Abstract
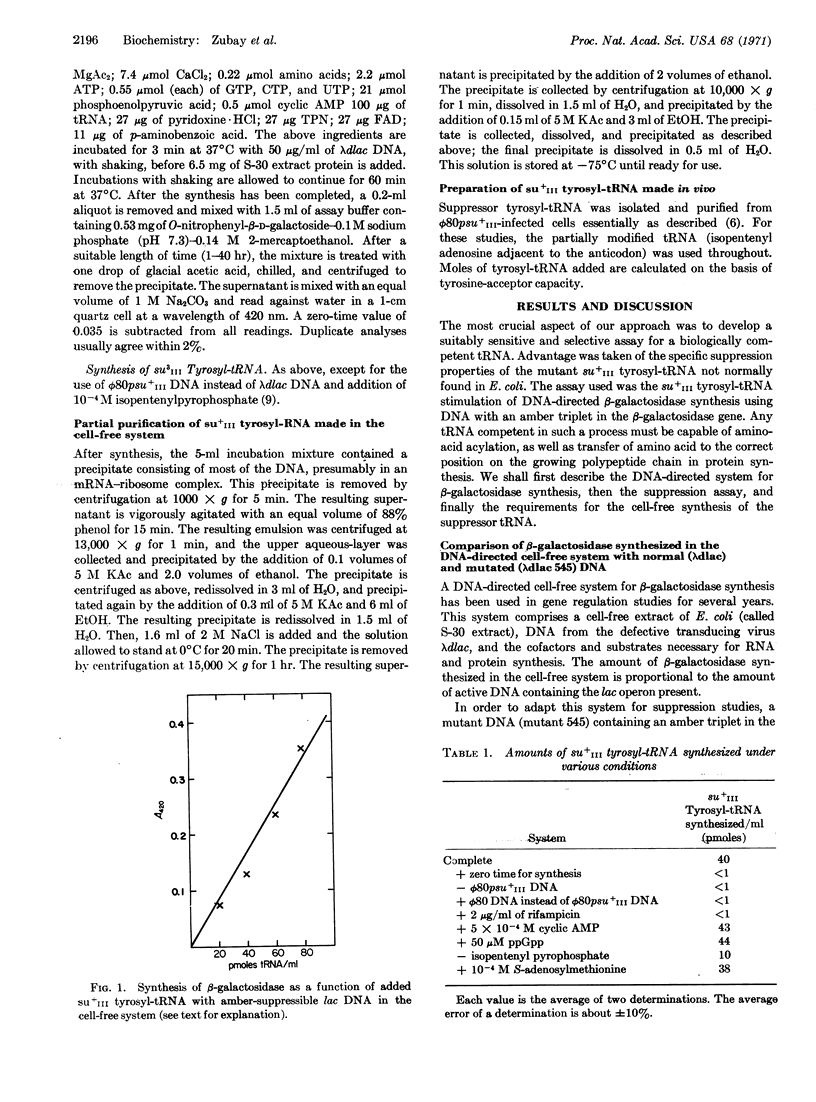
Biologically active su+III tyrosyl-tRNA has been synthesized in a DNA-directed cell-free system from Escerichia coli. Such a system should be most useful for studying the mechanism of tRNA synthesis. This tRNA is capable of suppressing amber mutations in the gene coding for β-galactosidase (EC 3.2.1.23) and, therefore, must be capable of being charged and transferring amino acids. A 4-fold stimulation in the activity of the tRNA formed de novo is obtained with isopentenyl pyrophosphate, a compound involved in the post-transcriptional acylation of an adenine base adjacent to the anticodon. It has been suggested elsewhere that formation of RNA subject to stringent control may be inhibited by guanosine tetraphosphate (ppGpp). However, guanosine tetraphosphate did not affect the synthesis of su+III tyrosyl-tRNA, even though the synthesis of this tRNA is subject to stringent control.
Keywords: E. coli, β-galactosidase, bacteriophage
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniel V., Sarid S., Beckmann J. S., Littauer U. Z. In vitro transcription of a transfer RNA gene. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1260–1266. doi: 10.1073/pnas.66.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Russell R. L. Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J Mol Biol. 1969 Jan 14;39(1):145–157. doi: 10.1016/0022-2836(69)90339-8. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Ippen K., Scaife J. G., Beckwith J. R. The promoter-operator region of the lac operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):413–420. doi: 10.1016/0022-2836(68)90395-1. [DOI] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]


