Abstract
Previous studies suggest that carotid body responses to long-term changes in environmental oxygen differ between neonates and adults. In the present study we tested the hypothesis that the effects of chronic intermittent hypoxia (CIH) on the carotid body differ between neonates and adult rats. Experiments were performed on neonatal (1–10 days) and adult (6–8 wk) males exposed either to CIH (9 episodes/h; 8 h/day) or to normoxia. Sensory activity was recorded from ex vivo carotid bodies. CIH augmented the hypoxic sensory response (HSR) in both groups. The magnitude of CIH-evoked hypoxic sensitization was significantly greater in neonates than in adults. Seventy-two episodes of CIH were sufficient to evoke hypoxic sensitization in neonates, whereas as many as 720 CIH episodes were required in adults, suggesting that neonatal carotid bodies are more sensitive to CIH than adult carotid bodies. CIH-induced hypoxic sensitization was reversed in adult rats after reexposure to 10 days of normoxia, whereas the effects of neonatal CIH persisted into adult life (2 mo). Acute intermittent hypoxia (IH) evoked sensory long-term facilitation of the carotid body activity (sensory LTF, i.e., increased baseline neural activity following acute IH) in CIH-exposed adults but not in neonates. The effects of CIH were associated with hyperplasia of glomus cells in neonatal but not in adult carotid bodies. These observations demonstrate that responses to CIH differ between neonates and adults with regard to the magnitude of sensitization of HSR, susceptibility to CIH, induction of sensory LTF, reversibility of the responses, and morphological remodeling of the chemoreceptor tissue.
Keywords: sensory long-term facilitation, hyperplasia, glomus cells
CHRONIC INTERMITTENT HYPOXIA (CIH) is experienced in many situations, including sleep-disordered breathing manifested as recurrent apneas. Nearly 4–5% of adult males and 2–4% of females after menopause are subject to recurrent apneas (16, 26). Patients with recurrent apneas, and rodents exposed to CIH, exhibit elevated blood pressures, increased sympathetic nerve activity, and altered breathing (23). It has been proposed that carotid bodies, the primary chemoreceptors for detecting changes in arterial blood O2, constitute the front line of defense during CIH, and the ensuing reflexes mediate cardiorespiratory changes (6). Direct recording of the carotid body sensory activity showed that CIH sensitizes chemoreceptor response to acute hypoxia in adult rats (18), cats (24), and mice (21) and evokes a long-lasting increase in baseline sensory activity in response to acute repetitive hypoxia in rats (17) and mice (21). This long-lasting elevation of baseline sensory activity was termed sensory long-term facilitation (LTF; 17). The effects of CIH on the carotid body are time dependent and reversible after reexposure to normoxia and were not associated with changes in morphology of the chemoreceptor tissue (17). Besides adult humans, nearly 50% of premature infants also experience CIH as a consequence of recurrent apneas (22). Carotid bodies, however, are immature at birth and respond poorly to hypoxia (4, 5, 7). Recently, Peng et al. (19) reported that CIH also sensitizes the response of neonatal carotid bodies to hypoxia. However, whether CIH also evokes sensory LTF of neonatal carotid bodies, i.e., increased baseline activity during normoxia, as seen in adults, is not known. Also it is unknown if the effects of CIH are reversible in neonatal carotid bodies.
Previous studies suggest that carotid body responses to long-term changes in environmental oxygen differ between neonates and adults. For example, continuous hypoxia in the neonatal period attenuates the hypoxic sensory response (27), whereas it augments the sensory response in adults (2). Likewise, neonatal hyperoxia not only depresses the hypoxic sensory response of the neonatal carotid body but the effects persist even in adult life (3). Given these previously reported differences between neonatal and adult carotid body responses, in the present study we tested the hypothesis that the effects of CIH also differ between neonatal and adult carotid body function. Our results demonstrate striking differences between the CIH-exposed neonatal and adult carotid bodies with respect to the magnitude of sensitization of the hypoxic sensory response, susceptibility to CIH, induction of sensory LTF, and reversibility of the responses, as well as the occurrence of morphological remodeling of the chemoreceptor tissue.
MATERIALS AND METHODS
Experiments were performed on neonatal (~2–6 h of age) and adult (~2–3 mo of age) male Sprague-Dawley rats. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the Case Western Reserve University.
Exposure to CIH
Adult rats or pups along with their mothers were exposed to CIH (15 s of hypoxia and 5 min of normoxia per cycle) as described previously (18). Briefly, animals housed in feeding cages were placed in a chamber designed for exposure to CIH. The animals were unrestrained, freely mobile, and fed ad libitum. The chamber was flushed with alternating cycles of nitrogen gas and room air. Inspired O2 levels reached a nadir of 5% O2 during hypoxia and a peak of 21% O2 during normoxia. Animals were exposed to nine episodes of intermittent hypoxia (IH) per hour for 8 h/day between 9:00 AM and 5:00 PM. O2 and CO2 levels in the chamber were continuously monitored, and ambient CO2 levels were maintained between 0.2 and 0.5%. Both groups of rats were exposed to 1, 3, or 10 days of CIH, which correspond to 72, 216, and 720 episodes, respectively. Control experiments were performed on age-matched rats exposed to normoxia. Acute experiments were performed after 6–10 h following either CIH or normoxia.
