Abstract
S-nitrosylation, or the replacement of the hydrogen atom in the thiol group of cysteine residues by a −NO moiety, is a physiologically important posttranslational modification. In our previous work we have shown that S-nitrosylation is involved in the disruption of the endothelial nitric oxide synthase (eNOS) dimer and that this involves the disruption of the zinc (Zn) tetrathiolate cluster due to the S-nitrosylation of Cysteine 98. However, human eNOS contains 28 other cysteine residues whose potential to undergo S-nitrosylation has not been determined. Thus, the goal of this study was to identify the cysteine residues within eNOS that are susceptible to S-nitrosylation in vitro. To accomplish this, we utilized a modified biotin switch assay. Our modification included the tryptic digestion of the S-nitrosylated eNOS protein to allow the isolation of S-nitrosylated peptides for further identification by mass spectrometry. Our data indicate that multiple cysteine residues are capable of undergoing S-nitrosylation in the presence of an excess of a nitrosylating agent. All these cysteine residues identified were found to be located on the surface of the protein according to the available X-ray structure of the oxygenase domain of eNOS. Among those identified were Cys 93 and 98, the residues involved in the formation of the eNOS dimer through a Zn tetrathiolate cluster. In addition, cysteine residues within the reductase domain were identified as undergoing S-nitrosylation. We identified cysteines 660, 801, and 1113 as capable of undergoing S-nitrosylation. These cysteines are located within regions known to bind flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), and nicotinamide adenine dinucleotide (NADPH) although from our studies their functional significance is unclear. Finally we identified cysteines 852, 975/990, and 1047/1049 as being susceptible to S-nitrosylation. These cysteines are located in regions of eNOS that have not been implicated in any known biochemical functions and the significance of their S-nitrosylation is not clear from this study. Thus, our data indicate that the eNOS protein can be S-nitrosylated at multiple sites other than within the Zn tetrathiolate cluster, suggesting that S-nitrosylation may regulate eNOS function in ways other than simply by inducing dimer collapse.
Introduction
Nitric oxide synthase (NOS) produces NO, a key signaling molecule in the nervous, immune, and cardiovascular systems. There are three isoforms of the enzyme NOS, of which endothelial nitric oxide synthase (eNOS) is expressed in endothelial cells, cardiac myocytes, and blood platelets (Michel and Feron, 1997). NO is synthesized in mammals by NOS from L-arginine and molecular oxygen in presence of NADPH, producing L-citrulline as a coproduct (Ignarro, 1990; Moncada et al., 1991; Stuehr, 1999). The main role of endothelium-derived NO is to regulate vascular tone. Besides this, the released NO is also involved in the suppression of smooth muscle cell proliferation, platelet aggregation, and modulation of leukocyte activation and adhesion (Bredt and Snyder, 1990; Bredt et al., 1991; Nathan and Xie, 1994).
In general, the structure of a mammalian NOS enzyme consists of two domains and a linker peptide with calmodulin (CaM) binding sequence. The reductase domain consists of two units: one that binds NADPH and FAD, and the other that binds FMN. NADPH acts as an electron donor, and FAD and FMN serve as an electron storage pool and transfer agent. Oxygenase domain contains a thiolate-linked heme group and binds to both L-arginine and tetrahydrobiopterin (BH4) (McMillan and Masters, 1993; Renaud et al., 1993; Chen et al., 1994; Nishimura et al., 1995; Stuehr, 1999). The activity of eNOS is regulated by the reversible Ca2+-dependent binding of CaM (Rodriguez-Crespo and Ortizde Montellano, 1996).
Our previous studies have shown that presence of exogenous NO has an inhibitory effect on the activity of eNOS in cultured cells (Wedgewood et al., 2005), in the purified eNOS protein (Ravi et al., 2004), and in lambs exposed to inhaled NO (Wedgewood et al., 2005; Mata-Greenwood et al., 2006). Further, we have shown that the inhibitory effect of NO is mediated, at least in part, through the disruption of the eNOS dimer that is associated with the release of zinc (Zn) due to destruction of the Zn tetrathiolate cluster (Hemmens et al., 2000; Govers and Rabelink, 2001).
NO is known to chemically modify cysteines to nitrosothiols, which are highly labile in presence of reducing agents like ascorbates and glutathione (Stamler, 1994; Kashiba-Iwatsuki et al., 1997; Stamler et al., 2001; Liu et al., 2004). NO can produce both single cysteine S-nitrosylations as in case of Ha-Ras (Lander et al., 1997), caspase 3 (Rossig et al., 1999), and nuclear factor-kappa B (Marshall and Stamler, 2001) and multiple cysteine S-nitrosylations in case of N-ethylmaleimide Sensitive Factor (NSF) (Matsushita et al., 2003) and the ryanodine receptor (Voss et al., 2004). Consensus S-nitrosylation motifs have been postulated by Stamler et al. (1997). These motifs have been suggested to contain hydrophilic residues adjacent to the specific cysteine either in the primary structure (Stamler et al., 1997) or brought together by three-dimensional (3D) conformations (Ascenzi et al., 2000, 2001). However, our previous studies, utilizing short peptide sequences, suggested that S-nitrosylation may be independent of the amino acids surrounding the cysteine residue. Nevertheless, from our data we could not exclude the possibility that the Stamler motifs (Stamler et al., 1997) could play a role in native proteins. Defining whether the intramolecular cysteines, tetrathiolate cysteines, or both are involved in the S-nitrosylation is likely to be a key step in unraveling the regulatory effects of S-nitrosylation on eNOS activity. Thus, the goal of this study was to identify the sites of S-nitrosylation within the native eNOS protein. Utilizing a modified biotin switch assay coupled with mass spectrometry, we were able to definitively identify eight unique cysteine residues susceptible to S-nitrosylation (Cys-93, −98, −211, −234, −381, −660, −801, and −852). Further, we identified a further four regions that were S-nitrosylated. These sequences were found to contain two cysteine residues that were S-nitrosylated. These identified regions were Cys14/25, Cys975/990, Cys1047/1049, and Cys1104/1113.
