Abstract
Recurrent apnea with intermittent hypoxia (IH) is a major clinical problem in infants born preterm. Carotid body chemo-reflex and catecholamine secretion from adrenal medullary chromaffin cells (AMC) are important for maintenance of cardio-respiratory homeostasis during hypoxia. This article highlights studies on the effects of IH on O2 sensing by the carotid body and AMC in neonatal rodents. Neonatal IH augments hypoxia-evoked carotid body sensory excitation and catecholamine secretion from AMC which are mediated by reactive oxygen species (ROS)-dependent recruitment of endothelin-1 and Ca2+ signaling, respectively. The effects of neonatal IH persist into adulthood. Evidence is emerging that neonatal IH initiates epigenetic mechanisms involving DNA hypermethylation contributing to long-lasting increase in ROS levels. Since adult human subjects born preterm exhibit higher incidence of sleep-disordered breathing and hypertension, DNA hypomethylating agents might offer a novel therapeutic intervention to decrease long-term cardio-respiratory morbidity caused by neonatal IH.
Keywords: Apnea of prematurity, Cardio-respiratory morbidities, DNA methylation, Histone modifications, Exocytosis, Neurotransmitters/modulators, Oxidative stress
1. Introduction
Recurrent apnea with intermittent hypoxia (IH) is a major clinical problem in infants born preterm (Abu-Shaweesh and Martin, 2008). Infants with recurrent apnea exhibit autonomic dysfunction including: (a) clinical signs of increased sympathetic nerve activity (Lagercrantz et al., 1990), (b) altered sympatho-adrenal function (Lagercrantz and Sjöquist, 1980), (c) augmented ventilatory response to hypoxia (Nock et al., 2004), and (d) cardiac arrhythmias (Poets et al., 1994). Young adults (~10 years of age) who were born preterm exhibit higher incidence of sleep-disordered breathing (Rosen et al., 2003; Paavonen et al., 2007; Hibbs et al., 2008). Notably, adults (30 years of age) born pre-term have higher incidence of hypertension and insulin resistance than those born full-term (Dalziel et al., 2007). These studies taken together suggest that recurrent apnea in pre-term infants leads to cardio-respiratory abnormalities in adulthood.
Cardio-respiratory responses to hypoxia depend on reflexes arising from the carotid body, the primary sensory organ for monitoring arterial blood O2 levels. Carotid bodies, however, are immature at birth and the maturation of carotid body O2 sensing occurs during the first week of neonatal life (Blanco et al., 1984; Donnelly, 2000; Carroll, 2003). Too little (hypoxia) or too much environmental O2 (hyperoxia) during neonatal life profoundly impacts maturation of O2 sensing by the carotid bodies (see Donnelly, 2000; Carroll, 2003; for reference). Catecholamine (CA) secretion from adrenal medullary chromaffin cells (AMC) is an important mechanism for maintaining cardiovascular homeostasis under stress conditions including hypoxia (Lagercrantz and Bistoletti, 1977; Seidler and Slotkin, 1985). In adult animals, hypoxia-evoked CA secretion from AMC is neurogenic and requires activation of the sympathetic nervous system (Seidler and Slotkin, 1986; Yokotani et al., 2002). In neonates, sympathetic innervation to the target organs is incomplete (Lagercrantz and Bistoletti, 1977; Seidler and Slotkin, 1985), and hypoxia facilitates CA secretion by directly affecting the excitability of AMC (Thompson et al., 1997; Takeuchi et al., 2001). Recent studies on rodent models, albeit limited, have provided evidence that neonatal IH profoundly affect the O2 sensing by the carotid body and AMC and the effects persist into adulthood. In this article, we provide a brief review of studies addressing the mechanisms underlying the effects of neonatal IH on the carotid body and AMC in rodents and their potential physiological significance.