Recording of Carotid Body Sensory Activity
Sensory activity from carotid bodies ex vivo was recorded as previously described (17). Briefly, carotid bodies along with the sinus nerves were harvested from anesthetized rats (urethane; 1.5 g/kg ip) treated with heparin (200 USP units ip). Tissues were treated with 0.1% collagenase for 5 min to facilitate removal of connective tissue. The carotid body along with the sinus nerve was placed in a recording chamber (250-μl volume) and superfused with warm physiological saline (35°C) at a rate of 2 ml/min. The composition of the medium was (in mM) 125 NaCl, 5 KCl, 1.8 CaCl2, 2 MgSO4, 1.2 NaH2PO4, 25 NaHCO3, 10 D-glucose, and 5 sucrose, and the solution was bubbled with 95% O2-5% CO2. Carotid bodies were placed close to the inlet port of the superfusing solution to facilitate the diffusion of O2 into the glomus tissue. The time lag for the medium to reach from the reservoir to the recording chamber was ~15 s. To facilitate recording of clearly identifiable action potentials, the sinus nerve was treated with 0.1% collagenase for 5 min. Action potentials (2–5 active units) were recorded from one of the nerve bundles with a suction electrode and stored in a computer via an analog-to-digital translation board (PowerLab/8P, AD Instruments). The criteria for chemoreceptor activity include increased sensory activity in response to hypoxia and return to baseline after resuming the superfusion with hyperoxic medium. “Single” units were selected based on the height and duration of the individual action potentials using a spike discrimination program (Spike Histogram Program, Power Laboratory, AD Instruments). In each carotid body, at least two chemoreceptor units were analyzed. The PO2 and PCO2 of the superfusion medium were determined by a blood gas analyzer (ABL 5, Radiometer, Copenhagen, Denmark).
Carotid Body Morphology
Carotid body morphology was analyzed as described previously (14). Briefly, carotid bifurcations were harvested from anesthetized pups and adult rats. Adult rats were transcardially perfused with 4% paraformaldehyde; in neonates, carotid bifurcations were immersed in 4% paraformaldehyde for 4 h at room temperature. All bifurcations were, subsequently, washed in PBS, and cryoprotected in 30% sucrose/PBS at 4°C for 24 h. Tissue specimens were frozen in OCT compound (Tissue Tek, VWR Scientific), serially sectioned at a thickness of 8 μm, washed three times in PBS, and exposed to 20% normal goat serum and 0.2% Triton X-100 in PBS for 30 min. Sections were incubated at room temperature for 2 h with rabbit anti-tyrosine hydroxylase (1:8,000, Pel-Freez) antibody in PBS with 1% normal goat serum and 0.2% Triton X-100. After washing with PBS, sections were incubated for 1 h with Texas Red-X goat anti-rabbit IgG (1:200, Molecular Probes) in PBS with 1% normal goat serum and 0.2% Triton X-100. After washing with PBS, sections were mounted in DAPI-containing media and visualized using a fluorescent microscope (Eclipse E600, Nikon). Carotid body morphology and glomic volumes were analyzed in age-matched control and CIH-exposed rats using IMAGE software (Scion, Frederick, MD).
Protocols
Series 1
In this series of experiments, the effects of graded isocapnic hypoxia (PCO2 = 36 ± 3 mmHg) on carotid body sensory activity were analyzed in neonatal pups and adult rats exposed to 720 episodes of CIH or normoxia (n = 7 carotid bodies from 7 rats/pups each). Baseline sensory activity was recorded for 5 min while super-fusing carotid bodies equilibrated with 95% O2-5% CO2 (medium PO2 = 400 ± 20 mmHg, and PCO2 = 36 ± 3 mmHg). At the beginning of a given experiment (adults as well as neonates), we first established the time required for evoking sensory activity that reached either a plateau or started to decline from the peak activity in response to severe hypoxia (~35 mmHg). In control pups it took about 4–5 min, and 2–3 min in adults to elicit “steady-state” response. This optimal duration of hypoxia was subsequently employed in the remainder of the experiment. Carotid body responses were taken with varying levels of hypoxia (medium PO2 = 140 ± 2 mmHg, 66 ± 2 mmHg, 34 ± 5 mmHg; PCO2 = 36 ± 3 mmHg). At least a 5-min interval was given between each hypoxic challenge.
Series 2
The impact of increasing the number of CIH episodes (36, 72, 216, and 720 CIH episodes) on the hypoxic sensory response (PO2 = 35 ± 5 mmHg, and PCO2 = 36 ± 3 mmHg) was examined in neonatal pups and adult rats (n = 7 carotid bodies from 7 pups and adult rats with each duration of CIH). Parallel experiments were performed on age-matched rat pups reared under normoxia (n = 7 carotid bodies from 7 pups at each age).
