Abstract
Physiological responses to hypoxia either continuous (CH) or intermittent (IH) depend on the O2-sensing ability of the peripheral arterial chemoreceptors, especially the carotid bodies, and the ensuing reflexes play important roles in maintaining homeostasis. The purpose of this article is to summarize the effects of CH and IH on carotid body function and the underlying mechanisms. CH increases baseline carotid body activity and sensitizes the response to acute hypoxia. These effects are associated with hyperplasia of glomus cells and neovascularization. Enhanced hypoxic sensitivity is due to alterations in ion current densities as well as changes in neurotransmitter dynamics and recruitment of additional neuromodulators (endothelin-1, ET-1) in glomus cells. Morphological alterations are in part due to up-regulation of growth factors (e.g. VEGF). Hypoxia-inducible factor-1 (HIF-1), a transcriptional activator might underlie the remodeling of carotid body structure and function by CH. Chronic IH, on the other hand, is associated with recurrent apneas in adults and premature infants. Two major effects of chronic IH on the adult carotid body are sensitization of the hypoxic sensory response and long-lasting increase in baseline activity i.e., sensory long-term facilitation (LTF) which involve reactive oxygen species (ROS) and HIF-1. In neonates, chronic IH leads to sensitization of the hypoxic response but does not induce sensory LTF. Chronic IH-induced sensitization of the carotid body response to hypoxia increases the likelihood of unstable breathing perpetuating in more number of apneas, whereas sensory LTF may contribute to increased sympathetic tone and systemic hypertension associated with recurrent apneas.
Keywords: Heme proteins, Mitochondria, Ion channels, Transmitters, Reactive oxygen species, Sensory long-term facilitation, Plasticity, HIF-1 transcription factor
1 Introduction
Continuous hypoxia (CH) is experienced during sojourns at high altitude, whereas people residing at sea level encounter intermittent hypoxia (IH) more frequently than continuous hypoxia. Physiological responses to both forms of hypoxia depend on the O2-sensing ability of the peripheral arterial chemoreceptors, especially the carotid bodies, and the ensuing reflexes play important roles in maintaining homeostasis. The purpose of this article is to summarize the effects of CH and IH on carotid body function and the underlying mechanisms.
2 Effects of Continuous Hypoxia on Carotid Body
Carotid bodies are composed of two cell types: type I and type II. Type I cells (also called glomus cells) express a variety of neurotransmitters, and form synaptic contact with afferent nerve endings whose cell bodies lie in the petrosal ganglion. Type II cells resemble glial cells. Available evidence support the idea that type I cells are the primary site where changes in O2 are being sensed. Figure 1 illustrates the effects of acute hypoxia on carotid body sensory activity and ventilation. Lowering inspired PO2 is followed by an immediate increase of carotid chemoreceptor (CC) and petrosal ganglion nerve (PGN) activities. After a short delay, ventilation starts to increase. Terminating hypoxia results in prompt cessation of CC and PGN activities along with decreases in ventilation.
Fig. 1. Effect of acute hypoxia on ventilation, carotid body (CC) and petrosal ganglion nerve (PGN) activities in an anesthetized cat.
