Abstract
The increase of cell surface sialic acid is a characteristic shared by many tumor types. A correlation between hypersialylation and immunoprotection has been observed, but few hypotheses have provided a mechanistic understanding of this immunosuppressive phenomenon. Here, we show that increasing sialylated glycans on cancer cells inhibits human NK cell activation through the recruitment of Siglec-7. Key to these findings was the use of glycopolymers end-functionalized with phospholipids, which enable the introduction of synthetically defined glycans onto cancer cell surfaces. Remodeling the sialylation status of cancer cells affected the susceptibility to NK cell cytotoxicity via Siglec-7 engagement in a variety of tumor types. These results support a model in which hypersialylation offers a selective advantage to tumor cells under pressure from NK immunosurveillance by increasing Siglec ligands. We also exploited this finding to protect allogeneic and xenogeneic primary cells from NK-mediated killing suggesting the potential of Siglecs as therapeutic targets in cell transplant therapy.
Introduction
Natural killer (NK) cells play a central role in the innate immune response against cancer cells and are vital to the containment of tumor growth and metastasis.1,2 NK cells use both activating and inhibitory receptors to distinguish healthy “self” cells from diseased cells.3 Tumor cells or virally infected cells are then killed through the release of lytic granules and engagement of cell apoptotic receptors (Fig. 1a). Yet, cancer is a microevolutionary process that can select for tumor cells capable of avoiding recognition and destruction by innate immune cells.4–6 In this regard, many aggressive cancers evade detection from NK cells by shedding NK activating ligands or overexpressing ligands for NK cell inhibitory receptors.7,8
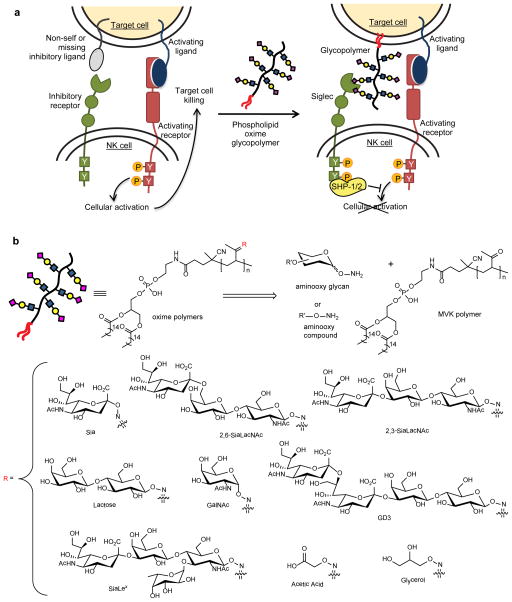
Figure 1. A glycocalyx engineering approach to studying sialoside dependent NK inhibition.
(a) In the presence of activating ligands and absence of inhibitory ligands on the target cell, NK cells are activated to release cytotoxic effectors and cytokines. Coating cancer cells with sialylated glycopolymers by membrane insertion can emulate cancer associated glycosylation changes that engage the Siglec family of inhibitory receptors. Localization of Siglecs to the site of activation enhances SHP-1/2 phosphatase recruitment to halt the phosphorylation cascade before cellular activation. (b) The methyl vinyl ketone (MVK) polymer consists of a polyketone backbone that is end-functionalized with a DPPE phospholipid. Oxime-linked polymers were generated from the chemoselective reaction of aminooxy compounds with the MVK scaffold (See Supplementary Information for abbreviations).
The upregulation of sialic acid on the surface of malignant cells is known to correlate with poor prognosis and decreased immunogenicity in a variety of cancers.9,10 However, beyond early studies invoking physical and electrostatic repulsion, few reports have provided the molecular details by which hypersialylation may promote tumor immunoevasion.11,12 Recent evidence suggests that NK cells are involved in selecting for cancer cell hypersialylation. Chemically induced tumors in IFN-γ−/− or IL-1α−/− mice, which have defective immunosurveillance, do not develop a hypersialylated phenotype.13 In vitro studies have also revealed a positive correlation between target cell sialylation state and NK cell resistance, which suggests there is a specific receptor in this evasive mechanism, though a candidate has yet to be fully elucidated.14–16
The Sialic acid-binding Immunoglobulin-like Lectin (Siglec) family of cell surface receptors may provide the missing mechanistic link between cancer hypersialylation and immunoevasion.17 The expression of each Siglec is restricted to a distinct set of leukocytes. Though all Siglecs bind glycans containing sialic acid, they differ in their recognition of the linkage regiochemistry and spatial distribution.18 Human NK cells ubiquitously express Siglec-7 (p75/AIRM1) while a smaller subset expresses Siglec-9.17,19 Both Siglecs contain a cytosolic Immunoreceptor Tyrosine-based Inhibitory Motif (ITIM) which recruits SHP phosphatases to the site of activation and halts the kinase phosphorylation cascade (Fig. 1a).20,21 As inhibitory receptors that recognize sialic acid ligands, the Siglecs are likely candidates for driving sialic acid-dependent protection of carcinomas from NK cells. Several reports have shown that various Siglecs can bind cancer-associated sialylated mucins,22–24 but establishing their roles in cancer immunoevasion has been undermined by difficulties in controlling, with molecular precision, the target cell’s glycosylation status. This challenge is inherent to studies of cell surface glycans, as they are heterogeneous and their structures are difficult to precisely modulate by genetic manipulation.25
Synthetic glycopolymers have been successfully used as functional mimics of cell-associated glycans for studies in glycobiology.26,27 For example, several labs have employed soluble glycopolymers and multivalent ligands to suppress antigen-induced B cell activation via binding to Siglec-2.28,29 Our laboratory has previously developed a platform to engineer a cell’s glycocalyx with synthetic glycans by generating glycopolymers end-functionalized with phospholipids that can passively insert into cell membranes.30,31 This technique enables the introduction of chemically defined glycan structures onto live human cell surfaces, which is demanding to achieve through conventional biological methods alone. We reasoned that this glycocalyx engineering approach could be applied to elucidate the roles of specific sialosides in mediating Siglec-based immunoevasion.
Herein, we report that cancer cells engineered to display sialylated glycopolymers are protected from NK cell killing via engagement of Siglec-7 (Fig. 1a). Our in vitro data supports a model in which tumor hypersialylation results from glycome evolution under the selective pressure of NK cell immunosurveillance. As well, glycocalyx engineering of allogeneic hematopoietic stem cells and xenogeneic porcine cells with synthetic glycopolymers provided protection from NK cell cytotoxicity. Thus, the natural protection afforded by hypersialylation might be exploited in cell-based therapies.
Results
Engineering Cell Surfaces With Synthetic Glycopolymers
Defining the role of sialylated epitopes in regulating cell-mediated NK activation necessitates the ability to introduce specific glycan structures onto cell surfaces. Unfortunately, glycan biosynthesis is not template driven and its complex regulation is difficult to manipulate by genetics alone especially in human cells.25 Regrettably, most conventional methods are constrained in their precise molecular control over structure and produce widespread changes in cell surface glycans. We sought to establish a complementary method that could introduce cancer-associated sialosides onto target cancer cell surfaces without the risk of altering the function of a multitude of cell surface glycoproteins and glycolipids.
An enticing solution was the use of synthetic glycopolymers to endow cell surfaces with chemically defined glycans. To do so, we generated synthetic polymers end-functionalized with phospholipids for passive insertion into cellular membranes (Fig. 1b).30,31 The glycopolymers were prepared by condensation of aminooxy glycans to a polymethyl vinyl ketone scaffold via oxime formation. Our recent advances in aminooxy glycan synthesis32 enabled us to generate a panel of polymers with a range of glycan structures (Fig. 1b). To address the validity of early models invoking physical and electrostatic repulsion in hypersialylation-mediated immunoevasion,11 we also made control polymers lacking sialic acid or sugars altogether. These control polymers were decorated with aminooxy carboxylate or glycerol moieties to maintain the same overall charge or steric bulk and hydrophilicity as a sialoside polymer, but without the potential for recognition by specific carbohydrate receptors.