Series 3
In this series, we examined the reversibility of the effects of CIH. To this end, adult and neonatal rat pups (n = 7 rats in each group) were exposed to 720 episodes of CIH followed by exposure to 10 days of normoxia. Subsequently, rats were anesthetized and hypoxic sensory responses of the ex vivo carotid bodies were recorded as described above from both groups. In addition, another group of rat pups (n = 7) was exposed to 720 CIH episodes soon after birth and then was reared in normoxia for an additional 50 days. Control experiments were performed on age-matched rats reared under normoxia for 60 days.
Series 4
The effects of CIH on sensory LTF of the carotid body were examined in adult and neonatal rat pups exposed to 720 CIH episodes (n = 6 carotid bodies from 6 adult rats; 7 carotid bodies from 7 rat pups). Baseline sensory activity was recorded for 15 min while superfusing carotid bodies with medium equilibrated with 95% O2-5% CO2 (medium PO2 = 390 ± 17 mmHg, and PCO2 = 36 ± 2 mmHg). Then carotid bodies were challenged with 10 episodes of 30 s (including the lag time of 15 s) hypoxia (medium PO2 = 35 ± 3 mmHg, and PCO2 = 35 ± 5 mmHg) interspersed with 5 min of baseline medium (PO2 = 390 ± 17 mmHg). After terminating the 10th episode of hypoxia, the sensory activity was continuously recorded for 1 h while superfusing the carotid bodies with medium equilibrated with hyperoxia.
Series 5
The effects of CIH (720 episodes) on carotid body morphology was examined in neonatal rat pups and adult rats (n = 3 rats each). Control experiments were performed on age-matched rat pups and adult rats exposed to a comparable number of days of normoxia (n = 3 rats each).
Data Analysis
Carotid body sensory activity (discharge from single units) was analyzed every 10 s for 5 min during the baseline as well as during the entire period of hypoxia, and the data were averaged and expressed as impulses per second (imp/s). In the experiments involving sensory LTF, baseline sensory activity was analyzed every 10 s for 15 min during each hypoxic episode and every 5 min during 60 min of the post-acute IH period. Sensory activity was averaged and expressed as impulses per second. The data were analyzed as absolute values or as Δimp/s (i.e., hypoxia – baseline activity). All data are presented as means ± SE. Statistical significance was assessed by two-way ANOVA with repeated measures followed by Tukey’s test. P values < 0.05 were considered significant.
RESULTS
Effect of CIH on Hypoxic Sensory Response in Neonates and Adult Rats
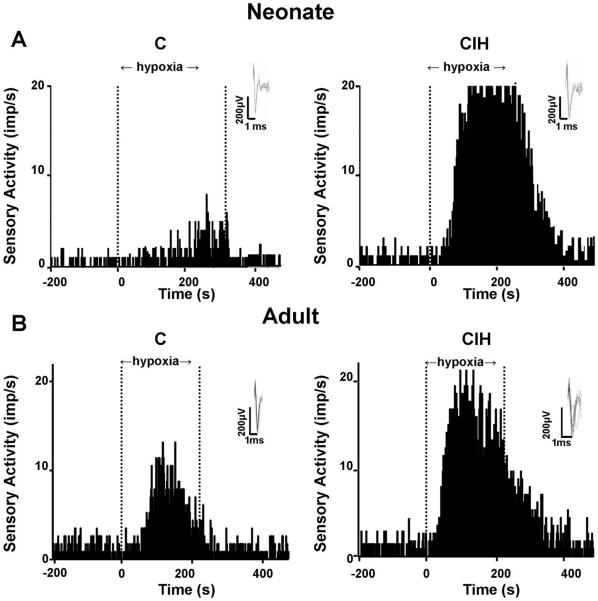
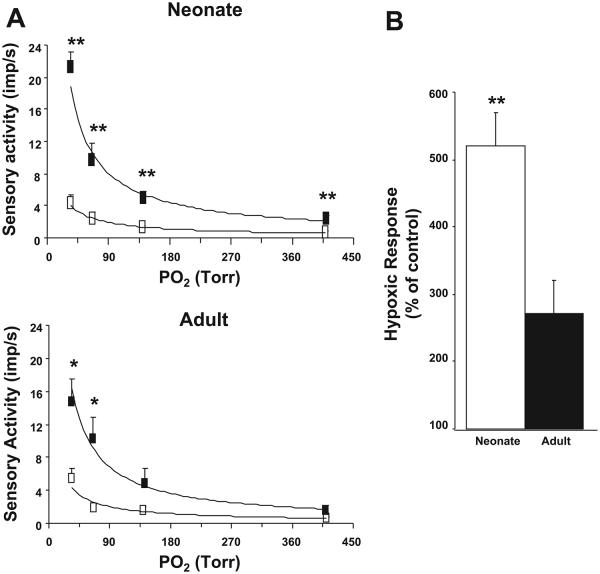
Figure 1 illustrates examples of hypoxic sensory responses in neonatal and adult carotid bodies after 720 episodes of CIH. The hypoxic sensory response was augmented in both groups of rats exposed to CIH. However, the magnitude of the hypoxic sensory response was more pronounced in CIH-exposed neonate than adult rats. Average data of the sensory response to graded isocapnic hypoxia in both groups of rats with and without CIH is summarized in Fig. 2A. In CIH-exposed rat pups, hypoxic sensory response was significantly greater at all levels of PO2 tested compared with age-matched control pups. On the other hand, in CIH-exposed adult rats, significant potentiation of the hypoxic response was seen only at severe levels of hypoxia. The response tended to be higher at modest levels of hypoxia in CIH-exposed adult rats, but it was not significant (P > 0.05).