Materials and Methods
Reagents
Methyl methanethiosulfonate (MMTS), UltraLink Immobilized Neutravidin, and EZ-Link N-[6-(biotinamido) hexyl]-30′-(20′-pyridyldithio) propionamide (biotin-HPDP) were obtained from Pierce (Rockford, IL). BioSpin P-30 Tris columns were obtained from BioRad (Hercules, CA). HEPES free acid, dimethyl sulfoxide (DMSO), ammonium bicarbonate, dithiothretol, neocuproine, S-nitroso-N-acetyl penicillamine (SNAP), S-nitrosoglutathione (GSNO), sodium dodecyl sulfate, sodium ascorbate, sodium bicarbonate (NaHCO3), sodium chloride (NaCl), sodium phosphate, and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma-Aldrich (St. Louis, MO), and modified trypsin was obtained from Promega (Madison, WI). C18 ZipTips were obtained from Millipore (Bedford, MA).
Expression and purification of human eNOS
The poly-His-pCWeNOS plasmid was transformed into the protease-deficient Escherichia coli strain BL21 (DE3) pLysS (Novagen, San Diego, CA). Cells were grown in Luria broth with 1% glycerol containing 200 μg/mL ampicillin and 40 μg/mL chloramphenicol. Cultures were grown at 28°C until an OD600 of 0.8 was reached. Approximately 1 h before that, heme precursor δ-aminolevulinic acid (0.5 mM final concentration) was added. Cells were then induced by adding Isopropyl β-D-1 thiogalactopyranoside (IPTG) (0.8 mM final concentration); 0.5 mM adenosinetriphosphate (ATP) and 3 μM riboflavin were also added, and the cells were then grown at 22°C for a further 48 h in the dark. Cells were then harvested by centrifugation (15 min at 4000 g at 4°C). The cell pellet was resuspended in lysis buffer [40 mM N-(2-hydroxyethyl) piperazine-N-(3-propane sulfonic acid) (EPPS), pH 7.6, containing 1 mg/mL lysozyme, 150 mM NaCl, 0.5 mM L-arginine, 4 μM BH4, 2 μM FAD, 10% glycerol. A protease inhibitor cocktail (Sigma, St.Louis, MO) was added according to manufacturer's recommendation. The bacterial suspension was incubated with mild shaking at 4°C for 30 min to ensure complete cell lysis. Cells were broken by sonication using three 25 s pulses followed by three cycles of freezing and thawing. Cell debris was removed by centrifugation at 30,000 g for 30 min at 4°C. The supernatant was then applied to an Ni-NTA His-Bind Superflow (Novagen, San Diego, CA) column preequilibrated with Buffer A (40 mM EPPS, pH 7.6, containing 150 mM NaCl, 10% glycerol, and 0.5 mM L-arginine). The column was washed with five bed volumes of Buffer A followed by Buffer B (Buffer A with 25 mM imidazole). The bound protein was then eluted with Buffer C (Buffer A+200 mM imidazole). The heme-containing fractions were pooled and concentrated using Centriprep-100 YM-10 (Millipore). The concentrated protein was dialyzed against three changes of Buffer A containing 4 μM BH4 and 1 mM dithiothreitol (DTT). The protein was further purified by using a 2′,5′-adenosine-2-5-diphosphate (ADP)-sepharose column equilibrated with 40 mM Tris-buffer, pH 7.6, containing 1 mM L-arginine, 3 mM DTT, 4 μM BH4, 4 μM FAD, 10% glycerol, and 150 mM NaCl (Buffer D) and washed with Buffer D containing 400 mM NaCl to prevent nonspecific binding. eNOS was then eluted with Buffer E (Buffer D with 5 mM adenosine-2′-monophosphate (2′-AMP)). The heme-containing fractions were pooled, concentrated, and dialyzed at 4°C against Buffer D containing 1 mM DTT, 4 μM BH4, 4 μM FAD, and 10% glycerol, and stored at −80°C until used. The DTT, BH4, and FAD were removed by repeated buffer exchange using a centricon filter when required.
Biotin switch assay
Biotin switch assay was modified to incorporate peptide mapping experiments. Briefly, 100 μL of the human eNOS (0.8 μg/μL approx.) was passed through a BioSpin column preequilibrated with HEN buffer [250 mM HEPES (pH 7.7), 1 mM EDTA, and 0.1 mM neocuproine] to remove the presence of DTT. For the SNAP experiments, SNAP was added to bring the final concentration to 10 mM and incubated for 30 min in the dark at room temperature. For the GSNO experiments, a 100 mM stock of GSNO in NaHCO3 (200 mM) buffer was utilized. The solution was incubated at 37°C for 1 h. The sample was centrifuged again with a BioSpin column to remove excess nitrosylating agent (SNAP or GSNO). Excess unreacted MMTS was removed by desalting twice with the BioSpin column preequilibrated in HEN buffer. Five μL of freshly prepared 4 mM biotin-HPDP in DMSO and 4 μL of freshly prepared 100 mM sodium ascorbate were added to the sample and incubated for 1 h at 25°C. Modified protein was passed through a BioSpin column preequilibrated with 50 mM ammonium bicarbonate (pH 7.5–8.5) solution to remove excess biotin. The 0.1 mg/mL trypsin solution was prepared in 50 mM ammonium bicarbonate and added to the protein in a 1:25 (w:w) trypsin:protein ratio. Digestion was done at 37°C for 18 h. Digested peptides were lyophilized to dryness and reconstituted in water.