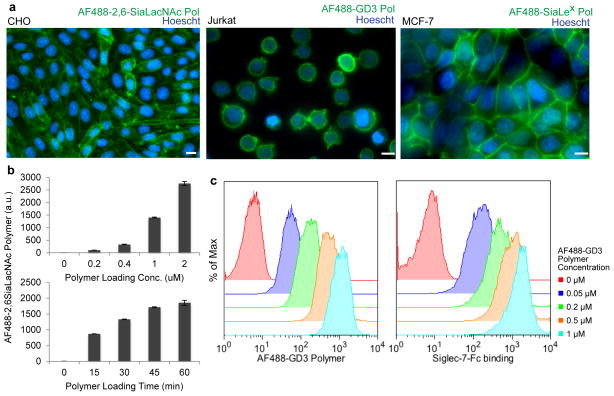
To probe the incorporation of synthetic glycopolymers into the cell membranes, we included small amounts of aminooxy-Alexa Flour 488 or aminooxy-biotin along the polymer backbone. The polymers were incubated with a variety of cancer cell lines, and the abundance and distribution of cell-bound polymers were determined by flow cytometry and fluorescence microscopy. The glycopolymers incorporated robustly into the plasma membranes of all cell lines studied (e.g. CHO, Jurkat, MCF-7, Fig. 2a; K562, DU145, Supplementary Results, Supplementary Fig. 1). Polymer densities were controlled by altering the initial polymer concentration in the cell media, while incubation time had a lesser effect (Fig. 2b). Importantly, the incorporation efficiencies were not noticeably affected by glycan structure (Supplementary Fig. 2).
Figure 2. Glycopolymers enable controlled manipulation of cellular glycosylation status.
(a) Fluorescence microscopy of CHO, Jurkat, and MCF-7 cells labeled with AF488 conjugated glycopolymers demonstrate good incorporation across cell types. Scale bars, 10 μm. (b) K562 cells were incubated with increasing concentrations of AF488-2,6-SiaLacNAc polymer for 45 min at room temperature or 1 μM polymer for increasing time points. Incorporation was measured by flow cytometry. a.u., arbitrary units. (c) Glycopolymers show concentration dependent labeling that correlates with Siglec-7-Fc lectin binding. Jurkat cells were coated with increasing concentrations of AF488-GD3 polymer at room temperature for 45 min and labeled with Siglec-7-Fc and anti-humanFc-647 on ice. For (b) data are presented as mean ± s.d. (n = 3).
The dynamic nature of the cell surface imposes a finite surface lifetime on the glycopolymers as components of the plasma membrane are continually internalized and refreshed. To determine their cell surface half-life, we used biotin-conjugated glycopolymers and labeled remaining surface-associated polymers with fluorescent anti-biotin antibody over 24 h. The surface half-life was 4–7 hours depending on cell type and was independent of glycan structure (Supplementary Fig. 3). Notably, NK cell activation and cytotoxicity are detectable on a shorter timeframe.2,3 Next, we established whether the cell surface-bound glycopolymers were accessible to exogenous receptor binding. As expected, 2,6-SiaLacNAc polymer incorporation on Jurkat cells increased binding to both the lectin SNA and a Siglec-7-Fc chimera (Supplementary Fig. 4). In addition, cell surface incorporation of glycopolymers containing GD3, a reported Siglec-7 ligand,33 bound to exogenous Siglec-7-Fc in a dose-dependent manner (Fig. 2c). These results establish that glycopolymer density can be controlled which directly correlates with the amount of glycan epitopes available for receptor binding.
Sialoside Glycopolymers Protect Cells from NK Cytotoxicity
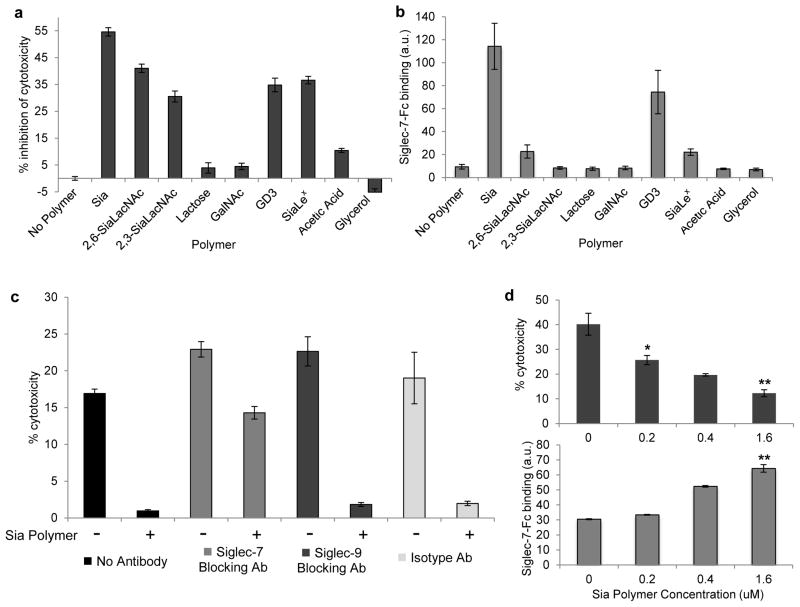
We next tested the effects of glycocalyx engineering on NK cell-mediated cytotoxicity. Jurkat cells provided an ideal initial target as they are readily lysed by primary NK cells and showed drastic changes in Siglec-7-Fc binding upon polymer incorporation (Supplementary Fig. 4). In preliminary studies, freshly isolated human peripheral blood mononuclear cells (PBMC) were used as a source of NK effector cells. Target Jurkat cells were pretreated with various polymers and their susceptibility to NK cell killing was assessed after 4 h of coincubation. The sialoside-functionalized glycopolymers provided protection against cell killing in a structure-specific manner, whereas control polymers lacking sialic acid or sugars altogether did not inhibit cell cytotoxicity (Supplementary Fig. 5). That the acetic acid-functionalized polymer had a minimal impact on cytotoxicity suggests that the activity of sialylated polymers was not simply due to charge effects. Interestingly, despite equal loading on cells, the polymer containing the monosaccharide sialic acid alone (abbrev. Sia polymer) offered the strongest protection against killing, with over 50% inhibition. This was confirmed by repeating the cytotoxicity assays with purified human NK cells where the Sia polymer offered the best protection at various effector to target ratios (Fig. 3a, Supplementary Fig. 6).
Figure 3. Sialoside glycopolymers protect target cells from NK cell-mediated cytotoxicity.
(a) Glycopolymers protect target cells from NK-mediated cytotoxicity in a structure dependent manner. Jurkat cells were labeled with 1 μM indicated polymer and incubated with purified NK cells at an effector to target ratio of 3:1 in a 4 h cytotoxicity assay. (b) To assess if Siglec-7 binding correlated with protection seen in (a), Jurkat cells were treated with indicated polymer and Siglec-7-Fc binding was assessed by flow cytometry. (c) Dependence of Siglec-7 for Sia polymer protection was probed by preincubating purified NK cells with 10 μg/mL of Siglec-blocking or isotype antibody and mixing with Jurkat cells at a 4:1 NK cell to target ratio in a 4 h cytotoxicity assay. (d) K562 target cells were labeled with increasing concentrations of Sia polymer and labeled with Siglec-7-Fc and anti-hFc-647 or incubated with PBMC at a 10:1 effector to target ratio. Data are presented as mean ± s.d. (n = 3; *P < 0.05, **P < 0.01 for polymer coated versus no polymer control). a.u., arbitrary units.