Fig. 1.
Chronic intermittent hypoxia (CIH) augments carotid body response to hypoxia in both adult and neonatal rats. Examples of carotid body responses to hypoxia in rat pups (A) and adult rats (B) exposed either to 720 episodes of CIH or to normoxia (C) are shown. Area between dashed lines indicates duration of hypoxic stimulus. Hypoxia: medium PO2 = 32 and 33 mmHg (A); medium PO2 = 33 and 34 mmHg (B). Insets: superimposed action potential from a single unit. imp/s, impulses per second.
Fig. 2.
Effect of CIH on carotid body responses to graded hypoxia in neonates and adults. A, top: average data of the carotid body response to graded hypoxia from rat pups conditioned with 720 episodes of CIH (closed box) and age-matched control pups (open box). A, bottom: similar data in adult rats conditioned with 720 episodes of CIH (closed box) and normoxic controls (open box). Medium PCO2 was kept at 35–36 mmHg in both groups. B: average data of hypoxic sensory response (medium PO2 = 35 ± 3 mmHg) presented as percentage of age-matched controls in neonatal rat pups and adult rats exposed to 720 episodes of CIH. Data presented are means ± SE from 7 carotid bodies from 7 rats in each group, respectively. *P < 0.05, **P < 0.005, CIH vs. control.
Sensory response to severe hypoxia (medium PO2 = 35 ± 3 mmHg) was compared between CIH-exposed neonates and adults, and the results are summarized in Fig. 2B. Hypoxic sensory response was 519% in CIH compared with control pups, whereas it was 272% in CIH-exposed adult rats. Thus the magnitude of the hypoxic response was 247% higher in CIH-exposed neonates than in CIH-exposed adults (P < 0.001).
The effect of an increasing number of CIH episodes on the hypoxic sensory response (PO2 = 35 ± 5 mmHg) was assessed in neonates and adults. Control experiments were performed on age-matched pups and adult rats exposed to normoxia. The results are summarized in Fig. 3. In neonatal pups, 36 episodes of CIH had no significant effect on the hypoxic sensory response. However, increasing the number of CIH episodes from 72 to 720 resulted in progressive augmentation of the hypoxic response (Fig. 3A). In contrast, in adult rats, significant augmentation of the hypoxic sensory response was elicited only after 720 episodes of CIH (Fig. 3B).
Fig. 3.
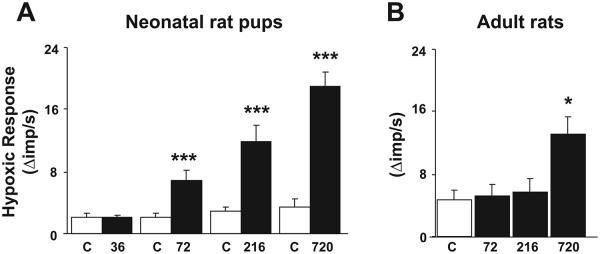
Effect of increasing number of CIH episodes on carotid body response to hypoxia in neonatal and adult rats. A: average data of delta (hypoxia minus baseline) hypoxic sensory response in age-matched controls (C) and pups exposed to 36, 72, 216, and 720 episodes of CIH. B: data from control (C) and 72, 216, and 720 episodes of CIH-conditioned adult rats. Hypoxia: medium PO2 = 35 ± 5 mmHg (A); medium PO2 = 35 ± 3 mmHg (B). Data are means ± SE from 7 carotid bodies from 7 rats, respectively. *P < 0.05, ***P < 0.001, CIH vs. control.
