M, Petrie HT, Murre C. Gene expression patterns define novel roles for E47 in cell cycle progression, cytokine-mediated signaling, and T lineage development. Proc Natl Acad Sci U S A. 2006;103:9976–9981. doi: 10.1073/pnas.0603728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreiros-Vidal I, Carroll T, Taylor B, Terry A, Liang Z, Bruno L, Dharmalingam G, Khadayate S, Cobb BS, Smale ST, Spivakov M, Srivastava P, Petretto E, Fisher AG, Merkenschlager M. Genome-wide identification of Ikaros targets elucidates its contribution to mouse B-cell lineage specification and pre-B-cell differentiation. Blood. 2013;121:1769–1782. doi: 10.1182/blood-2012-08-450114. [DOI] [PubMed] [Google Scholar]
- 16.Henning K, Heering J, Schwanbeck R, Schroeder T, Helmbold H, Schafer H, Deppert W, Kim E, Just U. Notch1 activation reduces proliferation in the multipotent hematopoietic progenitor cell line FDCP-mix through a p53-dependent pathway but Notch1 effects on myeloid and erythroid differentiation are independent of p53. Cell Death Differ. 2008;15:398–407. doi: 10.1038/sj.cdd.4402277. [DOI] [PubMed] [Google Scholar]
- 17.O’Riordan M, Grosschedl R. Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity. 1999;11:21–31. doi: 10.1016/s1074-7613(00)80078-3. [DOI] [PubMed] [Google Scholar]
- 18.Lukin K, Fields S, Lopez D, Cherrier M, Ternyak K, Ramirez J, Feeney AJ, Hagman J. Compound haploinsufficiencies of Ebf1 and Runx1 genes impede B cell lineage progression. Proc Natl Acad Sci U S A. 2010;107:7869–7874. doi: 10.1073/pnas.1003525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bain G, Robanus Maandag EC, te Riele HP, Feeney AJ, Sheehy A, Schlissel M, Shinton SA, Hardy RR, Murre C. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- 20.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 21.Borghesi L, Hsu LY, Miller JP, Anderson M, Herzenberg L, Schlissel MS, Allman D, Gerstein RM. B lineage-specific regulation of V(D)J recombinase activity is established in common lymphoid progenitors. J Exp Med. 2004;199:491–502. doi: 10.1084/jem.20031800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taniguchi Ishikawa E, Gonzalez-Nieto D, Ghiaur G, Dunn SK, Ficker AM, Murali B, Madhu M, Gutstein DE, Fishman GI, Barrio LC, Cancelas JA. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc Natl Acad Sci U S A. 2012;109:9071–9076. doi: 10.1073/pnas.1120358109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boettcher S, Ziegler P, Schmid MA, Takizawa H, van Rooijen N, Kopf M, Heikenwalder M, Manz MG. Cutting edge: LPS-induced emergency myelopoiesis depends on TLR4-expressing nonhematopoietic cells. J Immunol. 2012;188:5824–5828. doi: 10.4049/jimmunol.1103253. [DOI] [PubMed] [Google Scholar]
- 24.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 25.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Pietras EM, Warr MR, Passegue E. Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 2011;195:709–720. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda Y, Cain DW, Kuraoka M, Kondo M, Kelsoe G. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J Immunol. 2009;182:6477–6484. doi: 10.4049/jimmunol.0803961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bain G, Maandag EC, Izon DJ, Amsen D, Kruisbeek AM, Weintraub BC, Krop I, Schlissel MS, Feeney AJ, van Roon M, et al. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 31.Barndt RJ, Dai M, Zhuang Y. Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol Cell Biol. 2000;20:6677–6685. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansson R, Welinder E, Ahsberg J, Lin YC, Benner C, Glass CK, Lucas JS, Sigvardsson M, Murre C. Positive intergenic feedback circuitry, involving EBF1 and FOXO1, orchestrates B-cell fate. Proc Natl Acad Sci U S A. 2012;109:21028–21033. doi: 10.1073/pnas.1211427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang Y, Soriano P, Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 34.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan Y, Shen H, Franklin DS, Scadden DT, Cheng T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol. 2004;6:436–442. doi: 10.1038/ncb1126. [DOI] [PubMed] [Google Scholar]
- 36.Niu H, Fang G, Tang Y, Xie L, Yang H, Morel L, Diamond B, Zou YR. The function of hematopoietic stem cells is altered by both genetic and inflammatory factors in lupus mice. Blood. 2013;121:1986–1994. doi: 10.1182/blood-2012-05-433755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 38.Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27(kip1) Nat Med. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 39.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 40.Georgescu C, Longabaugh WJ, Scripture-Adams DD, David-Fung ES, Yui MA, Zarnegar MA, Bolouri H, Rothenberg EV. A gene regulatory network armature for T lymphocyte specification. Proc Natl Acad Sci U S A. 2008;105:20100–20105. doi: 10.1073/pnas.0806501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Os R, Kamminga LM, Ausema A, Bystrykh LV, Draijer DP, van Pelt K, Dontje B, de Haan G. A Limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells. 2007;25:836–843. doi: 10.1634/stemcells.2006-0631. [DOI] [PubMed] [Google Scholar]
- 42.Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, Su X, Pui CH, Relling MV, Evans WE, Shurtleff SA, Downing JR. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 43.Steininger A, Mobs M, Ullmann R, Kochert K, Kreher S, Lamprecht B, Anagnostopoulos I, Hummel M, Richter J, Beyer M, Janz M, Klemke CD, Stein H, Dorken B, Sterry W, Schrock E, Mathas S, Assaf C. Genomic loss of the putative tumor suppressor gene E2A in human lymphoma. J Exp Med. 2011;208:1585–1593. doi: 10.1084/jem.20101785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathas S, Janz M, Hummel F, Hummel M, Wollert-Wulf B, Lusatis S, Anagnostopoulos I, Lietz A, Sigvardsson M, Jundt F, Johrens K, Bommert K, Stein H, Dorken B. Intrinsic inhibition of transcription factor E2A by HLH proteins ABF-1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nat Immunol. 2006;7:207–215. doi: 10.1038/ni1285. [DOI] [PubMed] [Google Scholar]