The observation that only sialoside glycopolymers elicited NK inhibition suggested Siglec family members as likely candidates in target protection. Glycan array data have indicated that Siglec-7 preferentially binds GD3 and other disialylated glycans while Siglec-9 has broad specificity among sialosides.34,35 However, no obvious correlations with previous binding data were seen in our cytotoxic inhibition screen. To test Siglec binding preferences with our panel of glycopolymers on cell surfaces, we treated Jurkat cells with polymers and assessed the binding of soluble Siglec-7-Fc or Siglec-9-Fc fusion proteins (Fig. 3b, Supplementary Fig. 7). The trends seen in NK cell inhibition strongly correlated with soluble Siglec-7 binding while Siglec-9 seemed to heavily prefer the SiaLex polymer, which was not the most potent NK cell inhibitor. In addition, Siglec-7 was expressed ubiquitously on NK cells from all donors while Siglec-9 was found at lower levels in a subset of NK cells (Supplementary Fig. 8).
The biggest surprise from our results rested in the GD3 glycan, which bound to Siglec-7 in previous reports, and in our hands, on cell surfaces but did not provide inhibition of cytotoxicity greater than other sialylated ligands. This was especially true when PBMC rather than purified NK cells where used (Fig. 3a, Supplementary Fig. 5). These differences convey a possible divergence between binding preference and biological activity for Siglec-7 that could reflect specific ligand-induced conformational changes or geometrical requirements.35 Alternatively, the highly conjugated GD3 polymers may be difficult for Siglec-7 to bind in a congested cell-cell synapse compared to a soluble Siglec-7-Fc. Nonetheless, the Sia polymer bound most avidly to Siglec-7-Fc and also offered optimal protection from NK-mediated killing. The Sia polymer may serve as a mimic for the preferred branched disialylated ligand of Siglec-7 or, more intriguingly, Siglec-7 may actually prefer binding to densely sialylated structures of various linkage types. Due to its potent binding to Siglec-7 and strong NK cell inhibitory activity, we focused our subsequent studies on the Sia polymer.
To directly assess whether Siglec-7 is the causative mediator of sialic acid-based NK cell inhibition, we performed the cytotoxicity assay in the presence of function blocking antibodies (Fig. 3c). As previously observed, target cells coated with the Sia polymer were protected from cytotoxicity; however, the Siglec-7 blocking antibody (S7.7) abrogated the inhibitory effect, providing comparable cytotoxicity as observed with uncoated target cells. In the presence of Siglec-9 blocking or control antibodies, the polymer was still able to protect target cells. We also demonstrated that this protection was dependent on the density of Sia polymer (Fig. 3d). Collectively, these data indicate that Siglec-7 allows NK cells to tune cytotoxic activation based on target cell sialylation status.
Siglec-7 Engagement Provides a Strong Inhibitory Signal
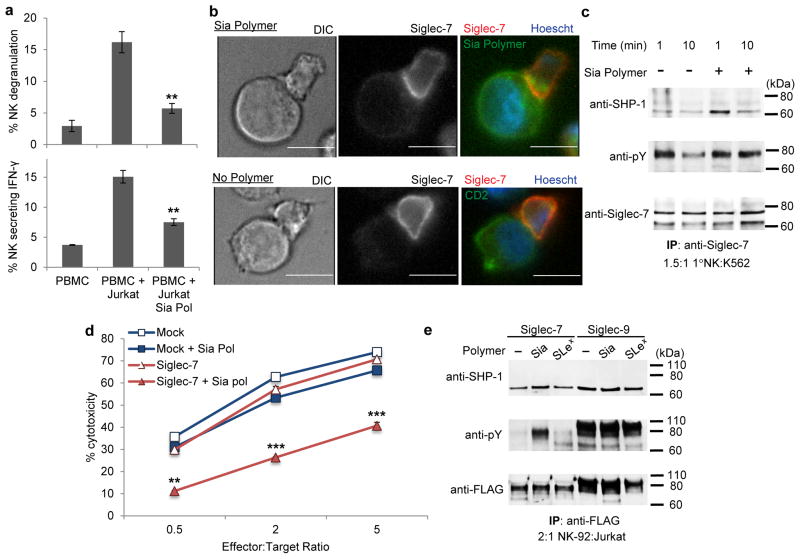
To demonstrate that Siglec-7 engagement decreases NK cell activity, we incubated sialic acid polymer-coated Jurkat cells with PBMC’s and monitored the presence of the activation markers, CD107a/LAMP1 and IFN-γ, on NK cells. The trafficking of CD107a/LAMP1 to the cell surface correlates with degranulation and the release of cytotoxic molecules such as perforin. Additionally, to recruit more lymphocytes to the site of encounter, stimulated NK cells secrete IFN-γ, which can be captured as it exits the cell and quantified. Upon coculture, Sia polymer-treated cells inhibited both degranulation and IFN-γ production by NK cells, consistent with a model in which polymer engagement suppresses NK cell activation (Fig. 4a).
Figure 4. Siglec-7 provides a strong NK inhibitory signal in response to a sialic acid glycopolymer.
(a) PBMC was incubated with Jurkat cells at a 1:1 effector to target ratio. CD107a and IFN-γ were detected by antibody labeling and reported as % positive on gated CD56+ cells. (b) Siglec-7 displayed recruitment to the NK synapse in fluorescence microscopy of NK-Jurkat target cell conjugates labeled with AF488-Sia polymers. Scale bars, 10 μm. (c) Western blot analysis of Siglec-7 activation. NK cells were incubated with K562 cells with or without Sia polymer and lysed at the indicated times followed by anti-Siglec-7 immunoprecipitation. An increase in SHP-1 recruitment and prolonged Siglec-7 phosphorylation was seen in NK cells treated with Sia polymer-coated K562 cells but not with untreated targets. (d) NK-92 cells were retrovirally transduced to overexpress FLAG-tagged Siglec-7 and incubated with polymer-treated Jurkat cells at various ratios. NK-92 cells expressing Siglec-7 were susceptible to Sia polymer inhibition while mock treated cells were uninhibited. (e) Western blot analysis of NK-92 cells stimulated for 5 min with target Jurkat cells coated with no, Sia, or SiaLex polymers. Only the Sia polymer coated targets elicited Siglec phosphorylation and increased SHP-1 in NK-92 cells overexpressing Siglec-7. Siglec-9 was phosphorylated in all instances. Data are presented as mean ± s.d. (n = 3; *P < 0.05, **P < 0.01, ***P < 0.001 for polymer coated versus no polymer control, two-tailed, paired analysis). Full blots are shown in Supplementary Note 2.
Based on the ITIM model (Fig. 1b),20,28,36 the sialic acid glycopolymers should enhance recruitment of Siglec-7 to the NK–target immunological synapse. We preincubated Jurkat cells with or without the Sia polymer and coincubated them with NK cells to permit synapse formation. Cell conjugates were then fixed and stained for both Siglec-7 and the cell surface marker CD2, and imaged by fluorescence microscopy (Fig. 4b). In the absence of polymer, Siglec-7 was widely distributed across the NK cell surface while in conjugation with target cells. Conversely, Siglec-7 localized to the site of synapse formation in NK conjugates with glycopolymer-coated target cells, implying its interaction with the sialic acid polymer on the target cell surface. This observation is consistent with previous reports demonstrating that recruitment of Siglec-7 to the NK–target synapse via antibody binding can inhibit NK killing.37 Moreover, artificially crosslinking Siglec-7 by primary and secondary antibody treatment provided no inhibition of NK cytotoxicity (Supplementary Fig. 9). These results suggest that Siglec-7 must localize and engage at the immune synapse with a target cell to promote a strong inhibitory signal and dampen NK cell-promoted cytotoxicity.