CIH-Induced Sensitization of the Hypoxic Response is Reversible in Adult but not in Neonatal Rats
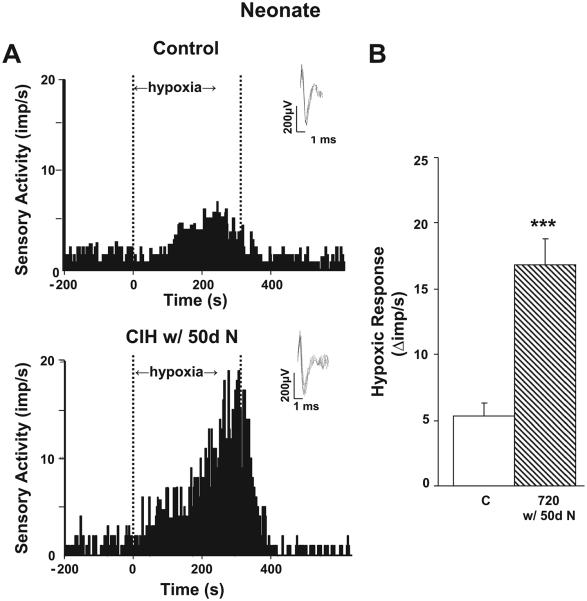
In adult rats CIH-induced sensitization of the hypoxic sensory response was completely reversed after exposing CIH rats to 10 days of normoxia (Fig. 4). In contrast, CIH-evoked sensitization of the hypoxic sensory response persisted even after 10 days of normoxia in neonatal rats. To test whether longer exposures to normoxia are required for reversing CIH-induced sensitization of the hypoxic sensory response in neonates, another group of rat pups was conditioned with 720 episodes of CIH (i.e., 10 days of CIH) soon after birth and then were reared in a normoxic environment until 2 mo of age. Age-matched rat pups reared in normoxia for 2 mo served as controls. As shown in Fig. 5, even after 50 days of normoxia, the carotid body response to hypoxia was significantly greater in rats that were exposed to CIH as neonates than the corresponding controls.
Fig. 4.
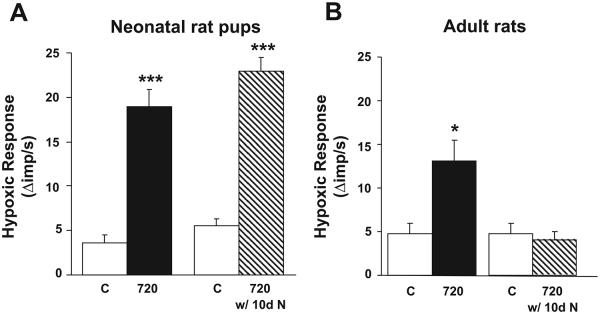
CIH-induced sensitization of the hypoxic response is reversible in adult rats but not in neonatal rats. Hypoxic sensory response in neonatal rats (A) and adult rats (B) exposed to 720 episodes of CIH and following reexposure to 10 days of normoxia (N). Delta hypoxia sensory response = sensory activity during hypoxia minus baseline activity. Data presented are means ± SE from 7 carotid bodies from 7 rats, respectively. *P < 0.05, ***P < 0.001, CIH vs. control.
Fig. 5.
Carotid body response to hypoxia in 2-mo-old rats exposed to neonatal CIH. A: examples of carotid body responses to hypoxia from an age-matched control (A, top) and from rats exposed to neonatal CIH (720 CIH episodes) and then reared in normoxia for 50 days (A, bottom) are shown. Area between dashed lines indicates duration of the hypoxic stimulus. Medium PO2 during hypoxia was 32 and 31 mmHg in top and bottom panels, respectively. Insets: superimposed action potential from a single unit. B: average data of HSR. Results are presented as Δimp/s, i.e., sensory activity during hypoxia minus baseline activity. Age-matched controls (C) and rat pups conditioned with 720 episodes of CIH and then reared up in normoxia for 50 days (720 w/50d N) are shown. Data presented are means ± SE from 7 carotid bodies from 7 rats, respectively. ***P < 0.001, CIH vs. control.
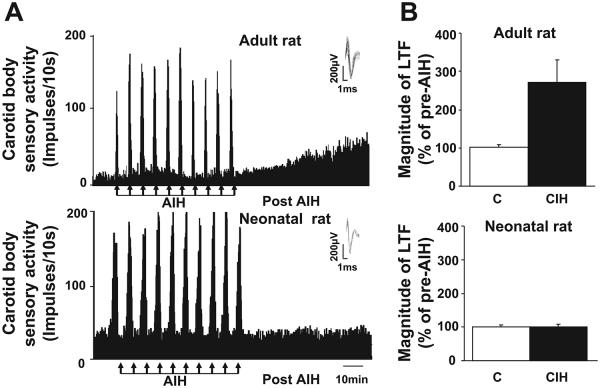
Absence of CIH-Evoked Sensory LTF in Neonatal Carotid Bodies
The effects of acute IH on sensory LTF were examined in neonatal and adult rat carotid bodies exposed to 720 episodes of CIH. Acute IH evoked robust sensory LTF in CIH-exposed adult rats. In contrast, acute IH was ineffective in evoking sensory LTF in CIH-exposed neonatal rats (Fig. 6).
Fig. 6.