2. Effects of neonatal IH on hypoxic sensing by the carotid body
Carotid bodies from neonatal rat pups respond poorly to hypoxia (Donnelly, 2000; Carroll, 2003; Peng et al., 2004). Neonatal rats exposed to chronic hypoxia exhibit reduced carotid body response to hypoxia (Donnelly and Doyle, 1994; Sterni et al., 1999). In striking contrast, rat pups exposed to IH from ages P0–P10 (15 s of hypoxia followed by 5 min of normoxia, 9 episodes/h, 8 h/day) exhibit augmented carotid body response to hypoxia (Peng et al., 2004; Pawar et al., 2008, 2009). The augmented sensory response to hypoxia could be seen in ex vivo carotid bodies (Peng et al., 2004; Pawar et al., 2008, 2009), suggesting that at least part of this response is independent of circulatory changes. Although IH leads to a similar augmentation of the carotid body response to hypoxia in adult rats (Peng et al., 2003, 2004), there are some notable differences between the effects of IH in neonates versus adult carotid bodies. First, the augmented hypoxic sensitivity in neonates is seen with exposures to as little as 72 episodes of IH; whereas adult rats require as many as 720 IH episodes, suggesting that neonates are relatively more sensitive to IH than adults (Pawar et al., 2008). Second, in IH exposed adult rats, repetitive hypoxia leads to long-lasting increase in base line sensory activity of the carotid body, a phenomenon termed as sensory long term facilitation (sensory LTF; Peng et al., 2003). In striking contrast, IH is ineffective in evoking sensory LTF in neonatal carotid bodies (Pawar et al., 2008). Third, IH has no significant effect on carotid body morphology in adult rats (Peng et al., 2003); whereas it caused hyperplasia of glomus cells (Pawar et al., 2009). Fourth, in adult rats, the augmented carotid body response to hypoxia is completely reversed after the cessation of IH (Peng et al., 2003); whereas the effects of neonatal IH persisted into adulthood (Pawar et al., 2008).
2.1. Significance of the heightened carotid body response to hypoxia by IH in neonates
IH exposed rat pups exhibit augmented hypoxic ventilatory response (HVR), a hallmark reflex response initiated by the carotid body (Peng et al., 2004; Julien et al., 2011). A similar increase in the HVR was also seen in preterm infants with recurrent apneas compared to infants without apneas (Nock et al., 2004). The enhanced HVR evoked by neonatal IH, on one hand, may be beneficial in the initial stages, as it provides adequate oxygenation in infants with apnea, thereby preventing deleterious effects of hypoxia on the central nervous system. On the other hand, if the apneas persist, instead of being beneficial, the heightened hypoxic sensitivity of the carotid body may lead to breathing instability and increased incidence of apneas. Indeed, neonatal rats exposed to several days of IH exhibit greater number of apneas than control rat pups (Julien et al., 2008; Nanduri et al., 2012).
3. Effects of neonatal IH on hypoxia-evoked catecholamine (CA) secretion from adrenal medullary chromaffin cells (AMC)
Souvannakitti et al. (2009) examined the effects of IH on CA secretion from AMC in neonatal rats in response to hypoxia. CA secretion is monitored from dissociated chromaffin cells by carbon fiber ampermetry. The number of chromaffin cells responding to hypoxia and the magnitude of CA secretion for a given level of hypoxia are greater in IH exposed rats than the controls. The increased CA secretion by hypoxia is due to a greater number of secretory events as well as greater amount of CA released per secretory event. IH increased both norepinephrine and epinephrine contents in neonatal adrenal medullae. In striking contrast, hypoxia-evoked CA secretion is reduced in rat pups exposed to continuous hypobaric hypoxia (0.4 ATM), suggesting that the augmented secretory response of AMC is unique to IH. Like the carotid body, the enhanced AMC response to hypoxia is not reversed after the cessation of IH, and persisted into adulthood. Since sympathetic innervation to the target organs is incomplete in neonatal rats, it is likely that CA secretion from AMC contributes to cardiovascular changes during hypoxia associated with apnea, a possibility that requires further investigation.