We next evaluated the signaling status of Siglec-7 in response to glycopolymer engagement. K562 cells were functionalized with and without Sia polymer and coincubated with NK cells for the indicated times followed by cell lysis. Cell lysates were subjected to Siglec-7 immunoprecipitation and western blot analysis (Fig. 4c). After 1 min, a large increase in SHP-1 coimmunoprecipation was observed only when target cells were coated with the glycopolymer. Additionally, tyrosine phosphorylation of Siglec-7 was prolonged in the presence of targets bearing glycopolymer while untreated target cells elicited only initial phosphorylation (Fig. 4c). These data fit the established model in which localization of Siglec-7 to the NK-target synapse is necessary for SHP-1 phosphatase recruitment and dampening of the kinase phosphorylation cascade. Therefore, Sia glycopolymers promote the localization of Siglec-7 to the NK synapse, which increases phosphorylation of the ITIM domain and recruitment of SHP-1.
NK-92 is a highly cytotoxic human NK line currently being investigated for cancer treatment because of its high expression of many NK activating receptors but limited expression of inhibitory receptors including Siglec-7 and Siglec-9.38,39 If Siglec-7 is a potent inhibitory receptor in NK cells then its overexpression in the NK-92 line should dampen cytotoxicity and make the NK-92 cells susceptible to inhibition by hypersialylated targets. We generated viable NK-92 clones that overexpressed FLAG-tagged Siglec-7 or Siglec-920 and tested their cytotoxicity against Jurkat cells with and without the Sia polymer (Fig. 4d, Supplementary Fig. 10). The mock transduced NK-92 cells demonstrated high cytotoxic activity against Jurkat cells irrespective of treatment with Sia polymer. However, only NK-92 cells overexpressing Siglec-7 showed an appreciable decrease in cytotoxic activity against the Sia polymer-coated Jurkat cells (Fig. 4d). Interestingly, Siglec-9 overexpressing NK-92 cells had an overall lower activity level but were not affected by sialic acid polymer-coated target cells (Supplementary Fig. 10).
FLAG immunoprecipitation and western blot analysis of the Siglec expressing NK-92 cells demonstrated again that incubation with Sia polymer-coated target cells increased phosphorylation of Siglec-7 and coimmunoprecipitation of SHP-1 (Fig. 4e). Jurkat cells lacking Sia polymer or instead coated with SiaLex polymer, which had lower cytotoxic inhibition, did not elicit substantial Siglec-7 phosphorylation. In agreement with the lower cytotoxic activity seen in the Siglec-9 overexpressing NK-92 cells, Siglec-9 remained highly phosphorylated independent of the target cell’s glycopolymer.
Siglec-7 Alters Cancer Cell Susceptibility to NK Killing
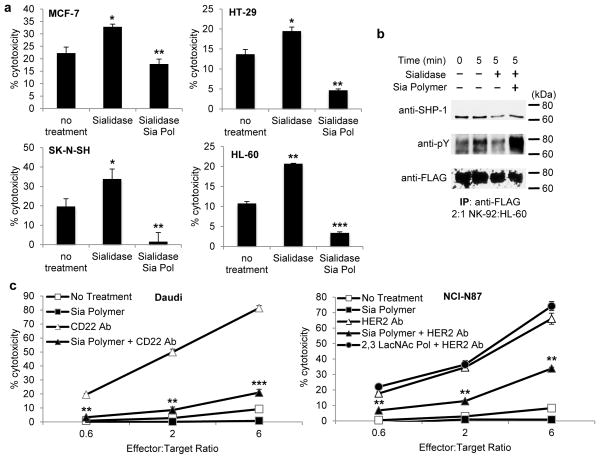
While the studies with glycopolymers on Jurkat and K562 cells were valuable in establishing the capacity of Siglec-7 ligands to provide a strong inhibitory signal in NK cells, our observations suggest that these cell lines do not inherently possess an abundance of Siglec-7 ligands. Therefore, we evaluated the effects of sialic acid remodeling in a variety of other tumor lines to examine the role of natural Siglec-7 ligands in modulating NK cell susceptibility. Several carcinoma lines from breast, brain, colon, liver or lymphoid tissue exhibited an increased susceptibility to NK cell killing after sialidase treatment (Fig. 5a, Supplementary Fig. 11). Protection against NK cell cytotoxicity was completely recovered by decoration of the target cells with the Sia polymer after sialidase treatment. Coating target cancer cells with the other sialylated or control polymers after sialidase treatment produced effects that followed the same trends as previously observed (Fig. 3, Supplementary Fig. 12). Western blot analyses confirmed that sialidase treatment of target cancer cells decreased Siglec-7 phosphorylation and SHP-1 recruitment in NK-92 and primary NK cells during incubation with HL-60, HT-29, and MCF-7 cell lines (Fig. 5b, Supplementary Fig. 13).
Figure 5. Sialylation status affects susceptibility to native and antibody-dependent NK cytotoxicity in multiple cancer lines.
(a) Removal of cell surface sialic acid from cancer cell lines increases NK cell-mediated cytotoxicity. NK immunoprotection is recovered by treatment with the Sia polymer (Sia Pol). Cancer target cells were treated with VC sialidase for 1 h at 37 °C before polymer incorporation at room temperature and coculture with purified NK (5:1, effector:target) (b) Western blot analysis of Siglec-7 phosphorylation after target cell sialylation remodeling. Promyelocytic HL-60 cells treated with sialidase and coated with Sia polymer were cocultured with NK-92 cells overexpressing Siglec-7 at a 2:1 effector to target ratio and lysed immediately or at 5 min. (c) Cytotoxicity assays performed with Daudi B lymphoma and NCI-N87 gastric carcinoma cells in the presence of 10 μg/mL anti-CD22 or 2 μg/mL anti-HER2 antibody respectively in increasing NK:target ratios. Data are presented as mean ± s.d. (n = 3; *P < 0.05, **P < 0.01, ***P < 0.001 for Sia polymer coated versus no polymer control, two-tailed, paired analysis). Full blots are shown in Supplementary Note 2.
To further demonstrate that Siglec-7 engagement mediates sialylation-dependent protection from NK cell killing, sialidase and Sia polymer treated cells were cocultured with primary NK cells preincubated with Siglec-7 blocking or isotype antibodies (Supplementary Fig. 14). Again, blocking Siglec-7 abrogated the influence of target sialylation status on NK killing but not in the isotype control. Furthermore, NK-92 cell cytotoxicity was not affected by target cell sialidase treatment and Sia polymer recovery; however, expression of Siglec-7 in NK-92 cells recapitulated the sialylation dependency seen with primary NK cells (Supplementary Fig. 15). Therefore, cell surface sialylation can modulate NK cell killing of many cancer cell types, suggesting that Siglec-7 engagement is a mechanism for cells to avoid NK destruction irrespective of tissue origin.
In addition to “natural” killing, NK cells express the FcγIII receptor, CD16, which allows them to mediate antibody-dependent cell cytotoxicity (ADCC), bridging the activities of the innate and adaptive immune response.6,40,41 Considering the importance of antibody-based antitumor therapies, we reasoned that hypersialylated cancer cells may also escape from NK-mediated ADCC. To address the role of cell surface sialosides in NK ADCC, we coated Burkitt’s B lymphoma (Daudi), breast adenocarcinoma (SK-BR-3) and gastric carcinoma (NCI-N87) cell lines with the Sia polymer. NK cells were then coincubated with the Daudi cells alongside a humanized anti-CD22 (Siglec-2) antibody, or with SK-BR-3 or NCI-N87 cells alongside an antibody against HER2/NEU, marketed as Herceptin. In all cases, the Sia polymer was able to abrogate antibody-dependent killing (Fig. 5c, Supplementary Fig. 16). In contrast, the less effective 2,3-SiaLacNAc polymer provided poor protection of target cells from ADCC. This result suggests an active role of Siglec-7 engagement in protection against ADCC rather than a simple steric blocking effect on antibody binding. We conclude that cell surface Siglec-7 ligands can protect cancer cells from both innate responses and therapeutically relevant ADCC. Additionally, these findings support a role for sialylation status in modulating the efficacy of therapeutic antibody treatment. Indeed, hypersialylation may represent a natural antibody resistance mechanism.