CIH conditioning does not elicit sensory long-term facilitation (LTF) in neonatal carotid bodies. A: representative examples of carotid body sensory activity in response to 10 brief episodes of acute intermittent hypoxia (AIH; at arrows) from a CIH-conditioned (720 CIH episodes) adult (A, top) and neonatal rat (A, bottom). Insets: superimposed action potential from a single unit. B: average data of the magnitude of sensory LTF. Results are presented as percentage of pre-AIH. Top and bottom represent data from adult and neonatal rats, respectively, along with respective controls (C). Data presented are means ± SE from 6 adult and 10 neonatal carotid bodies.
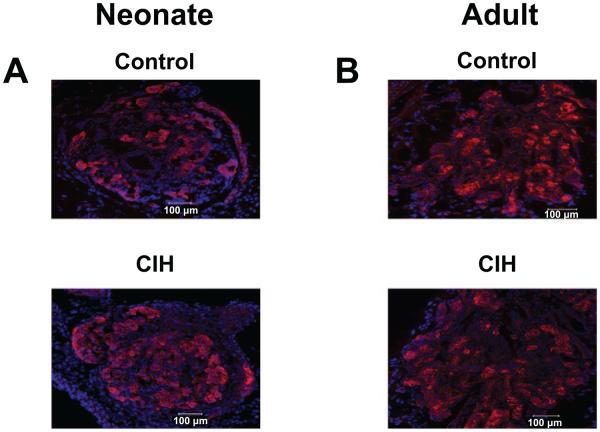
CIH Affects Carotid Body Morphology in Neonatal Rats
The effects of CIH (720 episodes) on carotid body morphology were examined in neonates and adult rats. Control studies were performed on age-matched neonates and adult rats exposed to normoxia. Carotid body sections were stained for tyrosine hydroxylase (TH), an established marker of glomus cells. Morphometric analysis revealed that the number of TH-positive glomus cells and the ratio of glomic to total carotid body volume were significantly greater in CIH-exposed rat pups compared with controls (Fig. 7, Table 1). The total volume of the carotid body, however, was not significantly altered in CIH-exposed neonates compared with controls (Fig. 7, Table 1). In contrast, there were no significant changes in the number of glomus cells, or the ratio of glomic to total carotid body volume or total volume of the carotid body in adult rats exposed to CIH (Fig. 7, Table 1).
Fig. 7.
CIH induces hyperplasia of glomus cells in neonatal carotid bodies but not in adult carotid bodies. Tyrosine hydroxylase (TH)-like immunoreactivity in the carotid body. Carotid body sections from a control and neonatal rat pup exposed to 720 episodes of CIH (A, top and bottom, respectively), and control and adult rat exposed to 720 episodes of CIH (B, top and bottom, respectively) are shown.
Table 1.
Morphometric analysis of carotid bodies from control and CIH-conditioned neonatal and adult rats.
| Total Volume, μm3 | Glomic Volume, μm3 | Glomic Volume/Total Volume | |
|---|---|---|---|
| Neonatal rat | |||
| Control | (2.96±0.18)×106 | (0.62±0.07)×106 | 0.21 ±0.02 |
| CIH | (3.62±0.27)×106NS | (1.52±0.14)×106*** | 0.42±0.02*** |
| Adult rat | |||
| Control | (3.75±0.34)×106 | (0.75±0.07)×106 | 0.22±0.03 |
| CIH | (3.70±0.57)×106NS | (0.82±0.13)×106NS | 0.23±0.22NS |
Data are means ± SE from 6 carotid bodies [n = 4 sections in each carotid body from 3 rats (adults and neonatal rats) and in each group]. CIH, chronic intermittent hypoxia. NS, not significant
P < 0.001, CIH vs. control.
DISCUSSION
In the present study we investigated whether carotid body responses to CIH differ between neonates and adult rats. Consistent with previous reports (18, 19), CIH exposure led to sensitization of the carotid body response to hypoxia in both adults and neonates. However, for a given level of hypoxia, the magnitude of sensitization was more pronounced in neonates than in adults. The pronounced sensitization of the hypoxic sensory response in neonates could be attributed to several factors, including cardiovascular alterations, which are known to influence the hypoxic sensory response of the carotid body (9). The present study was, however, performed on carotid bodies ex vivo, wherein the influence from cardiovascular changes on the chemoreceptor activity was effectively absent. Thus changes in cardiovascular variables are unlikely to account for the observed enhancement in the hypoxic sensory response in neonates. The amount of connective tissue around the carotid bodies would affect the diffusion of oxygen and the amount of oxygen dissolved around the tissue. Although great care was taken in removing the connective tissue around the carotid bodies in both groups of rats, we cannot exclude the contribution, if any, from variations in oxygen diffusion between the preparations. Nonetheless, these findings demonstrate that CIH evokes greater sensitization of the hypoxic response in neonatal carotid bodies than in adults.