4. Reactive oxygen species (ROS): an important cellular mechanism mediating the effects of neonatal IH on the carotid body and AMC
The above outlined studies demonstrate that intermittent but not continuous hypoxia leads to augmented hypoxic sensing by the carotid body and AMC in neonatal rats. The major difference between intermittent and continuous hypoxia is the periodic oxygenation in the former but not the latter. In this respect, intermittent hypoxia resembles ischemia–reperfusion. It is well known that during reperfusion, there is increased generation of ROS (Ambrosio et al., 1995). The following observations demonstrate that ROS mediate the effects of neonatal IH on hypoxic sensing by the carotid body and AMC: (a) IH increased ROS levels in neonatal carotid bodies and adrenal medullae as evidenced by elevated malondialdehyde (MDA) levels (Pawar et al., 2009; Souvannakitti et al., 2010), which represent oxidized lipids and proteins (Hiroshi et al., 1979), and (b) antioxidant treatment prevented the augmented hypoxic response of the carotid body and AMC evoked by neonatal IH (Pawar et al., 2009; Souvannakitti et al., 2009, 2010). Interestingly, antioxidant treatment had no effect on hyperplasia of glomus cells by IH (Pawar et al., 2009), suggesting that IH-induced augmented hypoxic sensitivity of the neonatal carotid body is not secondary to increased number of glomus cells.
The elevated ROS levels by neonatal IH could be due to either increased ROS generation by pro-oxidant enzymes or decreased ROS degradation by anti-oxidant enzymes. The family of NADPH oxidases (Nox) constitutes one of the major sources of ROS in mammalian cells (see Bedard and Krause, 2007; for reference). IH exposed neonatal rat adrenal medulla show up regulation of Nox2 and 4 mRNAs and increase in Nox enzyme activity (Souvannakitti et al., 2010). On the other hand, mRNAs encoding anti-oxidant enzymes such as the manganese superoxide dismutase (Sod-2), catalase (CAT1), and glutathione peroxidase 1 (GPX1) were down regulated in IH exposed neonatal rat carotid bodies and adrenal medullae (Nanduri et al., 2012). These observations suggest that both decreased activity of anti-oxidant enzymes and increased activity of NADPH oxidases contribute to elevated ROS levels by neonatal IH.
5. Neonatal IH initiates epigenetic programming of the red-ox state
The increased ROS levels are not reversed after the cessation of neonatal IH, but persisted into adulthood (Pawar et al., 2008; Souvannakitti et al., 2009). A recent study examined the molecular mechanisms underlying the long-lasting effects of neonatal IH on ROS levels in the carotid body and adrenal medulla (Nanduri et al., 2012). In this study, rat pups are exposed to IH from ages P0–P10 and then reared under room air environment (normoxia) for 40 days. Analysis of mRNAs show increased expression of genes encoding pro-oxidant enzymes and decreased expression of genes encoding anti-oxidant enzymes in carotid bodies and adrenal medullae of adult rats exposed to neonatal IH compared to controls.
Epigenetic mechanisms are heritable modifications of DNA and include DNA methylation and histone modifications. Epigenetic changes result in long-term changes in gene expression (Feinberg, 2007). Using the Sod2 as a model gene, Nanduri et al. (2012) showed that DNA hypermethylation contributes to neonatal IH-induced down regulation of Sod2 mRNA, protein and the enzyme activity. These authors further identified a single CpG dinucleotide within the Sod2 gene close to the transcription initiation site that was hypermethylated in response to neonatal IH. Neonatal rats exposed to IH were treated with decitabine, an inhibitor of DNA methylation. Decitabine treatment prevented DNA hypermethylation of the Sod2 gene and restored ROS levels to control values. Further studies are needed to establish whether DNA hypermethylation also mediates the down regulation of anti-oxidant enzyme genes other than the Sod2 by neonatal IH. Molecular mechanisms mediating the persistent up regulation of pro-oxidant enzymes by neonatal IH, however, remain to be elucidated. Not withstanding these limitations, the study by Nanduri et al. (2012) demonstrate that neonatal IH initiates epigenetic changes that lead to long-lasting increase in ROS levels in the carotid body and adrenal medulla.
Adult rats that were exposed to IH in neonatal period exhibited (a) augmented carotid body and AMC responses to hypoxia; (b) enhanced hypoxic ventilatory response, a hall mark response of the carotid chemoreflex; (c) irregular breathing; (d) greater number of apneas; and (e) elevated blood pressures and plasma catecholamines compared to control rats. Remarkably, decitabine treatment prevents these cardio-respiratory changes (Nanduri et al., 2012). These observations suggest that neonatal IH predisposes to cardio-respiratory dysfunction in adulthood involving epigenetic regulation of the red-ox state. These findings might be of clinical relevance in view of the recent studies showing greater incidence of sleep disordered breathing with apnea (Rosen et al., 2003; Paavonen et al., 2007; Hibbs et al., 2008) and hypertension (Dalziel et al., 2007) in young adults and adults, respectively who were born preterm.