Glycopolymers Protect Xenogeneic and Allogeneic Cells
NK cell recognition of MHC-mismatch (“missing self”) plays an important role in linking the innate and adaptive immune responses that can lead to rejection of allogeneic transplants.42 The direct involvement of NK cells in mouse and human bone marrow stem cell transplant rejection is well documented.43,44 Likewise, NK cells have been shown to activate against foreign endothelial cells and play a major role in xenograft rejection.45 Reflecting on the ability of sialic acid glycopolymers to protect cancer cells from the NK response, we surmised that transplantation cells might be engineered to evade NK cell-mediated cytotoxicity through exogenous addition of Siglec-7-binding ligands to their cell surface.
We first coated CD34+ hematopoietic stem cells (HSC) isolated from human bone marrow with the sialic acid polymers and coincubated with allogeneic human NK cells at various effector to target ratios. The polymers inhibited NK cell killing of the HSC’s by over 75% at all ratios (Fig. 6a). Therefore, we propose that recruitment of Siglec-7 is able to protect allogeneic cells from the innate immune system and may provide a strategy for preventing acute rejection of adoptive transfer transplants. To test whether glycopolymers could also be used to protect xenogeneic cells from NK cell activation, we used the common in vitro model of pig aortic epithelial cells (PAOEC) which are susceptible to lysis by NK cells due to an incompatible MHC and the expression of NK activating ligands.46 Moreover, α-Gal antibodies naturally found within human serum further contribute through NK cell-mediated ADCC. Coating the porcine cells with the Sia polymer led to a two-fold decrease in NK-mediated killing (Fig. 6b) illustrating that Siglec-7 engagement may also inhibit NK recognition in the context of xenogeneic transplants.
Figure 6. Primary xenogeneic porcine and allogeneic hematopoietic stem cells are protected by Sia polymer incorporation.
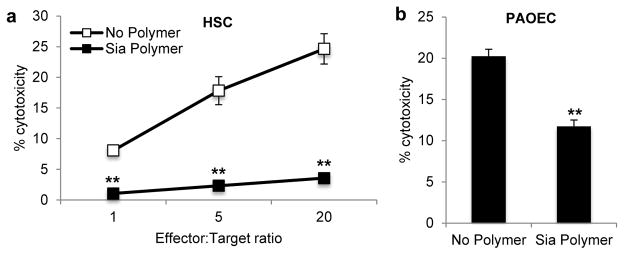
Sia polymer cell surface incorporation protects transplant cell targets from NK cell-mediated cytotoxicity. (a) Bone marrow derived CD34+ hematopoietic stem cells (HSC) were treated with 4 μM Sia polymer for 1 h at room temperature followed by coculture with purified NK at indicated effector to target ratios in a 4 h cytotoxicity assay (b) The Sia polymer also dampened the NK cytotoxic response to pig aortic endothelial cells (PAOEC) at a 10:1 effector:target ratio. Data are presented as mean ± s.d. (n = 3; **P < 0.01 for polymer coated versus no polymer control, two-tailed, paired analysis).
Discussion
Regulation of the immune system is heavily influenced by glycosylation.47 The Siglec family members are known to be manipulated by bacterial pathogens to escape immunological detection.17 We show here that tumor cells may also exploit this mechanism to evade the immune system. NK cells are part of the human body’s first line of defense against tumorigenic cells and widely express Siglec-7.1,2,19 Glycans on the same cell surface (cis ligands) are believed to dominate Siglec occupancy in resting cells, whereas activation or high affinity/avidity ligands are necessary to permit trans ligand binding and recruit Siglecs to the site of receptor activation.17,29 Previous efforts have shown that Siglec-7 on NK cells could bind to soluble α2,8-disialylated polymers or target cells with high amounts of the disialylated ligands, DSGb5 or GD3.33,48,49 However, this interaction was only possible after treating the NK cells with sialidase to release the Siglec from cis ligand binding. This dependency on sialic acid removal has hindered efforts to assess the role and activity of Siglec-7 in a biologically relevant context.
Utilizing our glycocalyx engineering approach, we demonstrated that Siglec-7 on NK cells can indeed bind to trans ligands on the target cell surface under native conditions (i.e. without enzymatic pretreatment). Incorporation of synthetic glycopolymers onto cancer cell membranes successfully emulated a hypersialylated phenotype and inhibited NK cell-mediated cytotoxicity via engagement of Siglec-7 in a variety of tumor types. Our in vitro observation that sialylation status can also reduce the efficacy of antibodies in mediating ADCC implies that hypersialylation may also be used by cancers to afford resistance to therapeutic treatment. Indeed, it would be interesting to examine the cell surface sialylation status of therapeutic antibody-refractive cancers whose resistance mechanism is not otherwise obvious.
Our findings offer a new perspective on the role of Siglec-7 in NK immunobiology and provide the opportunity for new therapeutics that target this regulatory receptor. We hope that the insights gained from the glycopolymers will spur efforts to confirm the endogenous ligands for Siglec-7. In fact, disialylated glycosphingolipids which are proposed Siglec-7 ligands were recently reported to be overexpressed in cancer stem cells suggesting that these cells in particular may use this mechanism to escape early immune detection.50 As Siglec-7 is also expressed on most monocytes and classes of CD8+ cytotoxic T lymphocytes, this mode of immunosuppression likely applies beyond the realm of NK cells and opens new avenues in the defining the role of hypersialylation in cancer immunology.
Methods
General Synthetic Methods
Synthetic reagents were purchased from Sigma-Aldrich, Acros, and TCI and used without purification unless noted otherwise. Anhydrous DMF and MeOH, 99.9% purity were purchased from Acros in sealed bottles; all other anhydrous solvents (Fisher brand; HPLC grade) were obtained from an alumina column solvent purification system.
Aminooxy Glycan-Conjugated Polymers
All aminooxy compounds were conjugated to the DPPE-MVK polymer backbone by the following method: A mixture of MVK polymer (1 equiv.), aminooxy compound (1.5 equiv. to ketone), and formic acid (1% v/v) in MeCN/MeOH (2:1, v/v) was stirred at 50 °C for 20 h. To the reaction mixture was added H2O (to 25% v/v) and continued to stir at 32 °C for 18 h. The reaction was diluted in H2O and MeOH and concentrated under vacuum. The resulting residue was dissolved in H2O and dialyzed against aqueous 5mM NH4HCO3 thrice and H2O once over 72 h to afford the corresponding oxime phospholipid polymer after lyophilization. Yields and characterization as well the synthesis of aminooxy compounds can be found in Supplementary Note 1. All glycopolymers were conjugated at 70–85% of ketone sites depending on glycan size and charge.
Reagents and Antibodies
Dulbecco’s phosphate-buffered saline pH 7.4 (PBS), Ham’s F-12, DMEM, and RPMI-1640 media were purchased from Hyclone/Thermo Scientific. Hank’s balanced salt solution with Ca2+ and Mg2+ (HBSS), α-MEM, and DMEM/F12 media were obtained from Invitrogen Life Technologies, Inc. Fetal bovine serum (FBS) was obtained from HyClone Laboratories and heat-inactivated before use. Hoechst 33342 nuclear stain and Alexa Fluor 488 hydroxylamine were purchased from Molecular Probes Life Technologies. Halt Protease Inhibitor Cocktail was purchased from Thermo Scientific. Phosphatase Inhibitor Cocktail B and 6-Carboxyfluorescein succinimidyl ester (CFSE, 5 mg/mL in DMSO) was purchased from Santa Cruz Biotechnology. Brefeldin A (GolgiPlug) was purchased from BD Biosciences. Anti-FLAG M2 Affinity Gel and Triton X-100 were purchased from Sigma-Aldrich. Human recombinant IL-2 was purchased from Peprotech and stored as a 0.5 mg/mL solution in 100 mM AcOH.
