Abstract
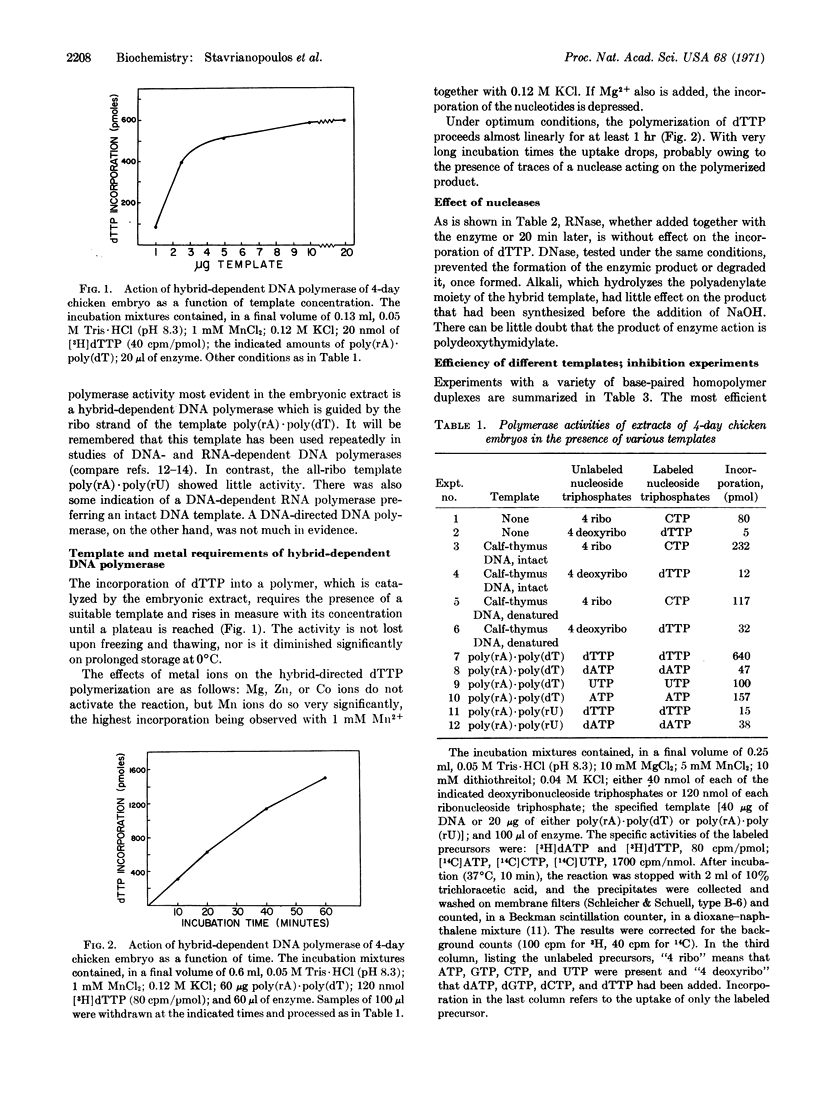
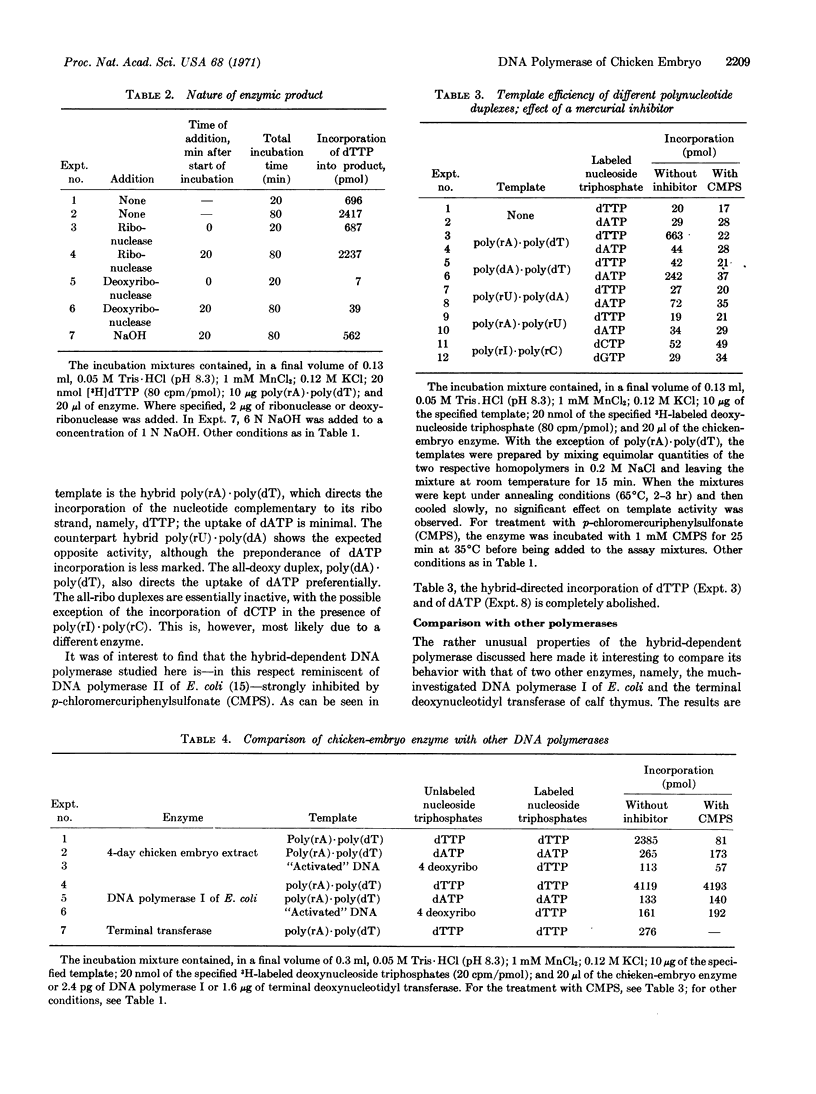
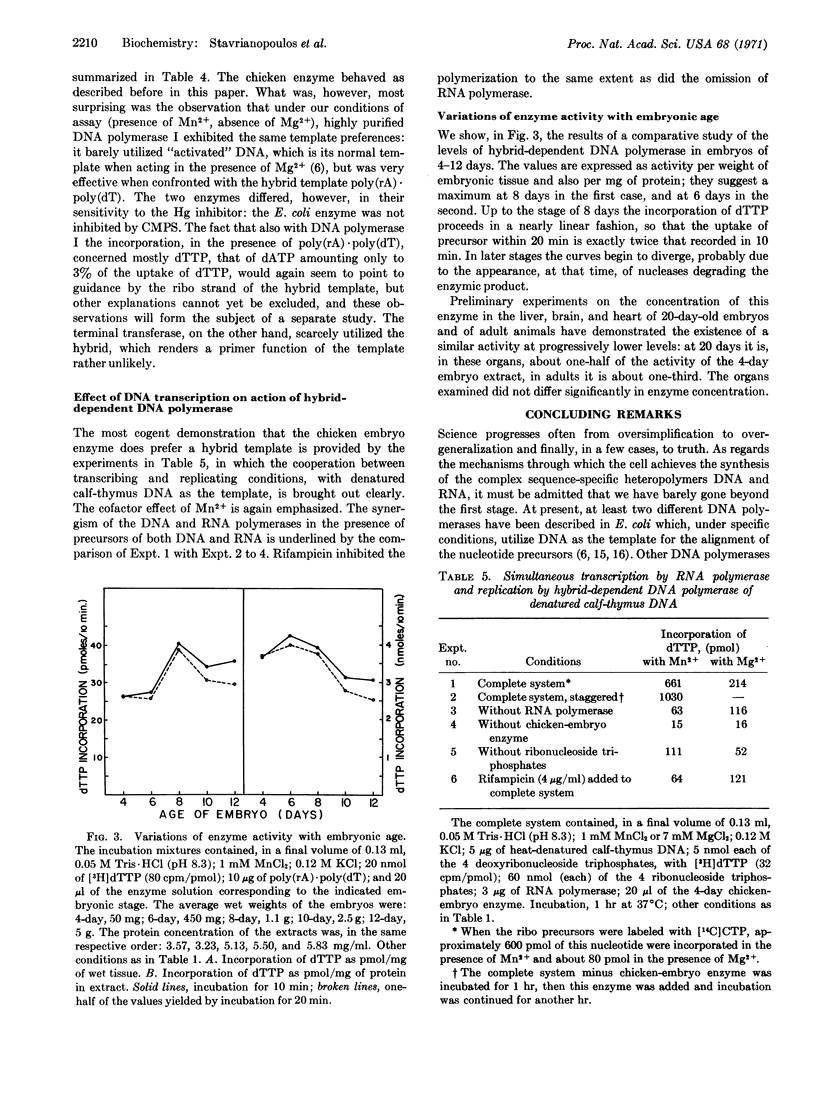
This paper presents a preliminary survey of the nucleic acid polymerases of the developing chicken embryo, especially of the 4-day stage. The predominant activity is that of a DNA polymerase preferring a DNA-RNA hybrid as the template. The enzyme, which is activated by Mn2+ ions and inhibited by p-chloromercuriphenylsulfonate, copies preferentially the ribo strand of a hybrid, such as poly(rA)·poly(dT), but is relatively inactive with all-ribo duplexes. DNA polymerase I of Escherichia coli was also found to use the hybrid template with high efficiency, copying preferentially the ribo strand. With the chicken enzyme, the template activity of denatured DNA was increased tenfold by simultaneous transcription with RNA polymerase. DNA polymerase activity reaches a maximum in the 6- to 8-day chicken embryo and then declines progressively to about one-third of the maximal value in the adult chicken.