6. Mechanisms by which ROS mediate the effects of intermittent hypoxia on O2 sensing by the carotid body and AMC
6.1. Carotid body
Carotid bodies are comprised of glomus or type I cells and sustentacular or type II cells. Much of the available evidence suggests that hypoxia is transduced by the glomus cell, which by releasing excitatory transmitter(s) leads to excitation of the nearby afferent nerve ending (see Kumar and Prabhakar, 2012; for reference). Rey and Iturriaga (2004) reported that in adult rats, endothelin-1 (ET-1), a peptidergic modulator plays an important role in IH-induced augmented carotid body response to hypoxia. Neonatal rat carotid bodies expressed higher levels of ET-1 than the adults, and much of the peptide was localized to glomus cells (Pawar et al., 2009). The following findings suggest that ET-1 plays an important role in mediating the enhanced hypoxic sensory response of the carotid body in neonatal rats. These include: (a) IH exposed carotid bodies exhibit enhanced basal ET-1 release; (b) exogenous application of ET-1 as little as femtomolar concentrations markedly augments the hypoxic sensory response in IH exposed but not in control neonatal carotid body; (c) IH up regulated ETA mRNA expression in the carotid body; and (d) ETA but not ETB receptor antagonist prevents IH-evoked sensitization of the hypoxic sensory response of the carotid body (Pawar et al., 2009). Notably, anti-oxidant treatment prevents IH-evoked increase of basal ET-1 release and the up regulation of the ETA receptor mRNA (Pawar et al., 2009), suggesting that ROS mediates the effects of neonatal IH on the carotid body, which is in part due to its effects on ET-1 signaling.
Although the mechanisms by which ET-1 mediates augmented hypoxic sensing by neonatal IH have not been studied, a previous study (Chen et al., 2000) reported that exogenous application of ET-1 augments adult ex vivo carotid body response to hypoxia, and this effect was attributed to phosphorylation of Ca2+ channel protein and enhanced Ca2+ influx in glomus cells. Further studies are needed to establish the mechanisms by which ROS facilitate the basal ET-1 release and transcriptional up regulation of ET-1A receptor mRNA. Recently, Del Rio et al. (2011) reported that in adult rat carotid body several days of IH exposure leads to transient ET-1 up regulation, decreased endothelial nitric oxide synthase (eNOS) expression, delayed but progressive increase in inducible NOS (iNOS) and pro-inflammatory cytokines. Whether in addition to ET-1, other neurotransmitter/modulators also contribute to the effects of IH in neonatal carotid body remains to be determined.