The following antibodies were used: anti-CD56-PE mAb (clone AF12-7H3; Miltenyi Biotec), anti-CD56-FITC mAb (clone HCD56; BioLegend) anti-CD3-FITC mAb (clone BW264/56; Miltenyi Biotec), anti-CD34-FITC mAb (clone AC136; Miltenyi Biotec), anti-Siglec-7-PE mAb (clone 6–434, BioLegend), anti-Siglec-9-PE mAb (clone K8, BioLegend), anti-CD2-FITC mAb (clone RPA-2.10, BD Pharmingen), mouse IgG1 κ-PE isotype (clone MOPC-21, BioLegend), goat anti-mouse IgG κ-AF647 (lot 101944, Jackson ImmunoResearch), goat anti-human IgG, Fc-DyLight649 (lot 98136, Jackson ImmunoResearch), anti-biotin-DyLight649 mAb (clone 200-492-211, Jackson ImmunoResearch), anti-SHP-1/SH-PTP-1 mAb (clone D-11, Santa Cruz Biotech), anti-phosphotyrosine-HRP mAb (clone 4G10, Millipore), rabbit anti-Siglec-7 (clone H-48, Santa Cruz Biotech), anti-CD107a/LAMP-1 mAb (clone H4A3, BioLegend), blocking anti-Siglec-7 mAb(clone S7.7, BioLegend), blocking anti-Siglec-9 mAb (clone 191240, R&D Systems), humanized anti-CD22 mAb52 and anti-HER2 mAb53 (gift from D. Rabuka, Emeryville, CA), goat anti-mouse IgG-HRP (lot 97909, Jackson ImmunoResearch), goat anti-rabbit IgG-FITC (lot 1984680, Millipore), mouse IgG1 κ isotype (clone MG1-45, BioLegend). Human recombinant Siglec-7-Fc chimera was purchased from R&D Systems. Fluorescein labeled elderberry bark Sambucus nigra lectin (SNA-FITC) was purchased from Vector Laboratories.
Cell culture and primary leukocyte isolation
All cell lines were obtained from the American Type Culture Collection (ATCC) and maintained at 37 °C and 5% CO2 in a water-saturated incubator. Cell densities were counted using a hemocytometer and maintained between 1 × 105 and 1 × 106 cells/mL. Chinese Hamster Ovary (CHO) cells were maintained in Ham’s F12 media supplemented with 10% FBS, 100 units/ml penicillin and 0.1 mg/ml streptomycin (pen/strep). Jurkat, Daudi, K562, HL-60, H9, DU145, NCI-N87 and SK-BR-3 cells were maintained in RPMI-1640 supplemented with 10% FBS and pen/strep. MCF-7, SK-N-SH, HT-29, WiDr, and HepG2 cells were maintained in high glucose DMEM supplemented with 10% FBS and pen/strep. NK-92 cells were maintained in α-MEM modified with ribonucleosides and deoxyribonucleotides and supplemented with 10% FBS, 10% horse serum, 0.1 mM β-mercaptoethanol, 0.02 mM folic acid, 0.2 mM inositol and 200 IU/mL human recombinant IL-2. Primary Porcine Aortic Endothelial Cells (PAOEC)54 were purchased from Genlantis (San Diego, CA) and maintained in PrimaPure Porcine Endothelial Cell Growth Medium. Human Bone Marrow CD34+ Hematopoietic Stem Cells were purchased from Stem Express (Placerville, CA) and expanded with StemSpan serum-free expansion medium supplemented with CC100 cytokine cocktail containing human recombinant Flt-3 Ligand, Stem Cell Factor, IL-3, and IL-6 (StemCell Technologies).
Primary leuokocytes were isolated from buffy coats obtained from the Red Cross (Oakland, CA) or the Blood Centers of the Pacific (San Francisco, CA). PBMC was obtained by dilution of buffy coats into PBS and density gradient centrifugation with Ficoll-Paque (GE Healthcare Life Sciences). PBMC was cultured in RPMI-1640 containing 10% FBS (v/v) and pen/strep and used within 48 h. NK cells were isolated from PBMC by negative selection using a NK cell isolation kit (Miltenyi Biotec) according to the manufacturer’s protocol and cultured in RPMI-1640 containing 10% FBS (v/v). Cell purity was verified by flow cytometry to be > 90–95% CD56+/CD3−. Viability of all cells in culture was > 90% for each experiment.
Glycopolymer loading and flow cytometry
The glycopolymers were aliquoted and stored at −20 °C in PBS at 50 μM as calculated by % conjugation and phospholipid amount. For labeling, cells were harvested and resuspended in HBSS at 107 cells/mL followed by incubation with glycopolymers at the indicated concentration (commonly 1 μM) for 45 min at room temperature. Cells were then washed in HBSS and complete media and kept on ice or used immediately for the desired experiment. For lectin labeling, cells were resuspended in 25 μL of 1% BSA/PBS containing Ca2+ and Mg2+ and labeled with 0.4 μg/mL Siglec-7-Fc and 1 μg/mL anti-hIgG Fc-647 or 10 μg/mL SNA-FITC for 40 min on ice. Flow cytometry was performed on a BD Biosciences FACSCalibur flow cytometer and data analyzed using FlowJo software (Tree Star).
Fluorescence Microscopy
Jurkat cells were labeled with 1 μM AF488-Sia or Sia polymers at room temperature for 1 h in HBSS. 2.5 × 105 NK cells were added to 1.25 × 105 target Jurkat cells (E:T ratio: 2:1) and incubated for 15 min in 100 μL DMEM at 37 °C. Cells were carefully transferred to a poly-L-lysine coated 8 chambered coverglass slide and allowed to settle for 15 min at 37 °C. The cells were fixed by incubating in 4% paraformaldehyde/PBS at room temperature for 20 min. Cells were washed in 1% FBS/PBS and stained with anti-Siglec-7 (1/100) followed by anti-MsLC-647 (1/1,000) and anti-CD2-FITC (1/1,000) for 30 min on ice each. Nucleus was stained with 5 μg/mL Hoescht 33342 for 20 min. Images were acquired on a Zeiss 200M epifluorescence microscope and deconvolved using a nearest neighbor deconvolution algorithm. All images were analyzed using Slidebook 5.0 (Intelligent Imaging Innovations).
NK cell degranulation and IFN-γ secretion
Polymer-coated target Jurkat cells were mixed with 5 × 105 PBMC at an effector to target ratio of 1 and incubated for 4 h at 37 °C. Control samples were incubated in 100 ng/mL PMA and 1μg/mL ionomycin to assess maximum activity. IFN-γ was measured by an IFN-γ secretion assay-detection kit (PE) (Miltenyi Biotech) according to the manufacturer’s directions. For the degranulation assay, PBMC was treated with GolgiPlug (1/200 dilution) and anti-CD107a (1/100 dilution) during coculture and labeled with anti-MsIgG-647 and anti-CD56-PE on ice. Percent degranulation was assessed by gating on CD56+ cells and measuring mean proportion of CD107a+.55
Cell cytotoxicity assay
Target cells were prelabeled by incubation with 5 μM CFSE in HBSS for 5 min, washed, and added to human PBMC or purified NK cells at the specified effector to target ratio in DMEM/F12, no phenol red with 10% FBS and cocultured for 4 h at 37 °C in 5% CO2. In a typical experiment 75 μL of target cells at 106 cells/mL were added to a V-bottom 96-well plate containing 100 μL of PBMC or NK cells at varying ratios. Propidium iodide (20 μg/mL, Sigma) was added to each sample and NK cell cytotoxicity was evaluated by flow cytometry as described.56,57 At least, 8,000 target cells were acquired after electronic gating on CFSE (FL-1) and the mean proportion of propidium iodide positive cells was determined. Percent cytotoxicity was calculated as 100 × (experimental % dead − spontaneous % dead)/(100 − spontaneous % dead). For antibody blocking experiments, NK cells were pre-incubated with 10 μg/mL Siglec-7 (S7.7),37 Siglec-9 (191240),58 or isotype control (IgG1, κ) for 30 min at 37 °C before addition of target cells. Sialidase treatment was performed by incubating target cells at 107 cells/mL in 0.25% BSA/2 mM CaCl/DMEM with 0.1 U/mL Vibrio cholerae sialidase for 1 h at 37 °C. The cells were washed twice in DMEM/F12 and HBSS before polymer coating and coculture with effector cells.