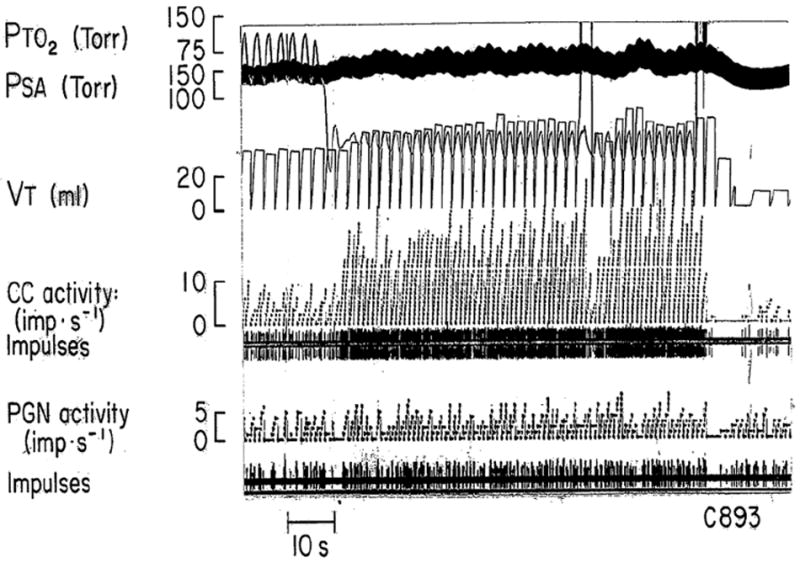
PTO2 = Partial pressure of tracheal oxygen; PSA = Arterial blood pressure; VT- tidal volume
The transduction of the hypoxic stimulus at the carotid body is complex and involves interactions between heme and/or redox-sensitive proteins and O2 sensitive K+ channels that regulate membrane potential (Gonzalez et al., 1994, Lahiri and Cherniack, 2001, Lopez-Barneo et al., 2001, Prabhakar, 2006). The transduction process leads to Ca2+-dependent release of neurotransmitter(s) from the glomus cells, which by acting on the nearby afferent nerve endings produce an increase in sensory nerve activity (sensory transmission).
If hypoxia continues for several days, the carotid chemoreceptor activity continue to increase at the same inspired oxygen levels, contributing to ventilatory acclimatization (Bisgard et al., 1987, Barnard et al., 1987). The following section summarizes the effects of CH on the sensory transduction and transmission at the carotid body.
2.1 Chronic Hypoxia and Ion Channel Expression in the Carotid Body
Ion channels, especially those conducting K+ and Na+ ions contribute to the excitability of glomus cells. Three to four weeks of hypoxia decreases the density of K+ currents in rat glomus cells (Wyatt et al., 1995); whereas 2 weeks of hypoxia increase Na+ current density via cAMP-dependent mechanisms (Stea et al., 1992). Acute hypoxia increases Ca2+ influx in the glomus cells and this effect is mediated entirely by opening of the voltage-dependent Ca2+ channels. Four types of voltage dependent Ca2+ channels have been identified in glomus cells, including L-, P/Q-, N-, and R- (resistant) type Ca2+ currents (Silva and Lewis, 1995; Overholt and Prabhakar, 1997). Hempleman (1996) reported that 5–8 days of hypobaric hypoxia (~0.4 ATM) increases Ca2+ channel density in glomus cells from neonatal carotid bodies and markedly attenuated their inactivation properties. Hempleman proposed that increased hypoxic sensitivity is mediated in part by augmented Ca2+ influx through voltage-gated Ca2+ channels leading to increased stimulus-secretion coupling in glomus cells.
2.2 Effect of Chronic Hypoxia on Heme and/or Redox-Sensitive Proteins
Carotid bodies express a variety of heme-containing proteins including NO synthases (NOS), heme-oxygenase-2 (HO-2), mitochondrial cytochromes, and NADPH-oxidases. Di Giulio et al. (1998) reported increases in nNOS protein in the carotid body after 12 days of hypoxia. Ye et al. (2002) found no changes in nNOS expression in the carotid body after 4 weeks of hypoxia, but there was a robust increase in iNOS expression, which was normally absent in the glomus tissue. These investigators further showed that chronic hypoxia increases NO production in the carotid body. NO being inhibitory to the carotid body activity, it was proposed that elevated NO might contribute to blunting of the hypoxic sensitivity of the carotid body during prolonged hypoxia. Little information is available on the effects of chronic hypoxia on HO-2, mitochondrial cytochromes, and NADPH-oxidases in the carotid body.