Separation of biotinylated peptides
A neutravidin gel-filled column was equilibrated with five gel-bed volumes of binding buffer (0.1 M sodium phosphate and 0.15 M NaCl; pH 7.2). The biotinylated sample was then applied to the column and allowed to enter the gel bed. The column flow was stopped for incubation for 30 min at room temperature. The column was then washed with 10 bed volumes of binding buffer. The biotinylated peptides bound to the column were eluted with two bed volumes of 45 mM DTT. The eluted peptides were concentrated by using C18 ZipTips prior to the mass spectrometry analysis.
Nano-LC-MS/MS analysis
Approximately 4 pmol of the modified eNOS digest was injected into a ThermoElectron (San Jose, CA) LTQ linear ion trap mass spectrometer with a nanospray ionization source with a 10 cm×75 μm, C18-packed nanospray tip. The stationary phase was Biobasic C18, 3μm particles. The flow rate was 200 nL/min with a gradient of 3–80% solution B over a 40 min period. Solvent A was 0.1% formic acid in water (v/v) and solvent B was 100% acetonitrile/0.1% (v/v) formic acid.
MALDI-TOF analysis
A Bruker Omniflex matrix-assisted laser desorption/ionization time-of-flight ( MALDI-TOF) mass spectrometer (Bruker Daltonics, Billerica, MA) equipped with a nitrogen laser was used. Mass spectra were obtained in the positive-ion mode at an acceleration voltage of 20 kV. Each mass spectrum was the sum of 100 laser shots. The matrix solution consisting of 10 mg/mL α-cyano-4-hydroxycinnamic acid (CHCA) in 50% acetonitrile/50% aqueous 0.1% trifluoroacetic acid (TFA) (v:v) was prepared fresh daily. Sample and matrix were combined in a 1:1 (v:v) ratio and immediately spotted on the target plate.
Analysis of 3D structure of eNOS
Three-dimensional structure of the oxygenase domain of eNOS was obtained from the Swissprot protein database (http://swissprot.ch) and inspected in the open-source software package Pymol version 0.97 (DeLano Scientific LLC, Palo Alto, CA; http://pymol.sourceforge.net).
Results
Determination of S-nitrosylation sites in eNOS treated with GSNO
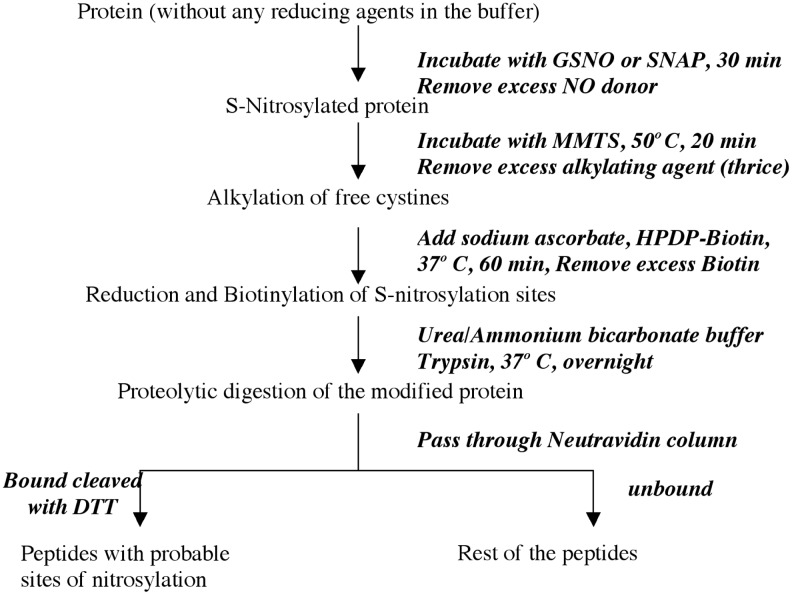
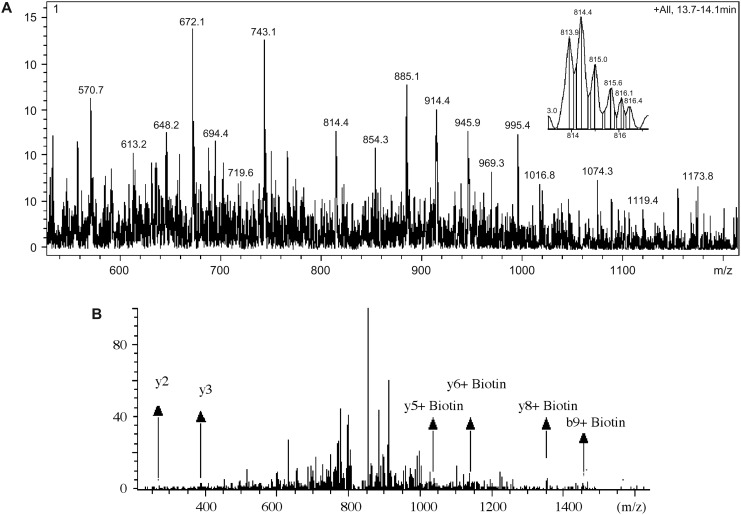
To determine the S-nitrosylation sites, the biotin switch assay was modified as shown in Figure 1. The biotin-tagged peptides were either analyzed directly or after elution from a neutravidin column. All samples were desalted with C18 ZipTips prior to analysis. Both MALDI mass spectrometry and liquid chromatography mass spectrometry (LC-MS) were used to analyze these digested peptides. Nano-LC-MS/MS of the tryptic digest of nitrosylated eNOS gave a total sequence coverage of 15%. Though the sequence coverage was low, a total of three modified tryptic peptides were identified in this digest. These peptides were m/z 597.1 and +2 charge (1102–1107) with Cys 1104, m/z 814.4 and +2 (846–855) with Cys 852, and m/z 945.8 and +2 (225–237) with Cys 234. Figure 2A shows the mass spectrum with peaks m/z 814.4 and m/z 945.8. Figure 2B shows the fragmentation (MS/MS) of the ion with m/z 814.4 in a data-dependent scan.