6.2. Adrenal medullary chromaffin cells (AMC)
Hypoxia-induced CA secretion from neonatal chromaffin cells requires depolarization of AMC, activation of voltage-gated Ca2+ channels and the ensuing elevations in [Ca2+]i (Takeuchi et al., 2001; Souvannakitti et al., 2010). IH exposed neonatal AMCs exhibited elevated baseline [Ca 2+]i and augmented [Ca 2+]i responses to hypoxia and these effects persisted in the presence of Ca2+-free medium as well as in the presence of cadmium chloride, a pan voltage-gated Ca2+ channel blocker (Souvannakitti et al., 2009). These observations suggest that elevation of [Ca 2+]i by IH involves voltage-gated Ca2+ flux as well as mobilization of intracellular Ca2+ stores. Mibefradil, a blocker of T-type Ca2+ channels attenuated the effects of hypoxia on [Ca2+]i and catecholamine secretion in IH-exposed AMC. Cav3.1 and Cav3.2 T-type Ca2+ channel mRNA levels were increased and T-type Ca2+ currents were augmented in IH-treated AMC (Souvannakitti et al., 2010). These observations suggest that activation of low threshold T-type Ca2+ by IH contributes in part to the augmented Ca2+ influx. The following findings by Souvannakitti et al. (2010) suggest that ryanodine receptors (RyRs) contribute to mobilization of Ca2+ stores by IH. First, IH up regulated mRNA levels of RyR2 and RyR3 isoforms in neonatal AMCs. Second, RyR2 was S-glutathionylated in IH exposed AMC, a post-translational modification, which is known to activate RyRs (Aracena et al., 2003). Third, in the presence of Ca2+-free medium, blockade of RyRs prevented the elevated baseline and hypoxia-evoked [Ca2+]i responses in IH exposed AMC. Remarkably, anti-oxidant treatment prevented the effects of IH on hypoxia-evoked CA secretion, exaggerated [Ca2+]i responses, transcriptional up regulation of T-type Ca2+ channels as well as RyRs and post-translational modification of RyRs by S-glutathionylation (Souvannakitti et al., 2009, 2010). These findings demonstrate that the augmented catecholamine secretion in response to neonatal IH requires ROS-mediated changes in Ca2+ signaling involving transcriptional and post-translational modifications of T-type Ca2+ channels and RyRs. The mechanisms by which ROS mediates the effects of neonatal IH on O2 sensing by the carotid body and AMC are illustrated in Fig. 1.
Fig 1.
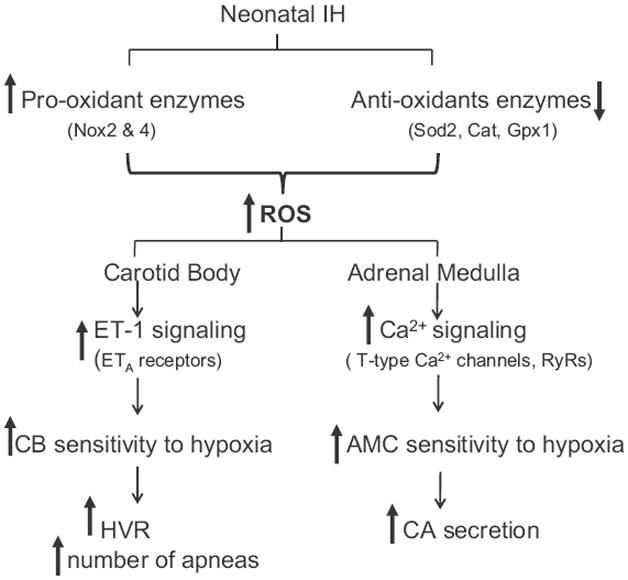
Schematic presentation of the effects of neonatal intermittent hypoxia (IH) on carotid body (CB) and adrenal medullary chromaffin cell (AMC) responses to hypoxia and the involvement of reactive oxygen species (ROS). Nox2 and 4 = NADPH oxidase 2 and 4; Sod2 = superoxide dismutase 2; Cat = catalase; Gpx1 = glutathione peroxidase 1; ET-1 = endothelin 1; ETA = endothelin 1A receptor subtype; RyRs = ryanodine receptors; HVR = hypoxic ventilatory response; CA = catecholamine.
7. Gaps in the knowledge and future directions
Recurrent apnea with intermittent hypoxia (IH) is a major clinical problem in preterm infants. In this review, we attempted to summarize the impact of neonatal IH on hypoxic sensing by the carotid body and AMC in a rodent model. Available evidence, albeit limited, demonstrates that exposure to IH in the neonatal period leads to heightened hypoxic sensitivity of the carotid body and enhanced CA secretion from neonatal AMC. Evidence is emerging that ROS signaling is a major cellular mechanism mediating the effects of neonatal IH on the carotid body and AMC. Virtually nothing is known on the effects of IH on O2 sensitive K+ channels(s) and Ca2+ signaling in glomus cells of the neonatal carotid bodies, although they play critical roles in the sensory transduction in adult carotid bodies (see Kumar and Prabhakar, 2012; for reference). Likewise, a variety of K+ channels have been implicated in hypoxia evoked CA secretion from neonatal AMC (Thompson et al., 1997). However, little is known on the effects of neonatal IH and ROS on K+ channels in neonatal AMC.