2.3 Chronic Hypoxia and Neurotransmitters in the Carotid Body
Carotid body expresses a variety of neurotransmitters/modulators. Some of them excite and others inhibit afferent nerve activity. Because chronic hypoxia sensitizes the carotid body to subsequent hypoxic stimuli, it was thought that prolonged exposure to low oxygen down regulate the “inhibitory” transmitters and up-regulate the “excitatory” ones. This idea has been extensively tested and the following section will briefly summarize these studies.
Carotid bodies express dopamine (DA), which may function as an inhibitory modulator (Gonzalez et al., 1994; Bisgard, 2000; Prabhakar, 2000). Tyrosine hydroxylase (TH), the enzyme responsible for DA synthesis, is expressed in glomus cells as well as in nerve fibers and ganglion cells of the carotid body (Gonzalez et al., 1994). Chronic hypoxia up-regulates TH mRNA, protein, as well as the enzyme activity (Czyzyk-Krzeska et al., 1992), and also increases DA turnover in the glomus tissue (Hanbauer et al., 1981). Dopaminergic D2 receptors which mediate the inhibitory actions of DA are initially down-regulated, but later up-regulated by chronic hypoxia in the carotid body (Huey and Powell, 2000). These observations indicate that although DA is inhibitory, chronic hypoxia up-regulates the dopaminergic system in the carotid body.
Acetylcholine (ACh) stimulates the carotid body activity and its stimulatory actions are mediated by nicotinic cholinergic receptors localized to glomus cells as well as the afferent fiber terminals (Shirahata et al., 1998). Chronic hypoxia (9–14 days) up-regulates nicotinic receptor expression (α3 and α7 subunits) on petrosal neurons that provide afferent innervation to the carotid body (Dinger et al., 2003). However, mecamylamine, a blocker of nicotinic receptors had little effect on chronic hypoxia-induced hypersensitivity of the carotid body. Endothelin-1 (ET-1) and its receptors are either undetectable or expressed in low abundance in glomus cells from normal carotid bodies. but are up-regulated by chronic hypoxia in glomus cells (He et al., 1996; Chen et al., 2002a, b). ET-1 by itself has no appreciable excitatory effect on carotid body afferent nerve activity but markedly augments the hypoxic sensory response (Chen et al., 2000). Blockade of ETA receptors prevents chronic hypoxia-induced hypersensitivity of the carotid body to acute low PO2. Taken together these observations suggest that the enhanced hypoxic sensitivity elicited by chronic hypoxia is mediated by recruiting excitatory neuromodulator(s), which are not normally expressed in the carotid body, rather than tipping the balance between the inhibitory and excitatory transmitters.
3 Molecular Mechanisms Underlying the Effects of Continuous Hypoxia on the Carotid Body
Transcriptional regulation of genes is one of the pivotal mechanisms that underlie long-term adaptations to chronic hypoxia (Bunn and Poyton, 1996). The following section summarizes studies on the transcriptional activator hypoxia-inducible factor-1 in mediating carotid body response to continuous hypoxia.
3.1 Role of HIF-1 on Carotid Body Changes by Continuous Hypoxia
HIF-1 is a heterodimeric protein composed of a constitutively expressed HIF-1β subunit and an O2-regulated HIF-1α subunit. HIF-1 is a global transcriptional regulator of oxygen homeostasis that controls multiple physiological processes (Semenza, 2000). Complete loss of function in Hif1a gene in mice results in embryonic lethality at mid-gestation with major malformations of the heart and vasculature (Iyer et al., 1998). On the other hand, HIF-1α heterozygous mice (i.e., partially deficient in HIF-1α) develop normally and are indistinguishable from wild type controls. However, upon exposure to chronic hypoxia, adult Hif1a+/− mice exhibit impaired physiological adaptations as evidenced by reduced erythropoietic response and pulmonary vascular remodeling (Yu et al., 1999). Kline et al. (2002) reported severely impaired hypoxic sensing of the carotid bodies, and absence of ventilatory acclimatization in Hif1a+/− mice. How might HIF-1 contribute to carotid body adaptations to chronic hypoxia? It is known that HIF-1 regulates several genes including ET-1 (Semenza, 2000). Therefore, HIF-1 might participate in functional changes in the carotid body by regulating ET-1.