Generation of Siglec expressing NK-92 cells
The human Siglec-7 and Siglec-9 coding sequences were PCR amplified from plasmids (a kind gift from Dr. James Paulson, Scripps Research Institute)20 using primers: 5′-GTGGCAGATCTACCTCCAACCCCAGATATGC-3′ and 5′-GCCTCGAGCTACTTGT ATCATCGTCCTTATAGTCGGGATCCTTGGGGAT-3′ for Siglec-7 and 5′-GTGGCAGATCTACCTCTAACCCCAGACATGC-3′ and 5′-GCCTCGAGCTACTTGTCATCA TCGTCCTTATAGTCGGGATCCCTGTGGAT-3′ for Siglec-9. The PCR fragments were cloned into the pMSCV-puro retroviral vector (a kind gift from Dr. Mike Boyce, Duke University) at the BglII/XhoI sites. Viruses were produced by cotransfection with vectors containing gag/pol and VSV-G into HEK293T cells as previously described.59 NK-92 cells at 4 × 105 cells/mL were inoculated with retroviral media and 6 μg/mL polybrene in α-MEM with IL-2 and cultured for 24 h. The media was then replaced and transduced cells were selected for by culturing in 2 μg/mL puromycin for 4 days. After growth recovery for 7 days, viable NK-92 cells were labeled with anti-Siglec antibody and a high Siglec expressing population was sorted for on a DAKO-Cytomation MoFlo High Speed Sorter.
Coimmunoprecipitation and western blot analysis
Primary NK (5 × 106) or NK-92 cells (6 × 106) and target cells were mixed in 250 μL HBSS at an effector to target ratio of 1.5:1 or 2:1 and pelleted by centrifugation. Cells were transferred to 37 °C for the indicated times, and lysed in 50 mM TrisHCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and phosphatase/protease inhibitors. Lysates were first incubated with 2.5 μg anti-Siglec-7 antibody followed by Protein A/G agarose or ANTI-FLAG M2 Affinity gel for 18 h at 4 °C. The beads were washed three times with the lysis buffer and eluted by boiling in 2x SDS loading dye with β-mercaptoethanol. For Western blotting, proteins were resolved by SDS-PAGE on Bis-Tris Criterion Gels (10% or 4–12%; Bio-Rad) and transferred to nitrocellulose by wet transfer (Tris-glycine, 20% MeOH) at 45V for 5 h. Protein loading was confirmed by Ponceau stain (0.2% ponceau in 3% AcOH). Blocking and antibody incubation conditions were conducted in 1x Dulbecco’s phosphate buffered saline with 0.05% Tween-20 (PBST). Blots were blocked in 5% BSA/PBST and probed with anti-SHP-1/SH-PTP-1 mAb (1:500 dilution) followed by goat anti-rabbit IgG-FITC (1:3,000) in 3% BSA/PBST. Tyrosine phosphorylation was probed with anti-phosphotyrosine-HRP mAb (1:2,000) in 6% BSA/PBST. Siglec-7 loading was analyzed with anti-Siglec-7 (1:500 dilution) followed by goat anti-mouse IgG κ-AF647 (1:3,000) in 2% BSA/PBST. Siglec loading from NK-92 cells was analyzed by mouse anti-FLAG-Cy3 (1:1,000) in 2% BSA/PBST or mouse anti-FLAG-HRP (1:10,000) in 2% milk/PBST. Membranes were developed by chemiluminescence using the SuperSignal West Pico kit (Thermo) or scanned for fluorescence by a Typhoon 9410 imaging system (Amersham).
Statistical analysis
Data are shown as the mean ± standard deviation of at least three experiments. P values were calculated using paired Student’s t-test. A P value of < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We would like to thank Prof. J. Paulson (Scripps Research Institute) for the gift of plasmids encoding Siglec-7 and Siglec-9. Prof. M. Boyce (Duke University) for the gift of pMSCV retroviral plasmids and advice. Prof. D. Raulet, M. Ardolino, A. Iannello, P. Drake, and C. Hudak for advice and expertise. B. Belardi and D. Rabuka for helpful discussion and manuscript critique. This work was funded by a grant from the US National Institutes of Health (R01 GM59907). J.E.H. was supported by a predoctoral fellowship from the US National Science Foundation. S.M.C. was supported by a postdoctoral fellowship from the US National Institutes of Health (F32DK095521).
Footnotes
Author Contributions
J.E.H. synthesized compounds, designed and performed the experiments, analyzed the data and prepared the manuscript. S.M.C. performed the experiments, analyzed the data, and revised the manuscript. C.R.B. directed the study and revised the manuscript.
Competing Financial Interests Statement
The authors declare no competing financial interests.