Keywords: ribonucleotide·deoxyribonucleotide hybrid
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- CRICK F. H. On protein synthesis. Symp Soc Exp Biol. 1958;12:138–163. [PubMed] [Google Scholar]
- Chamberlin M. J. Comparative properties of DNA, RNA, and hybrid homopolymer pairs. Fed Proc. 1965 Nov-Dec;24(6):1446–1457. [PubMed] [Google Scholar]
- FOX C. F., ROBINSON W. S., HASELKORN R., WEISS S. B. ENZYMATIC SYNTHESIS OF RIBONUCLEIC ACID. III. THE RIBONUCLEIC ACID-PRIMED SYNTHESIS OF RIBONUCLEIC ACID WITH MICROCOCCUS LYSODEIKTICUS RIBONUCLEIC ACID POLYMERASE. J Biol Chem. 1964 Jan;239:186–195. [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Karkas J. D., Chargaff E. Template functions in the enzymic formation of polyribonucleotides. 3. Apurinic acid as template. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1241–1246. doi: 10.1073/pnas.56.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R. DNA polymerase II. Nature. 1970 Dec 12;228(5276):1050–1053. doi: 10.1038/2281050a0. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. Purification and DNA synthesis in cell-free extracts: properties of DNA polymerase II. Proc Natl Acad Sci U S A. 1971 Apr;68(4):761–764. doi: 10.1073/pnas.68.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE-HUNG S., CAVALIERI L. F. ISOLATION AND PROPERTIES OF A NUCLEIC ACID HYBRID POLYMERASE. Proc Natl Acad Sci U S A. 1964 Jun;51:1022–1028. doi: 10.1073/pnas.51.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Scolnick E. M., Aaronson S. A., Todaro G. J., Parks W. P. RNA dependent DNA polymerase activity in mammalian cells. Nature. 1971 Jan 29;229(5283):318–321. doi: 10.1038/229318a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E., Rands E., Aaronson S. A., Todaro G. J. RNA-dependent DNA polymerase activity in five RNA viruses: divalent cation requirements. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1789–1796. doi: 10.1073/pnas.67.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. Characterization of the products of DNA-directed DNA polymerases in oncogenic RNA viruses. Nature. 1970 Aug 8;227(5258):563–567. doi: 10.1038/227563a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Trávnícek M., Watson K. Synthetic DNA-RNA hybrids and RNA-RNA duplexes as templates for the polymerases of the oncogenic RNA viruses. Nature. 1970 Oct 31;228(5270):430–432. doi: 10.1038/228430a0. [DOI] [PubMed] [Google Scholar]
- Stavis R. L., August J. T. The biochemistry of RNA bacteriophage replication. Annu Rev Biochem. 1970;39:527–560. doi: 10.1146/annurev.bi.39.070170.002523. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- YONEDA M., BOLLUM F. J. DEOXYNUCLEOTIDE-POLYMERIZING ENZYMES OF CALF THYMUS GLAND. I. LARGE SCALE PURIFICATION OF TERMINAL AND REPLICATIVE DEOXYNUCLEOTIDYL TRANSFERASES. J Biol Chem. 1965 Aug;240:3385–3391. [PubMed] [Google Scholar]