FIG. 1.
The protocol of biotin switch assay that was modified to incorporate peptide mass-mapping experiments for detection of sites of nitrosylation in proteins.
FIG. 2.
(A) The mass spectrum of the peaks eluted during nLC-MS/MS run of a modified eNOS digest eluted at 13.7–14.1 min. Two biotinylated peptides 814.4 Da and +2, and 945.8 and +2 were identified due to a mass shift of 428.3 Da. (B) To confirm the biotinylation, peak 814.4 Da was isolated and MS/MS of the peak was done using data-dependent scan as shown. From the partial sequencing, the peptide was confirmed to be DPRLPPCTLR with a single biotin tag attached to the cysteine residue.
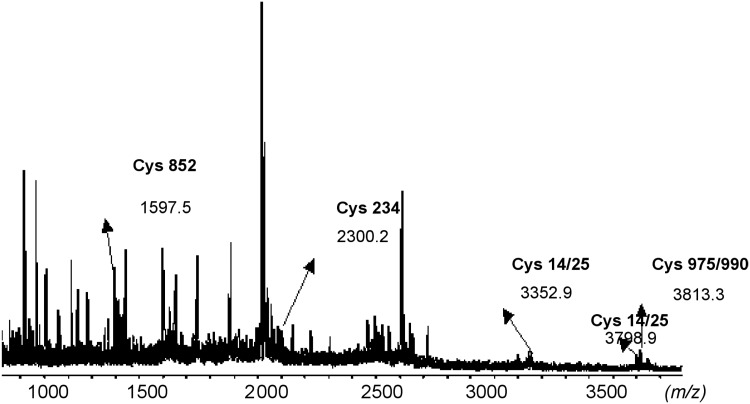
To improve the sequence coverage, MALDI-TOF mass spectrometry analysis was also performed on the same sample digest. With MALDI, the total sequence coverage was 30% and resulted in five biotinylated peptides that matched the theoretical digest. These peptides are 1597.5 Da (846–855), 2300.2 Da (221–237), 3352.7 Da (849–874), 3798.9 Da (5–39), and 3813.2 Da (963–993). The sites of these modifications were determined to be cysteines 14, 25, 234, 852, 975, and 990. As MALDI-MS seemed to give us more sequence coverage, it was decided to use this ionization technique for further analyses.
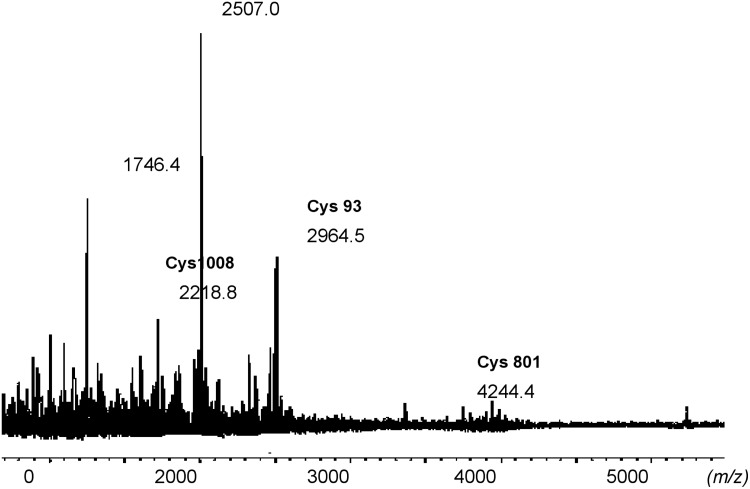
Mass spectrometry, especially MALDI-MS, is known to have suppression effects toward more hydrophobic analytes when analyzing complex mixtures (Amado et al., 1997; Krause et al., 1999; Belghazi et al., 2001; Burkitt et al., 2003). This could have an effect on our results due to the presence of highly hydrophobic moieties like biotin-HPDP on the peptides of interest. Thus, to reduce the potential suppression effects during mass spectrometry analysis and to enrich the peptides with a biotin tag, in our subsequent analyses, the digest was passed through a neutravidin column to bind the biotin-tagged peptides to the column. The biotin tag was then cleaved off with excess DTT. The eluted peptides were then analyzed with MALDI-TOF mass spectrometry. From the MALDI-MS analysis of the peptides eluted off the neutravidin column, four peptides matched the theoretical eNOS digest. These peptides were 2218.8 Da (1000–1021), 2507.0 Da (414–435), 2964.5 Da (70–96), and 4244.5 Da (776–815). These three peptides were each found to contain one cysteine. These cysteines were determined to be Cys 93, Cys 801, and Cys 1008.