References
- 1.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamai L, et al. NK Cells and Cancer. J Immunol. 2007;178:4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 7.Mamessier E, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996;56:2237–2244. [PubMed] [Google Scholar]
- 10.Leivonen M, Nordling S, Lundin J, von Boguslawski K, Haglund C. STn and prognosis in breast cancer. Oncology. 2001;61:299–305. doi: 10.1159/000055337. [DOI] [PubMed] [Google Scholar]
- 11.Dennis J, Waller C, Timpl R, Schirrmacher V. Surface sialic acid reduces attachment of metastatic tumour cells to collagen type IV and fibronectin. Nature. 1982;300:274–276. doi: 10.1038/300274a0. [DOI] [PubMed] [Google Scholar]
- 12.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 13.Cohen M, et al. Sialylation of 3-Methylcholanthrene–Induced Fibrosarcoma Determines Antitumor Immune Responses during Immunoediting. J Immunol. 2010;185:5869–5878. doi: 10.4049/jimmunol.1001635. [DOI] [PubMed] [Google Scholar]
- 14.Van Rinsum J, Smets LA, Van Rooy H, Van den Eijnden DH. Specific inhibition of human natural killer cell-mediated cytotoxicity by sialic acid and sialo-oligosaccharides. Int J Cancer. 1986;38:915–922. doi: 10.1002/ijc.2910380620. [DOI] [PubMed] [Google Scholar]
- 15.Yogeeswaran G, et al. Correlation of glycosphingolipids and sialic acid in YAC-1 lymphoma variants with their sensitivity to natural killer-cell-mediated lysis. Int J Cancer. 1981;28:517–526. doi: 10.1002/ijc.2910280419. [DOI] [PubMed] [Google Scholar]
- 16.Ogata S, Maimonis PJ, Itzkowitz SH. Mucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicity. Cancer Res. 1992;52:4741–4746. [PubMed] [Google Scholar]
- 17.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 18.Paulson JC, Macauley MS, Kawasaki N. Siglecs as sensors of self in innate and adaptive immune responses. Ann N Y Acad Sci. 2012;1253:37–48. doi: 10.1111/j.1749-6632.2011.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicoll G, et al. Identification and Characterization of a Novel Siglec, Siglec-7, Expressed by Human Natural Killer Cells and Monocytes. J Biol Chem. 1999;274:34089–34095. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 20.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004;279:43117–43125. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- 21.Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841–6849. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- 22.Nath, et al. Macrophage–tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor sialoadhesin. Immunology. 1999;98:213–219. doi: 10.1046/j.1365-2567.1999.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takamiya R, Ohtsubo K, Takamatsu S, Taniguchi N, Angata T. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12–Syk pathway. Glycobiology. 2013;23:178–187. doi: 10.1093/glycob/cws139. [DOI] [PubMed] [Google Scholar]
- 24.Belisle JA, et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer. 2010;9:118. doi: 10.1186/1476-4598-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiessling LL, Splain RA. Chemical Approaches to Glycobiology. Annu Rev Biochem. 2010;79:619–653. doi: 10.1146/annurev.biochem.77.070606.100917. [DOI] [PubMed] [Google Scholar]
- 26.Collins BE, Paulson JC. Cell surface biology mediated by low affinity multivalent protein–glycan interactions. Curr Opin Chem Biol. 2004;8:617–625. doi: 10.1016/j.cbpa.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Brown JM, et al. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci USA. 2012;109:4768–4773. doi: 10.1073/pnas.1121318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci USA. 2009;106:2500–2505. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins BE, et al. High-Affinity Ligand Probes of CD22 Overcome the Threshold Set by cis Ligands to Allow for Binding, Endocytosis, and Killing of B Cells. J Immunol. 2006;177:2994–3003. doi: 10.4049/jimmunol.177.5.2994. [DOI] [PubMed] [Google Scholar]
- 30.Rabuka D, Forstner MB, Groves JT, Bertozzi CR. Noncovalent Cell Surface Engineering: Incorporation of Bioactive Synthetic Glycopolymers into Cellular Membranes. J Am Chem Soc. 2008;130:5947–5953. doi: 10.1021/ja710644g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belardi B, O’Donoghue GP, Smith AW, Groves JT, Bertozzi CR. Investigating Cell Surface Galectin-Mediated Cross-Linking on Glycoengineered Cells. J Am Chem Soc. 2012;134:9549–9552. doi: 10.1021/ja301694s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudak JE, Yu HH, Bertozzi CR. Protein Glycoengineering Enabled by the Versatile Synthesis of Aminooxy Glycans and the Genetically Encoded Aldehyde Tag. J Am Chem Soc. 2011;133:16127–16135. doi: 10.1021/ja206023e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicoll G, et al. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur J Immunol. 2003;33:1642–1648. doi: 10.1002/eji.200323693. [DOI] [PubMed] [Google Scholar]
- 34.Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem. 2003;278:31007–31019. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 35.Attrill H, et al. Siglec-7 undergoes a major conformational change when complexed with the alpha(2,8)-disialylganglioside GT1b. J Biol Chem. 2006;281:32774–32783. doi: 10.1074/jbc.M601714200. [DOI] [PubMed] [Google Scholar]
- 36.Daëron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 37.Falco M, et al. Identification and Molecular Cloning of P75/Airm1, a Novel Member of the Sialoadhesin Family That Functions as an Inhibitory Receptor in Human Natural Killer Cells. J Exp Med. 1999;190:793–802. doi: 10.1084/jem.190.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10:369–383. doi: 10.1089/152581601750288975. [DOI] [PubMed] [Google Scholar]
- 39.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 40.Dall’Ozzo S, et al. Rituximab-Dependent Cytotoxicity by Natural Killer Cells Influence of FCGR3A Polymorphism on the Concentration-Effect Relationship. Cancer Res. 2004;64:4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 41.Triulzi C, et al. Antibody-Dependent Natural Killer Cell–Mediated Cytotoxicity Engendered by a Kinase-Inactive Human HER2 Adenovirus-Based Vaccination Mediates Resistance to Breast Tumors. Cancer Res. 2010;70:7431–7441. doi: 10.1158/0008-5472.CAN-10-0493. [DOI] [PubMed] [Google Scholar]
- 42.Van Der Touw W, Bromberg JS. Natural Killer Cells and the Immune Response in Solid Organ Transplantation. Am J Transplant. 2010;10:1354–1358. doi: 10.1111/j.1600-6143.2010.03086.x. [DOI] [PubMed] [Google Scholar]
- 43.Murphy WJ, Vinay K, Bennett M. Rejection of bone marrow allografts by mice with severe combined immune deficiency (SCID). Evidence that natural killer cells can mediate the specificity of marrow graft rejection. J Exp Med. 1987;165:1212–1217. doi: 10.1084/jem.165.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy WJ, Koh CY, Raziuddin A, Bennett M, Longo DL. Immunobiology of natural killer cells and bone marrow transplantation: merging of basic and preclinical studies. Immunol Rev. 2001;181:279–289. doi: 10.1034/j.1600-065x.2001.1810124.x. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Waer M, Billiau AD. Xenotransplantation: Role of natural immunity. Transpl Immunol. 2009;21:70–74. doi: 10.1016/j.trim.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Forte P, Lilienfeld BG, Baumann BC, Seebach JD. Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J Immunol. 2005;175:5463–5470. doi: 10.4049/jimmunol.175.8.5463. [DOI] [PubMed] [Google Scholar]
- 47.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avril T, North SJ, Haslam SM, Willison HJ, Crocker PR. Probing the cis interactions of the inhibitory receptor Siglec-7 with α2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of α2,8-sialyltransferase gene expression. J Leukoc Biol. 2006;80:787–796. doi: 10.1189/jlb.1005559. [DOI] [PubMed] [Google Scholar]
- 49.Kawasaki Y, et al. Ganglioside DSGb5, preferred ligand for Siglec-7, inhibits NK cell cytotoxicity against renal cell carcinoma cells. Glycobiology. 2010;20:1373–1379. doi: 10.1093/glycob/cwq116. [DOI] [PubMed] [Google Scholar]
- 50.Liang YJ, et al. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. Proc Natl Acad Sci. 2013;110:4968–4973. doi: 10.1073/pnas.1302825110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabuka D, et al. Hierarchical Assembly of Model Cell Surfaces: Synthesis of Mucin Mimetic Polymers and Their Display on Supported Bilayers. J Am Chem Soc. 2007;129:5462–5471. doi: 10.1021/ja067819i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leonard JP, et al. Epratuzumab, a Humanized Anti-CD22 Antibody, in Aggressive Non-Hodgkin’s Lymphoma Phase I/II Clinical Trial Results. Clin Cancer Res. 2004;10:5327–5334. doi: 10.1158/1078-0432.CCR-04-0294. [DOI] [PubMed] [Google Scholar]
- 53.Menard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570–6578. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 54.Sommaggio R, Pérez-Cruz M, Costa C. Cellular studies for in vitro modeling of xenogeneic immune responses. Methods Mol Biol. 2012;885:91–103. doi: 10.1007/978-1-61779-845-0_7. [DOI] [PubMed] [Google Scholar]
- 55.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy Among Receptors on Resting NK Cells for the Activation of Natural Cytotoxicity and Cytokine Secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Godal R, et al. Lymphomas are sensitive to perforin-dependent cytotoxic pathways despite expression of PI-9 and overexpression of bcl-2. Blood. 2006;107:3205–3211. doi: 10.1182/blood-2005-07-2880. [DOI] [PubMed] [Google Scholar]
- 57.Bryceson YT, et al. Functional analysis of human NK cells by flow cytometry. Methods Mol Biol. 2010;612:335–352. doi: 10.1007/978-1-60761-362-6_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aalto K, et al. Siglec-9 Is a Novel Leukocyte Ligand for Vascular Adhesion Protein-1 and Can Be Used in PET Imaging of Inflammation and Cancer. Blood. 2011;118:3725–3733. doi: 10.1182/blood-2010-09-311076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naviaux RK, Costanzi E, Haas M, Verma IM. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.