Chronic hypoxia increases the number and size of glomus cells and increased blood vessels in the carotid body (McGregor et al., 1984; Wilson et al., 2005). HIF-1 might also contribute to structural alterations of the chemoreceptor organ during chronic hypoxia by up-regulating growth factors. HIF-1 is a potent inducer of vascular endothelial growth factor (VEGF; Semenza, 2000). Carotid body expresses VEGF in type I cells, and PD-ECGF expression is confined to the extra-cellular stroma (Jyung et al., 2000; Chen et al., 2003). Carotid bodies also express VEGF receptors, Fit-1 and Flk-1 (Jyung et al., 2000; Chen et al., 2003). VEGF acting on Flk-1 regulates hyperplasia of the type I cells, and promotes neo-vascularization by acting on Fit-1. PD-ECGF contributes more to angiogenesis as it does elsewhere in the body including the lung (Jyung et al., 2000). Thus, HIF-1 might participate in both functional and structural re-organization of the carotid bodies during chronic hypoxia.
4 Intermittent Hypoxia and Carotid Body Function
Recurrent apneas are characterized by periodic cessations of breathing resulting in cyclical decreases in arterial blood O2 or intermittent hypoxia (IH). Studies in humans and rodents suggest that the carotid bodies constitute the “frontline” defense system for detecting IH. Recurrent apnea patients exhibit more pronounced ventilatory depression with brief hyperoxic challenge (Dejour’s test, a measure of peripheral chemoreceptor sensitivity) than control subjects (Tafil-Klawe et al., 1991; Kara et al., 2003). Furthermore, glomectomized subjects with sleep apneas do not develop hypertension (see discussion in Somers and Abboud, 1993). Likewise, rats exposed to 30d of IH develop hypertension and increased sympathetic nerve activity and chronic bilateral sectioning of sinus nerves prevented these responses (Lesske et al., 1997). The following section summarizes recent studies reporting the effects of IH on carotid body function in experimental animal models.
4.1 Effects of IH on Hypoxic Sensory Response of the Adult Carotid Body
Hypoxic but not hypercapnic sensory response of the carotid body was augmented in adult rats exposed to 10d of IH (Peng and Prabhakar, 2004). Similar augmentation of the hypoxic sensory response was also reported in cats (Rey et al., 2004, 2006) and mice (Peng et al., 2006), suggesting that chronic IH uniformly augments the hypoxic sensory response in three species studied thus far. The augmented hypoxic response was reversed by re-exposing IH rats to 10 d of normoxia (Peng et al., 2003). IH had no significant effect on carotid body morphology (Peng et al., 2003). These observations suggest that IH leads to selective sensitization of the hypoxic sensory response.
4.2 IH –Induces Sensory Long-Term Facilitation of the Carotid Body in Adults
When anesthetized rats were exposed to acute intermittent hypoxia (AIH; 15 s of hypoxia followed by 5 min of re-oxygenation, 10 episodes), sensory activity increased with each episode and promptly returned to baseline after terminating AIH. In striking contrast, in chronic IH exposed animals, AIH resulted in long-lasting increase in baseline activity that persisted for 60 min after terminating AIH. This long-lasting increase in baseline sensory activity has been termed sensory LTF (Peng et al., 2003). Thus, in addition to sensitizing the response to hypoxia, IH also induces hitherto uncharacterized form of plasticity manifested as sensory long-term facilitation (LTF; Peng et al., 2003, 2006).