Determination of S-nitrosylation sites in eNOS treated with SNAP
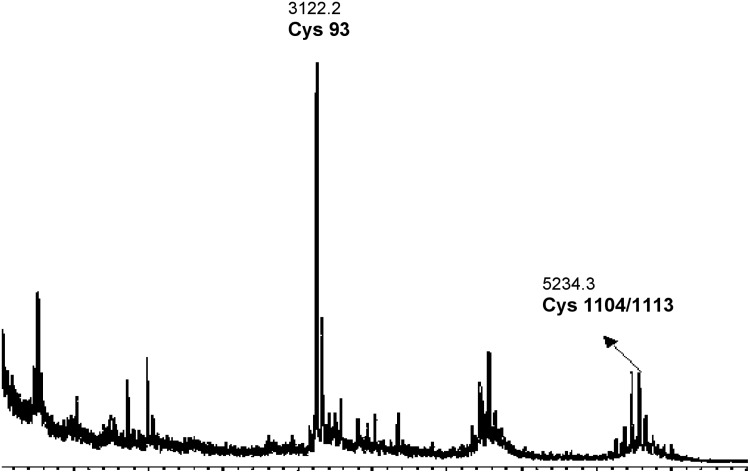
The modified biotin switch assay was also performed using SNAP as a nitrosylating agent. Only two peptides matched the theoretical eNOS digest. The peptide VKNWEV GSITYDTLSAQAQQDGPCTPRR of 3122.2 Da (70–97) had one cysteine at position 93, and peptide VLCLERGHMF VCGDVTMATNVLQTVQRILATEGDMELDEAGDVIGVLR 5234.3 Da consisted of two cysteines at positions 1104 and 1113.
The complete list of eNOS peptides with NO-modified cysteines detected in the experiments described above is given in Table 1.
Table 1.
List of All the Tryptic Peptides of Human eNOS Detected Using Mass Spectrometry Which Contained NO-Reactive Cysteine Residues
| Peptides detected | Position in eNOS sequence | Nitrosylated cysteine position | Sequence consensus motif (−2, −1, 0, 1, 2), with 0 being cysteine | Techniques used |
|---|---|---|---|---|
| 1048.1 Da, +1 | 98–106 | 98 | RRCLG | LC-MSa |
| 597.1 Da, +2 | 1102–1107 | 1104 | VLCLE | nLC-MS/MS |
| 814.4 Da, +2 | 846–855 | 852 | PPCTL | nLC-MS/MS |
| 945.8 Da, +2 | 225–237 | 234 | QRCPG | nLC-MS/MS |
| 1597.5 Da, +1 | 846–855 | 852 | PPCTL | MALDI-MS |
| 2300.2 Da, +1 | 221–237 | 234 | QRCPG | MALDI-MS |
| 3352.7 Da, +1 | 849–874 | 852 | PPCTL | MALDI-MS |
| 3798.9 Da, +1 | 05–35 | 14/25 | PPCGL/GLCGK | MALDI-MS |
| 3813.2 Da, +1 | 963–993 | 975 or 990 | GVCST or VPCFI | MALDI-MS |
| 2218.8 Da, +1 | 1000–1021 | 1008 | LPCIL | Neutravidin, GSNO, MALDI-MS |
| 2964.5 Da, +1 | 70–96 | 93 | GPCTP | Neutravidin, GSNO, MALDI-MS |
| 4244.5 Da, +1 | 776–815 | 801 | GVCPP | Neutravidin, GSNO, MALDI-MS |
| 3122.2 Da, +1 | 70–97 | 93 | GPCTP | Neutravidin, SNAP, MALDI-MS |
| 5234.3 Da, +1 | 1102–1149 | 1104 or 1113 | VLCLE or FVCGD | Neutravidin, SNAP, MALDI-MS |
Lu et al. (2005).
Discussion
NO donors like GSNO or SNAP have the potential to induce S-nitrosylation in proteins (Ji et al., 1999; Mallis and Thomas, 2000). Detection of S-nitrosylated proteins is commonly done by chemical assays like triiodide chemiluminescence and photolytic chemiluminescence (Stamler et al., 1992; Eu et al., 2000). Both these methods have limitations as they cannot be used for high-throughput analyses, require complex methodologies and equipment, and can be used for analysis of pure proteins only.
Mass spectrometry along with separation techniques like 2D gel electrophoresis has been routinely used to determine protein–protein interactions and presence of modifications and cross-linking sites in protein complexes (Kussmann and Roepstorff, 1998; Belghazi et al., 2001; Yamagata et al., 2002). A simple method to determine the tertiary structure or changes in tertiary structure of a protein complex due to physiological changes can be done by differential chemical modifications of specific sites in the protein complex followed by peptide mapping with mass spectrometry (Yang et al., 1996; Akashi et al., 1997; Hochleitner et al., 2000). In proteins, cysteines are known to be very sensitive to oxidation and can form different products under different conditions of oxidative stress, like formation of free cysteines, disulfide bonds, homocysteine, and/or nitrosothiols. Hence mass spectrometry in conjunction with differential alkylation has been commonly used to differentiate between various cysteine species in a given protein (Schniable et al., 2002; Schilling et al., 2004; Ueberheide et al., 2004; Lu et al., 2005). The biotin switch assay is a differential modification method especially developed for detecting S-nitrosylated proteins (Jaffrey et al., 2001). This method involves blocking the free cysteines in the protein or protein mixture with the thio-specific methylthiolating agent MMTS, specific decomposition of nitrosothiols using ascorbate, which results in reduction of nitrosothiols to thiols, and labeling the newly formed free thiol groups with a sulfhydryl-specific biotinylating reagent biotin-HPDP. This method is followed by either immunoblotting with antibodies against biotin or Western blotting and mass spectrometry for identifying the S-nitrosylated proteins in various cell extracts, including brain extracts, endothelial cells, and mesangial cells (Jaffrey et al., 2002; Kuncewicz et al., 2003; Martinez-Ruiz and Lamas, 2004; Lindermayr et al., 2005; Martinez-Ruiz et al., 2005; Yang and Loscalzo, 2005). Since our interest was to determine the sites of S-nitrosylation in a known protein rather than identifying the proteins that have undergone S-nitrosylation, we modified the biotin switch assay in the following fashion: after nitrosylation and following the biotin switch assay procedure, eNOS protein was digested by trypsin and peptide mass-mapping experiments were used to determine the sites of S-nitrosylation. Though nitrosylated proteins and peptides are known to be highly labile during mass spectrometry analysis, modification of nitrosothiols with biotin gives the advantage of improved stability of nitrosylated peptides in a mass spectrometer and the ease of enriching these peptides with avidin columns (Kaneko and Yoshinao, 2003). The complete list of sites of S-nitrosylation identified in this study and previous study (Taldone et al., 2005) carried out by our group is shown in Table 1. It is likely that multiple factors account for the differences in modified cysteines detected in our experiments: variations in the extent of nitrosylation; differences in MS ionization techniques used along with the peptide ionization efficiency; as well as the potential for sample loss during the cleanup and elution from the neutravidin column. The difference between the SNAP and GSNO results that employed the same analysis after nitrosylation is unclear. However, the differences may be attributed to variations in the extent of nitrosylation as well as the potential sample loss during the multiple steps in the experimental procedure.