In striking contrast to adult carotid bodies, CIH resulted in hyperplasia of glomus cells in neonates. Several lines of evidence suggest that glomus or type I cells are critical for sensing hypoxia by the carotid body (15). Therefore, it is conceivable that the greater sensitization of the hypoxic sensory response in CIH-exposed neonates could be attributed in part to hyperplasia of glomus cells. In early neonatal life, growth factors such as brain-derived nerve growth factor (BDNF) and glial-derived nerve factor (GDNF) play important roles in morphological development of the carotid body (8, 28). A recent study reported that intermittent hypoxia is a potent stimulus for BDNF release (29). The CIH-evoked hyperplasia of glomus cells in neonatal carotid bodies is likely due to enhanced growth factor expression, release, and/or upregulation of cognate receptors. In addition to hyperplasia, several other factors, including changes in cytosolic calcium (31), K+ channels (13), and transcriptional activators such as hypoxiainducible factors 1 and 2 (25), might contribute to the pronounced hypoxic sensitization of the neonatal carotid bodies. Further studies, however, are needed to establish which of these mechanisms play a role in CIH-induced facilitation of hypoxic sensing in neonatal carotid bodies.
Neonatal carotid bodies required as little as 72 episodes of CIH for sensitization of the hypoxic response compared with adult rats, which required as many as 720 CIH episodes. These observations demonstrate that neonatal carotid bodies are more sensitive to CIH than those of adults. Although changes in the environmental O2 level were similar, one might speculate as to whether the magnitude of IH was the same in both groups of rats. Neonates are generally stacked up in a pile for thermoregulation. However, this positioning might enhance the magnitude of the arterial PO2 fall, and the ensuing less efficient gas exchange could cause larger O2 excursions in the neonate, which may potentially account for the increased susceptibility of neonatal carotid bodies to CIH.
Another important difference between neonates and adults relates to the reversibility of CIH-evoked sensitization of the hypoxic sensory response. Following reexposure to normoxia, CIH-induced sensitization disappeared in adult rats, whereas hypoxic sensitization persisted up to 2 mo of age (the maximum duration studied) in rats exposed to 10 days of CIH as neonates. Further studies are needed to elucidate the mechanisms associated with the persistent effects of neonatal CIH.
Although CIH led to pronounced sensitization of the hypoxic sensory response in neonates, it was completely ineffective in evoking sensory LTF. In contrast, as reported previously (17), sensory LTF could readily be evoked in CIH-exposed adult carotid bodies. These observations suggest that the mechanisms responsible for induction of hypoxic sensitization by CIH differ from induction of sensory LTF. Recently it was reported that spaced application of 5-hydroxytryptamine (5-HT) evokes sensory LTF in adult rat carotid bodies, and ketanserin, a blocker of 5-HT2A/2C receptors, prevents this effect (20). Previous studies have shown that glomus cells express 5-HT in the adult rat carotid body (11). In addition, there is also evidence for the presence of 5-HT2 and 5-HT5a receptor subtypes in the adult rat carotid body (30, 32). Although the role of 5-HT in CIH-induced sensory LTF remains to be investigated, one conceivable explanation for the absence of sensory LTF in neonatal carotid bodies could be the absence of 5-HT or its receptor expression in the neonatal carotid body. What might be the significance for the absence of sensory LTF in neonatal carotid bodies exposed to CIH? Humans experiencing CIH as a consequence of sleep-disordered breathing exhibit markedly elevated sympathetic nerve activity with hypertension during daytime (12). It has been proposed that carotid body sensory LTF might play a role in daytime elevation in sympathetic nerve activity and hypertension in recurrent sleep apnea patients (16). In infants, development of the sympathetic nervous system, however, is slower and occurs in the first year of neonatal life (10), and in rat pups it occurs during the first few weeks of neonatal life (1). It is possible that in premature infants experiencing CIH as a consequence of apneas, the absence of CIH-induced sensory LTF may safeguard against sustained elevation of sympathetic nerve activity, which may otherwise produce deleterious effects.
In summary, the present study demonstrates that although CIH sensitizes the carotid body response to hypoxia in both neonatal and adult rats, there are notable differences between the groups with regard to susceptibility to CIH, magnitude of sensitization, reversibility of the response, and morphological changes of the glomus tissue. In addition, unlike adults, CIH was ineffective in evoking sensory LTF of the carotid body in neonatal rat pups, which may be of physiological significance in that the absence of sensory LTF could prevent sustained sympathetic excitation, which may not be beneficial in neonates.
Acknowledgments
GRANTS
The research was supported by National Heart, Lung, and Blood Institute Grants HL-25830 and HL-076537.