Unlike AIH, acute intermittent hypercapnia (10 episodes of 15 sec of 7% CO2 + 93% O2 interspersed with 5 min of 100% O2), another “physiological” stimulus to the carotid body was ineffective in evoking sensory LTF in IH exposed rats (Peng et al., 2003). The induction of chronic IH-induced sensory LTF is a time-dependent phenomenon, in that it was apparent after 3d of IH, and the magnitude further increased following 10d of IH. More interestingly, the magnitude of sensory LTF was not dependent on the severity of hypoxia used for IH conditioning, because it was indistinguishable with either FiO2 of 5 or 10% O2 used for chronic IH conditioning (Peng et al., 2003). Like the sensitization of the hypoxic response, sensory LTF was reversible after re-exposing chronic IH rats to 10d of normoxia.
4.3 Comparison of IH with Comparable Duration of Continuous Hypoxia in Adult Rodents
Studies described thus far suggest that IH exerts two major effects on the carotid body that include (a) selective sensitization of the hypoxic sensory response and (b) induction of sensory LTF. The total duration of hypoxia accumulated over 10d of chronic IH corresponds to 4h of low O2. A single episode of 4h of hypoxia or 4h of hypoxia/day for 10 days, however, were ineffective in inducing sensitization of the hypoxic sensory response and sensory LTF of the carotid body (Peng et al., 2003; Peng and Prabhakar, 2004). These findings demonstrate that for a given duration of hypoxic exposure, IH is more effective than continuous hypoxia in altering the carotid body function.
4.4 Effects of IH on Neonatal Carotid Body Activity
Carotid bodies are immature at birth and respond poorly to hypoxia compared with adults (Carroll, 2003). Clinical studies have shown that nearly 70–90% of prematurely born infants experience IH as a consequence of recurrent apneas (Poets et al., 1994). Recent studies examined carotid body response to hypoxia in neonatal rat pups (P0) exposed to 8h/day IH for 1-10d (Peng et al., 2004; Pawar et al., 2008a, b). Carotid body sensory response to hypoxia was markedly augmented in IH exposed pups and the magnitude was even greater compared to adults. A systematic comparison with adult carotid bodies showed that: (a) neonatal carotid bodies are more susceptible to IH than adults, (b) unlike adult carotid bodies, the effects of neonatal IH were not reversible following re-exposures to normoxia for ~2 months, and (c) the effects of neonatal IH were associated with increased number of glomus cells (Pawar et al., 2008a).
In striking contrast to adult rats, IH was ineffective in inducing sensory LTF in neonatal carotid bodies (Pawar et al., 2008a). Previous studies reported blunting of hypoxic sensory response in neonatal rats exposed to continuous hypoxia (Carroll, 2003). Thus, there are striking differences between the effects of intermittent and continuous hypoxia, in that the former facilitates, whereas the later reduces hypoxic sensing ability of neonatal carotid bodies.
4.5 Cellular Mechanism(S) Associated with IH
4.5.1 Role of Reactive Oxygen Species (ROS)
IH increases ROS generation in the carotid body as evidenced by decreased activity of the red-ox sensitive enzyme aconitase (Peng et al., 2003). Rats treated with a stable superoxide dismutase mimetic (MnTMPyP), a potent scavenger of ROS, attenuated or abolished sensitization of the hypoxic response as well as the sensory LTF in adult rats (Peng et al., 2003; Peng and Prabhakar, 2004) and the augmented hypoxic response in neonates (Pawar et al., 2008b).
Cellular sources of ROS generation involve inhibition of complex I and III of the mitochondrial electron transport chain (ETC) as well as activation of several oxidases. IH down-regulated mitochondrial complex I but not the complex III activity, whereas IH up-regulated NADPH oxidase activity in adult rat carotid bodies (Peng et al., 2003), suggesting that both mitochondrial electron transport chain at complex I and NADPH oxidase contribute to elevated levels of ROS. These observations demonstrate that chronic IH such as that seen during recurrent apneas facilitate hypoxic sensing in the carotid body by increasing the levels of ROS. In other words, ROS are acting as “amplifiers” of brief hypoxic signals associated with IH.