FIG. 3.
MALDI-MS of the tryptic digest of the modified eNOS with HCCA as matrix. Five peaks that have been labeled were identified to be biotinylated with single biotin mass tag.
FIG. 4.
MALDI-MS of the peptides eluted with neutravidin column with α-cyano-4-hydroxycinnamic acid (HCCA) as the matrix. Biotin tags have been cleaved off. Three peaks that have been labeled in bold were identified to be sites of S-nitrosylation in eNOS with GSNO as NO donor.
FIG. 5.
MALDI-MS of the peptides eluted from the neutravidin column with HCCA as matrix. The biotin tags have been cleaved off. The two peaks labeled in bold were identified to be sites of S-nitrosylation in eNOS with SNAP as the NO donor.
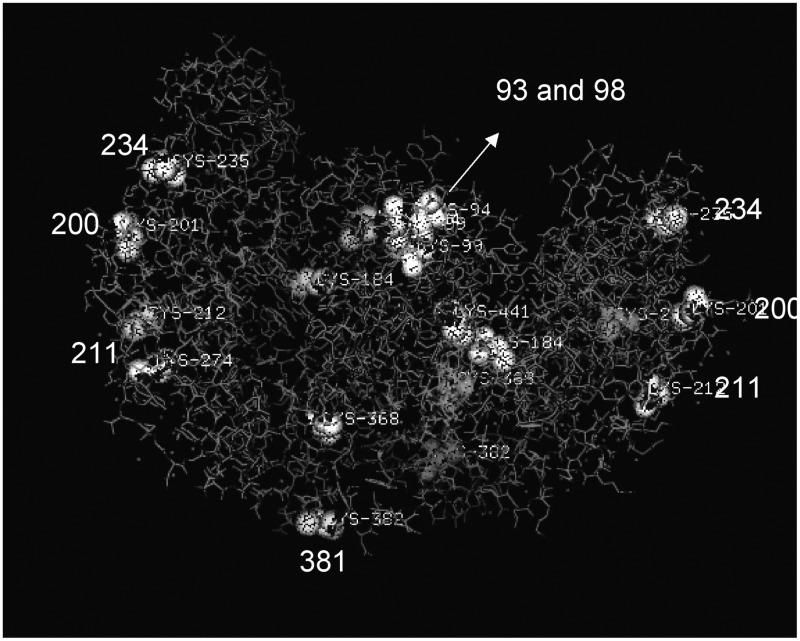
To investigate the relation of these cysteines with the 3D structure of eNOS, we obtained the X-ray structure from the Swissprot protein database and plotted it using Pymol software. Only the oxygenase domain of the human eNOS is available in the database. Figure 6 shows the X-ray structure of eNOS dimer, oxygenase domain only. All the surface cysteines (Cys 93, Cys 98, Cys 211, Cys 234, and Cys 381) were identified as sites of S-nitrosylation in our studies, except for Cys 200. In the reductase domain, we have found that regions containing cysteine-660, −801, and −1113 are known to bind with FMN, FAD, and NADPH (Mardsen et al., 1992). Other sites found to undergo S-nitrosylation (cysteines 852, 975/990, and 1047/1049) have not been implicated in any known bio-chemical functions.
FIG. 6.
X-ray structure of the oxygenase domain of human eNOS. The sequence was obtained from the Swissprot protein database. The surface cysteines have been labeled, and the structure is shown as a homodimer.
Cys 93, one of the cysteines involved in the Zn tetrathiolate dimer binding of eNOS, was found to undergo S-nitrosylation. The other cysteine involved in Zn binding, Cys 98, was detected as one of the nitrosylated sites by LC-MS and reported in our previous study (Taldone et al., 2005). We have not observed peptides containing this cysteine in MALDI-MS experiments. This might be because of the fact that the tryptic peptide containing Cys 98 (K)CLGSLVFPR has very high hydrophobicity (51.5% Bull and Breese hydrophobicity index with the mass of 991.5 Da). Further addition of a bulky hydrophobic biotin tag onto this peptide might make it either too difficult to elute off the C18 ZipTips or make it difficult to ionize in the mass spectrometer due to the suppression effects (Amado et al., 1997; Krause et al., 1999; Belghazi et al., 2001; Burkitt et al., 2003). In our previous study we modified the nitrosylated cysteines with iodoacetamide, and hence it was easier to detect that eNOS peptide containing Cys 98 (Taldone et al., 2005). Nonetheless, the fact that both cysteines involved in the formation of eNOS dimer are found nitrosylated supports our previous finding that the monomerization of eNOS homodimer in the presence of NO donors might be due to the S-nitrosylation of Zn-binding cysteines (Ravi et al., 2004).