Although a recent study (Nanduri et al., 2012) showed that epigenetic mechanisms involving DNA hypermethylation of the Sod-2 contribute to IH-induced persistent oxidative stress, the mechanism by which neonatal IH initiates DNA hypermethylation has not been studied. Besides DNA methylation, histone modifications represent another important epigenetic mechanism. The effects of neonatal IH on histone modifications in the carotid body and adrenal medulla remain to be investigated. Despite these gaps in the knowledge, it seems certain that neonatal IH leads to developmental programming of O2 sensing by the carotid body and AMC via epigenetic modulation of the red-ox state, which seems to be the underlying cause for cardio-respiratory dysfunctions in adulthood. Given that young adults born preterm exhibit higher incidence of not only sleep-disordered breathing with apneas (Rosen et al., 2003; Paavonen et al., 2007; Hibbs et al., 2008) but also higher incidence of hypertension (Dalziel et al., 2007), use of DNA hypomethylating agents in pre-term infants might offer novel therapeutic intervention to decrease long-term morbidity associated with neonatal IH.
Acknowledgments
This research is supported by National Institutes of Health grants HL-76537, HL-90554, and HL-86493. The authors gratefully acknowledge the participation of Drs. Anita Pawar, Dangjai Souvannakitti, and Ying-Jie Peng in various experiments outlined in this article. We thank Dr. Ganesh K. Kumar for critical review of the manuscript.
References
- Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what’s new? Pediatric Pulmonology. 2008;43:937–944. doi: 10.1002/ppul.20832. [DOI] [PubMed] [Google Scholar]
- Ambrosio G, Tritto I, Chiariello M. The role of oxygen free radicals in preconditioning. Journal of Molecular and Cellular Cardiology. 1995;27:1035–1039. doi: 10.1016/0022-2828(95)90072-1. [DOI] [PubMed] [Google Scholar]
- Aracena P, Sánchez G, Donoso P, Hamilton SL, Hidalgo C. S-glutathionylation decreases Mg2+ inhibition and S-nitrosylation enhances Ca2+ activation of RyR1 channels. Journal of Biological Chemistry. 2003;278:42927–42935. doi: 10.1074/jbc.M306969200. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. Journal of Physiology. 1984;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JL. Developmental plasticity in respiratory control. Journal of Applied Physiology. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Chen J, He L, Dinger B, Fidone S. Cellular mechanisms involved in rabbit carotid body excitation elicited by endothelin peptides. Respiration Physiology. 2000;121:13–23. doi: 10.1016/s0034-5687(00)00113-4. [DOI] [PubMed] [Google Scholar]
- Dalziel SR, Parag V, Rodgers A, Harding JE. Cardiovascular risk factors at age 30 following pre-term birth. International Journal of Epidemiology. 2007;36:907–915. doi: 10.1093/ije/dym067. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Moya EA, Iturriaga R. Differential expression of pro-inflammatory cytokines, endothelin-1 and nitric oxide synthases in the rat carotid body exposed to intermittent hypoxia. Brain Research. 2011;1395:74–85. doi: 10.1016/j.brainres.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. Developmental aspects of oxygen sensing by the carotid body. Journal of Applied Physiology. 2000;88:2296–2301. doi: 10.1152/jappl.2000.88.6.2296. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Doyle TP. Hypoxia-induced catecholamine release from rat carotid body, in vitro, during maturation and following chronic hypoxia. Advances in Experimental Medicine and Biology. 1994;360:197–199. doi: 10.1007/978-1-4615-2572-1_27. [DOI] [PubMed] [Google Scholar]
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- Hibbs AM, Johnson NL, Rosen CL, Kirchner HL, Martin R, Storfer-Isser A, Red line S. Prenatal and neonatal risk factors for sleep disordered breathing in school-aged children born preterm. Journal of Pediatrics. 2008;153:176–182. doi: 10.1016/j.jpeds.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroshi O, Nobuko O, Kunio Y. Assay for lipid peroxidases in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Julien C, Bairam A, Joseph V. Chronic intermittent hypoxia reduces ventilatory long-term facilitation and enhances apnea frequency in newborn rats. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2008;294:R1356–R1366. doi: 10.1152/ajpregu.00884.2007. [DOI] [PubMed] [Google Scholar]