4.5.2 Role of Neurotransmitters/Modulators in IH-Evoked Functional Changes in the Carotid Body
A study by Rey et al. (2006) on adult cat carotid body suggests that up-regulation of endothelin-1 (ET-1) in glomus cells and ET-1 receptors contribute to IH-induced sensitization of the hypoxic sensory response. Pawar et al. (2008b) showed that ET-1 and ETA receptors also important for IH-induced sensitization in neonatal carotid bodies. Furthermore, in neonates IH- enhances ET-1 release from the carotid body and up-regulates ETA receptor mRNA and both these responses to IH involve ROS-mediated signaling (Pawar et al., 2008b).
5-Hydroxytryptamine (5-HT) evokes long-term activation of neuronal activity elsewhere in the nervous system. Carotid bodies express 5-HT. Peng et al., (2006) reported that spaced (3 × 15 s of 100 nM at 5 min intervals) but not mass (300 nM, 45 s) application of 5-HT elicited LTF, whereas both modes of 5-HT application evoked initial sensory excitation of the carotid bodies in rats. Ketanserin, a 5-HT(2) receptor antagonist prevented sensory LTF but not the initial sensory excitation. Spaced application of 5-HT activated protein kinase C (PKC) and these effects were abolished by ketanserin as well as bisindolylmaleimide (Bis-1), an inhibitor of PKC. Bis-1 prevented 5-HT-evoked sensory LTF. Furthermore, 5-HT-evoked sensory LTF of the carotid body involves a novel signaling mechanism coupled to PKC-dependent activation of NADPH oxidase.
Further studies are needed to examine whether 5-HT signaling contributes to chronic IH-evoked sensory LTF.
4.6 Molecular Mechanisms Associated with IH
4.6.1 Hypoxia-Inducible Factor-1 (HIF-1) in IH-Induced Changes in the Carotid Body Function
IH up-regulates HIF-1α protein in glomus cells of the rat carotid body (Lam et al., 2008; and J. Nanduri, 2007 Unpublished observations). Peng et al (2006) examined the effects of chronic IH on carotid body activity in WT and HET mice partially deficient in HIF-1α. In IH exposed WT mice hypoxic sensory response of the carotid body was augmented, and acute intermittent hypoxia (AIH) evoked sensory LTF. In striking contrast, IH-evoked sensitization of the hypoxic sensory response and sensory LTF were absent in mice with heterozygous deficiency of HIF-1α. These observations suggest that HIF-1-mediated transcriptional activation is an important molecular mechanism underpinning the carotid body changes by IH. However, HIF-1 regulated down stream genes contributing to CIH-evoked changes in the carotid body remains to be determined.
Acknowledgments
The research is supported by grants from National Institutes of Health (Heart, Lung and Blood Institute) HL-90554 and HL-76537.