REFERENCES
- 1.Bartolome J, Bartolome M, Seidler J, Anderson TR, Slotkin TA. Effects of early postnatal guanethidine administration on adrenal medulla and brain of developing rats. Biochem Pharmacol. 1976;25:2387–2390. doi: 10.1016/0006-2952(76)90033-2. [DOI] [PubMed] [Google Scholar]
- 2.Bisgard GE. Increase in carotid body sensitivity during sustained hypoxia. Biol Signals. 1995;4:292–297. doi: 10.1159/000109455. [DOI] [PubMed] [Google Scholar]
- 3.Bisgard GE, Olson EB, Jr, Wang ZY, Bavis RW, Fuller DD, Mitchell GS. Adult carotid body chemoafferent responses to hypoxia after 1, 2, and 4 wk of postnatal hyperoxia. J Appl Physiol. 2003;95:946–952. doi: 10.1152/japplphysiol.00985.2002. [DOI] [PubMed] [Google Scholar]
- 4.Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol. 1984;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- 6.Cistulli PA, Sullivan CE. Pathophysiology of sleep apnea. In: Saunders NA, Sullivan CE, editors. Sleep and Breathing. Dekker; New York: 1994. pp. 405–448. [Google Scholar]
- 7.Donnelly DF. Developmental aspects of oxygen sensing by the carotid body. J Appl Physiol. 2000;88:2296–2301. doi: 10.1152/jappl.2000.88.6.2296. [DOI] [PubMed] [Google Scholar]
- 8.Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci. 2001;21:581–589. doi: 10.1523/JNEUROSCI.21-02-00581.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidone SJ, Gonzalez C. Handbook of Physiology. The Respiratory System. Control of Breathing. II. Am. Physiol. Soc.; Bethesda, MD: 1986. Initiation and control of chemoreceptor activity in the carotid body; pp. 247–312. sect. 3. pt. 1, chapt. 9. [Google Scholar]
- 10.Gagnon R, Campbell K, Hunse C, Patrick J. Patterns of human fetal heart rate accelerations from 26 weeks to term. Am J Obstet Gynecol. 1987;157:743–748. doi: 10.1016/s0002-9378(87)80042-x. [DOI] [PubMed] [Google Scholar]
- 11.Gronblad M, Liesi P, Rechardt L. Serotonin-like immunoreactivity in rat carotid body. Brain Res. 1983;276:348–350. doi: 10.1016/0006-8993(83)90745-x. [DOI] [PubMed] [Google Scholar]
- 12.Kara T, Narkiewicz K, Somers VK. Chemoreflexes–physiology and clinical implications. Acta Physiol Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim I, Boyle KM, Carroll JL. Postnatal development of E-4031-sensitive potassium current in rat carotid chemoreceptor cells. J Appl Physiol. 2005;98:1469–1477. doi: 10.1152/japplphysiol.01254.2003. [DOI] [PubMed] [Google Scholar]
- 14.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol. 2006;91:249–286. doi: 10.1016/j.pbiomolbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 17.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- 19.Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol. 2004;97:2020–2025. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- 20.Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006;576:289–295. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1 deficiency impairs carotid body-mediated cardio-respiratory responses and ROS generation in mice exposed to chronic intermittent hypoxia. J Physiol. 2006;577:705–16. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poets CF, Samuels MP, Southall DP. Epidemiology and pathophysiology of apnoea of prematurity. Biol Neonate. 1994;65:211–219. doi: 10.1159/000244055. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- 24.Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roux JC, Brismar H, Aperia A, Lagercrantz H. Developmental changes in HIF transcription factor in carotid body: relevance for O2 sensing by chemoreceptors. Pediatr Res. 2005;58:53–57. doi: 10.1203/01.PDR.0000163390.78239.EA. [DOI] [PubMed] [Google Scholar]
- 26.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 27.Sterni LM, Bamford OS, Wasicko MJ, Carroll JL. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am J Physiol Lung Cell Mol Physiol. 1999;277:L645–L652. doi: 10.1152/ajplung.1999.277.3.L645. [DOI] [PubMed] [Google Scholar]
- 28.Villadiego J, Mendez-Ferrer S, Valdes-Sanchez T, Silos-Santiago I, Farinas I, Lopez-Barneo J, Toledo-Aral JJ. Selective glial cell line-derived neurotrophic factor production in adult dopaminergic carotid body cells in situ and after intrastriatal transplantation. J Neurosci. 2005;25:4091–4098. doi: 10.1523/JNEUROSCI.4312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Yuan G, Prabhakar NR, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from PC12 cells in response to oxidative stress requires autocrine dopamine signaling. J Neurochem. 2006;96:694–705. doi: 10.1111/j.1471-4159.2005.03572.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang ZY, Keith IM, Beckman MJ, Brownfield MS, Vidruk EH, Bisgard GE. 5-HT5a receptors in the carotid body chemoreception pathway of rat. Neurosci Lett. 2000;278:9–12. doi: 10.1016/s0304-3940(99)00905-2. [DOI] [PubMed] [Google Scholar]
- 31.Wasicko MJ, Sterni LM, Bamford OS, Montrose MH, Carroll JL. Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells. J Physiol. 1999;514:493–503. doi: 10.1111/j.1469-7793.1999.493ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Nurse CA. Does endogenous 5-HT mediate spontaneous rhythmic activity in chemoreceptor clusters of rat carotid body? Brain Res. 2000;872:199–203. doi: 10.1016/s0006-8993(00)02499-9. [DOI] [PubMed] [Google Scholar]