- Julien C, Joseph V, Bairam A. Alteration of carotid body chemoreflexes after neonatal intermittent hypoxia and caffeine treatment in rat pups. Respiratory Physiology & Neurobiology. 2011;177:301–312. doi: 10.1016/j.resp.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Comprehensive Physiology. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz H, Bistoletti P. Catecholamine release in the newborn infant at birth. Pediatric Research. 1977;11:889–893. doi: 10.1203/00006450-197708000-00007. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Edwards D, Henderson-Smart D, Hertzberg T, Jeffery H. Autonomic reflexes in preterm infants. Acta Paediatrica Scandinavica. 1990;79:721–728. doi: 10.1111/j.1651-2227.1990.tb11546.x. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Sjöquist B. Deficient sympatho-adrenal activity–a cause of apnoea? Urinary excretion of catecholamines and their metabolites in preterm infants. Early Human Development. 1980;4:405–409. doi: 10.1016/0378-3782(80)90045-6. [DOI] [PubMed] [Google Scholar]
- Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng YJ, Kumar GK, Fox AP, Godley LA, Semenza GL, Prabhakar NR. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2515–2520. doi: 10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock ML, Difiore JM, Arko MK, Martin RJ. Relationship of the ventilatory response to hypoxia with neonatal apnea in preterm infants. Journal of Pediatrics. 2004;144:291–295. doi: 10.1016/j.jpeds.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Paavonen EJ, Strang-Karlsson S, Räikkönen K, Heinonen K, Pesonen AK, Hovi P, Andersson S, Järvenpää AL, Eriksson JG, Kajantie E. Very low birth weight increases risk for sleep-disordered breathing in young adulthood: the Helsinki study of very low birth weight adults. Pediatrics. 2007;120:778–784. doi: 10.1542/peds.2007-0540. [DOI] [PubMed] [Google Scholar]
- Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2009;296:R735–R742. doi: 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. Journal of Applied Physiology. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. Journal of Applied Physiology. 2004;97:2020–2025. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- Poets CF, Samuels MP, Southall DP. Epidemiology and pathophysiology of apnoea of prematurity. Biology of the Neonate. 1994;65:211–219. doi: 10.1159/000244055. [DOI] [PubMed] [Google Scholar]
- Rey S, Iturriaga R. Endothelins and nitric oxide: vasoactive modulators of carotid body chemoreception. Current Neurovascular Research. 2004;1:465–473. doi: 10.2174/1567202043361857. [DOI] [PubMed] [Google Scholar]
- Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, Martin RJ, Redline S. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. Journal of Pediatrics. 2003;142:383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Adrenomedullary function in the neonatal rat: responses to acute hypoxia. Journal of Physiology. 1985;358:1–16. doi: 10.1113/jphysiol.1985.sp015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Non-neurogenic adrenal catecholamine release in the neonatal rat: exocytosis or diffusion? Brain Research. 1986;393:274–277. doi: 10.1016/0165-3806(86)90031-3. [DOI] [PubMed] [Google Scholar]
- Souvannakitti D, Kumar GK, Fox A, Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. Journal of Neurophysiology. 2009;101:2837–2846. doi: 10.1152/jn.00036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. Journal of Neuroscience. 2010;30:10763–10772. doi: 10.1523/JNEUROSCI.2307-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterni LM, Bamford OS, Wasicko MJ, Carroll JL. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. American Journal of Physiology. 1999;277:L645–L652. doi: 10.1152/ajplung.1999.277.3.L645. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Mochizuki-Oda N, Yamada H, Kurokawa K, Watanabe Y. Non-neurogenic hypoxia sensitivity in rat adrenal slices. Biochemical and Biophysical Research Communications. 2001;289:51–56. doi: 10.1006/bbrc.2001.5913. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. Journal of Physiology. 1997;498(Pt 2):503–510. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani K, Okada S, Nakamura K. Characterization of functional nicotinic acetylcholine receptors involved in catecholamine release from the isolated rat adrenal gland. European Journal of Pharmacology. 2002;446:83–87. doi: 10.1016/s0014-2999(02)01819-8. [DOI] [PubMed] [Google Scholar]