References
- Barnard P, Andronikou S, Pokorski M, Smatresk N, Mokashi A, Lahiri S. Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol. 1987;63:685–691. doi: 10.1152/jappl.1987.63.2.685. [DOI] [PubMed] [Google Scholar]
- Bisgard GE. Carotid body mechanisms in acclimatization to hypoxia. Respir Physiol. 2000;121:237–246. doi: 10.1016/s0034-5687(00)00131-6. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Kressin NA, Nielsen AM, Daristotle L, Smith CA, Forster HV. Dopamine blockade alters ventilatory acclimatization to hypoxia in goats. Respir Physiol. 1987;69:245–55. doi: 10.1016/0034-5687(87)90031-4. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen Sensing and Molecular Adaptation to Hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Chen J, Dinger B, Jyung R, Stensaas L, Fidone S. Altered expression of vascular endothelial growth factor and FLK-1 receptor in chronically hypoxic rat carotid body. Adv Exp Med Biol. 2003;536:583–591. doi: 10.1007/978-1-4419-9280-2_74. [DOI] [PubMed] [Google Scholar]
- Chen J, He L, Dinger B, Fidone S. Cellular mechanisms involved in rabbit carotid body excitation elicited by endothelin peptides. Respir Physiol. 2000;121:13–23. doi: 10.1016/s0034-5687(00)00113-4. [DOI] [PubMed] [Google Scholar]
- Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002a;282:L1314–L1323. doi: 10.1152/ajplung.00454.2001. [DOI] [PubMed] [Google Scholar]
- Chen Y, Tipoe GL, Liong E, Leung S, Lam SY, Iwase R, Tjong YW, Fung ML. Chronic hypoxia enhances endothelin-1-induced intracellular calcium elevation in rat carotid body chemoreceptors and up-regulates ETA receptor expression. Pflugers Arch. 2002b;443:565–573. doi: 10.1007/s00424-001-0728-2. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Bayliss DA, Lawson EE, Millhorn DE. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem. 1992;58:1538–1546. doi: 10.1111/j.1471-4159.1992.tb11376.x. [DOI] [PubMed] [Google Scholar]
- Di Giulio C, Grilli A, De Lutiis MA, Di Natale F, Sabatino G, Felaco M. Does chronic hypoxia increase rat carotid body nitric oxide? Comp Biochem Physiol A Mol Integr Physiol. 1998;120:243–247. doi: 10.1016/s1095-6433(98)00023-3. [DOI] [PubMed] [Google Scholar]
- Dinger B, He L, Chen J, Stensaas L, Fidone S. Mechanisms of morphological and functional plasticity in the chronically hypoxic carotid body. In: Lahiri S, Semenza G, Prabhakar NR, editors. Oxygen Sensing: Responses and Adaptation to Hypoxia. Marcel Dekker; New York: 2003. pp. 439–465. [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: From natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Hanbauer I, Karoum F, Hellstrom S, Lahiri S. Effects of hypoxia lasting up to one month on the catecholamine content in rat carotid body. Neuroscience. 1981;6:81–86. doi: 10.1016/0306-4522(81)90245-1. [DOI] [PubMed] [Google Scholar]
- He L, Chen J, Dinger B, Stensaas L, Fidone S. Endothelin modulates chemoreceptor cell function in mammalian carotid body. Adv Exp Med Biol. 1996;410:305–311. doi: 10.1007/978-1-4615-5891-0_46. [DOI] [PubMed] [Google Scholar]
- Hempleman SC. Increased calcium current in carotid body glomus cells following in vivo acclimatization to chronic hypoxia. J Neurophysiol. 1996;76:1880–1886. doi: 10.1152/jn.1996.76.3.1880. [DOI] [PubMed] [Google Scholar]
- Huey KA, Powell FL. Time-dependent changes in dopamine D(2)-receptor MRNA in the arterial chemoreflex pathway with chronic hypoxia. Brain Res Mol Brain Res. 2000;75:264–270. doi: 10.1016/s0169-328x(99)00321-6. [DOI] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 Alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyung RW, LeClair EE, Bernat RA, Kang TS, Ung F, McKenna MJ, Tuan RS. Expression of angiogenic growth factors in paragangliomas. Laryngoscope. 2000;110:161–167. doi: 10.1097/00005537-200001000-00029. [DOI] [PubMed] [Google Scholar]
- Kara T, Narkiewicz K, Somers VK. Chemoreflexes – physiology and clinical implications. Acta Physiol Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci U S A. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S-Y, Tipoe GL, Liong EC, Fung ML. Differential expressions and roles of hypoxia-inducible factor-1α,-2α and 3α in the rat carotid body during chronic and intermittent hypoxia. Histol Histopathol. 2008;23:271–280. doi: 10.14670/HH-23.271. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Cherniack NS. Cellular and molecular mechanisms of O2 sensing with special reference to the carotid body. In: Hornbein T, Schoene RB, editors. High Altitude: An Exploration of Human Adaptation. Marcel Dekker Inc.; New York: 2001. pp. 101–130. [Google Scholar]
- Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia – influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Sánez P. Cellular mechanisms of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- McGregor KH, Gil J, Lahiri S. A morphometric study of the carotid body in chronically hypoxia rats. J Appl Physiol. 1984;57:1430–1438. doi: 10.1152/jappl.1984.57.5.1430. [DOI] [PubMed] [Google Scholar]
- Overholt JL, Prabhakar NR. Ca2+ current in rabbit carotid body glomus cells is conducted by multiple types of high-voltage-activated Ca2+ channels. J Neurophysiol. 1997;78:2467–2474. doi: 10.1152/jn.1997.78.5.2467. [DOI] [PubMed] [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol. 2008a;104:1287–94. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar A, Nanduri J, Khan SA, Wang N, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid bodies by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2008b doi: 10.1152/ajpregu.90490.2008. Am J Physiol (Regulatory, Comparative and Integrated). 2008 Dec 24. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. discussion 1196. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol. 2004;97:2020–2025. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006;576:289–295. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous Hif-1 deficiency impairs carotid body-mediated cardio-respiratory responses and Ros generation in mice exposed to chronic intermittent hypoxia. J Physiol. 2006;577:705–16. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poets CF, Samuels MP, Southall DP. Epidemiology and pathophysiology of apnoea of prematurity. Biol Neonate. 1994;65:211–219. doi: 10.1159/000244055. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–95. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol. 2006;91:17–23. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Del Rio R, Iturriaga R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res. 2006;1086:152–159. doi: 10.1016/j.brainres.2006.02.082. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Shirahata M, Ishizawa Y, Rudisill M, Schofield B, Fitzgerald RS. Presence of nicotinic acetylcholine receptors in cat carotid body afferent system. Brain Res. 1998;814:213–217. doi: 10.1016/s0006-8993(98)01015-4. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Lewis DL. L- and N-Type Ca2+ Channels in adult rat carotid body chemoreceptor type I cells. J Physiol. 1995;489(Pt 3):689–699. doi: 10.1113/jphysiol.1995.sp021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Abboud FM. Chemoreflexes–responses, interactions and implications for sleep apnea. Sleep. 1993;16(8 Suppl):S30–3. [PubMed] [Google Scholar]
- Stea A, Jackson A, Nurse CA. Hypoxia and N6, O2’-Dibutyryladenosine 3’, 5’-Cyclic Monophosphate, but not nerve growth factor, induce Na+ channels and hypertrophy in chromaffin-like arterial chemoreceptors. Proc Natl Acad Sci U S A. 1992;89:9469–9473. doi: 10.1073/pnas.89.20.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafil-Klawe M, Thiele AE, Raschke F, Mayer J, Peter JH, von Wichert W. Peripheral chemoreceptor reflex in obstructive sleep apnea patients; a relationship between ventilatory response to hypoxia and nocturnal bradycardia during apnea events. Pneumologie. 1991;45(Suppl 1):309–311. [PubMed] [Google Scholar]
- Wilson DF, Roy A, Lahiri S. Immediate and long-term responses of the carotid body to high altitude. High Alt Med Biol. 2005;6:97–111. doi: 10.1089/ham.2005.6.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Wright C, Bee D, Peers C. O2-Sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc Natl Acad Sci U S A. 1995;92:295–299. doi: 10.1073/pnas.92.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JS, Tipoe GL, Fung PC, Fung ML. Augmentation of hypoxia-induced nitric oxide generation in the rat carotid body adapted to chronic hypoxia: An involvement of constitutive and inducible nitric oxide synthases. Pflugers Arch. 2002;444:178–185. doi: 10.1007/s00424-002-0785-1. [DOI] [PubMed] [Google Scholar]
- Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]