Inspection of the sequence of peptides with active cysteines showed the presence of sequence consensus motif for Cys 200: (Ser)(Arg)Cys(Asp). The amino acid residues flanking the rest of the NO-reactive cysteines detected in these experiments did not conform to the (Gly/Ser/Thr/Cys/Tyr/Asn/Gln)(Lys/Arg/Asp/Gln)Cys(Asp/Gln), the (−2)(−1)Cys(1) sequence consensus motif proposed by Stamler et al. (1997) for effective catalysis of Cys-nitrosylation/denitrosylation processes. Further examination of the 3D structure of the oxygenase domain of eNOS, with focus on Cys 93, Cys 98, and Cys 234, revealed the presence of basic arginine residues in the proximity of all these cysteines. Cys 93 is flanked by Arg 97, Thr 94, and Cys 98. Similarly, Cys 234 is flanked by Arg 233 and Gln 232 on one side and Arg 237 and Gly 238 on the other side. Similar anomalies in Cys environments have been reported in other proteins containing of NO-reactive cysteines. For example, proteins human c-Fos and human c-Jun have basic residues flanking both sides of the NO-reactive cysteines, and human immunodeficiency virus-1 (HIV1) aspartic protease with NO-reactive Cys 67 is known to be flanked by His 69 and Thr 12 (Ascenzi et al., 2000). These results reaffirm the fact that Tyr/Lys/Arg/His/Asp/Glu may act as either acid or base depending on the microenvironment of the protein. Besides these amino acids, water molecules might also act as catalysts in Cys-nitrosylation and Cys-NO-denitrosylation processes (Ascenzi et al., 2000).
In conclusion, we have developed a modification of the biotin switch assay to optimize this method for the detection of sites of S-nitrosylation in proteins by peptide mapping. Multiple cysteine residues of human eNOS protein were identified as undergoing S-nitrosylation in the presence of an access of a nitrosylating agent. According to the crystal structure of the oxygenase domain of eNOS, these cysteine residues are located on the surface of the protein. Among the residues found to undergo S-nitrosylation are Cys 93 and Cys 98, the cysteines involved in the formation of eNOS homodimer through a Zn tetrathiolate cluster. This is in good agreement with our previous work that implicated the S- nitrosylation of these residues in the disruption of eNOS dimer. However, it should be noted that the functional significance of the other S-nitrosylated sites we have identified is not clear from these studies and warrants further study.
Acknowledgments
This research was supported in part by grants HL60190 (to S.M.B.), HL67841 (to S.M.B.), HL72123 (to S.M.B.), and HL70061 (to S.M.B.) all from the National Institutes of Health, and by a grant from the Fondation Leducq (to S.M.B.). The authors also thank Dr. Liam Moran and Mr. Gerald Koncar of ThermoElectron Corporation for the help with nano-LC-MS/MS experiments.
References
- Akashi S., Shirouza M., Terada T., Ito Y., Yokoyama S., and Takio K. (1997). Characterization of the structural difference between active and inactive forms of the ras protein by bio-chemical modification followed by mass spectrometric peptide mapping. Anal Biochem 248, 15–25 [DOI] [PubMed] [Google Scholar]
- Amado F.M.L., Dominggues P., Santana Marques M.G., Ferrer-Correia A.J., and Tomer K.B. (1997). Discrimination effects and sensitivity variations in matrix-assisted laser/ionization mass spectrometry. Rapid Commun. Mass Spectrom 11, 1347–1352 [Google Scholar]
- Ascenzi P., Colasanti M., Persichini T., Muolo M., Polticelli F., Venturini G., Bordo D., and Bolognesi M. (2000). Re-evaluation of amino acid sequence and structural consensus rules for cysteine-nitric oxide reactivity. Biol Chem 381, 623–627 [DOI] [PubMed] [Google Scholar]
- Ascenzi P., Salvati L., Bolognesi M., Colasanti M., Polticelli F., and Venturini G. (2001). Inhibition of cysteine protease activity by NO-donors. Curr Protein Pept Sci 2, 137–153 [DOI] [PubMed] [Google Scholar]
- Belghazi M., Bathany K., Hountondji C., Grandier-Vazeille X., Manon S., and Schmitter J.-M. (2001). Analysis of protein sequences and protein complexes by matrix-assisted laser desorption/ionization mass spectrometry. Proteomics 1, 946–954 [DOI] [PubMed] [Google Scholar]
- Bredt D.S., Huang P.M., Glatt C.E., Lowenstein C.J., Reed R.R., and Snyder S.H. (1991). Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 351, 714–718 [DOI] [PubMed] [Google Scholar]
- Bredt D.S., and Snyder S.H. (1990). Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 90, 6252–6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt W.I., Giannakopulos A.E., Sideridou F., Bashir S., and Derrick P.J. (2003). Discrimination effects in MALDI-MS of mixtures of peptides-analysis of the proteome. Aust J Chem 56, 369–377 [Google Scholar]
- Chen P.-F., Tsai A.-L., and Wu K.K. (1994). Cysteine 184 of endothelial nitric oxide synthase is involved in heme coordination and catalytic activity. J Biol Chem 269, 25062–25066 [PubMed] [Google Scholar]
- Eu J.P., Liu L., Zeng M., and Stamler J.S. (2000). An apoptotic model for nitrosative stress. Biochemistry 39, 1040–1047 [DOI] [PubMed] [Google Scholar]
- Govers R., and Rabelink T.J. (2001). Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 280, F193–F206 [DOI] [PubMed] [Google Scholar]
- Hemmens B., Goessler W., Schmidt K., and Mayer B. (2000). Role of bound zinc in dimer stabilization but not enzyme activity of neuronal nitric-oxide synthase. J Biol Chem 275, 35786–35791 [DOI] [PubMed] [Google Scholar]
- Hochleitner E.O., Borchers C., Parker C., Beinstock R.J., and Tomer K.B. (2000). Characterization of a discontinuous epitope of human immunodeficiency virus (HIV) core protein p24 by epitope excision and differential chemical modification followed by mass spectrometric peptide mapping analysis. Protein Sci 9, 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L.J. (1990). Annu Rev Pharmocol Toxicol 30, 535–560 [DOI] [PubMed] [Google Scholar]
- Jaffrey S.R., Erdjument-Bromage H., Ferris C.D., Tempst P., and Snyder S.H. (2001). Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3, 193–197 [DOI] [PubMed] [Google Scholar]
- Jaffrey S.R., Fang M., and Snyder S.H. (2002). Nitrosopeptide mapping: a novel methodology reveals S-nitrosylation of Dexras1 on a single cysteine residue. Chem Biol 9, 1329–1335 [DOI] [PubMed] [Google Scholar]
- Ji Y., Akerboom T.P., Sies H., and Thomas J.A. (1999). S-nitrosylation and S-glutathiolation of protein sulfhydryls by S-nitroso glutathione. Arch Biochem Biophys 362, 67–78 [DOI] [PubMed] [Google Scholar]
- Kaneko R., and Yoshinao W. (2003). Decomposition of protein nitrosothiol in matrix-assisted laser desorption/ionization and electrospray ionization mass spectrometry. J Mass Spectrom 38, 526–530 [DOI] [PubMed] [Google Scholar]
- Kashiba-Iwatsuki M., Kitoh K., Kasahara E., Yu H., Nisikawa M., Matsuo M., and Inoue M. (1997). Ascorbic acid and reducing agents regulate fate and function of S-nitrosothiols. J Biol Chem 122, 1208–1214 [DOI] [PubMed] [Google Scholar]
- Krause E., Wenschuh H., and Jungbult P.R. (1999). The dominance of arginine-containing peptides in MALDI-derived tryptic mass fingerprints of proteins. Anal Chem 71, 4160–4165 [DOI] [PubMed] [Google Scholar]
- Kuncewicz T., Sheta E.A., Goldknop I.L., and Kone B.C. (2003). Proteomic analysis of S-nitrosylated proteins in mesangial cells. Mol Cell Proteomics 2.3, 156–163 [DOI] [PubMed] [Google Scholar]
- Kussmann M., and Roepstorff P. (1998). Characterization of the covalent structure of proteins from biological material by MALDI mass spectrometry—possibilities and limitations. Spectroscopy 14, 1–12 [Google Scholar]
- Lander H.M., Hajjar D.P., Hempstead B.L., Mirza U.A., Chait B.T., Campbell S., and Quilliam L.A. (1997). A molecular redox switch on p21(ras). Structural basis for the nitric oxide-p21(ras) interaction. J Biol Chem 272, 4323–4326 [DOI] [PubMed] [Google Scholar]
- Lindermayr C., Saalbach G., and Durner J. (2005). Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137, 921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Yan Y., Zeng M., Zhang J., Hanes M.A., Ahearn G., McMahon T.J., Dickfeld T., Marshall H.E., Que L.G., and Stamler J.S. (2004). Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 116, 617–628 [DOI] [PubMed] [Google Scholar]
- Lu X.-M., Lu M., Tompkins R.G., and Fischman A.J. (2005). Site-specific detection of S-nitrosylation PKBα/Akt1 from rat soleus muscle using CapLC-Q-Tofmicro mass spectrometry. J Mass Spectrom 40, 1140–1148 [DOI] [PubMed] [Google Scholar]
- Mallis R.J., and Thomas J.A. (2000). Effect of S-nitrosothiols on cellular glutathione and reactive protein sulfhydryls. Arch Biochem Biophys 383, 60–69 [DOI] [PubMed] [Google Scholar]
- Mardsen P.A., Schappert K.T., Chen H.S., Flowers M., Sundell C.L., Wilcox J.N., Lamas S., and Michel T. (1992). Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett 307, 287–293 [DOI] [PubMed] [Google Scholar]
- Marshall H.E., and Stamler J.S. (2001). Inhibition of NF-kappa B by S-nitrosylation. Biochemistry 40, 1688–1693 [DOI] [PubMed] [Google Scholar]
- Martinez-Ruiz A., and Lamas S. (2004). Detection and proteomic identification of S-nitrosylated proteins in endothelial cells. Arch Biochem Biophys 423, 192–199 [DOI] [PubMed] [Google Scholar]
- Martinez-Ruiz A., Villanueva L., Orduna C.G., Lopez-Ferrer D., Higueras M.A., Tarin C., Rodriguez-Cresp I., Vazquez J., and Lamas S. (2005). S-nitrosylation of H90 promotes the inhibition of its ATPas endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci USA 102, 8525–8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Greenwood E., Jenkins C., Farrow K.N., Konduri G., Russell J., Lakshminrusimha S., Black S.M., and Steinhorn R.H. (2006). eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol 290, L232–L241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Morrell C.N., Cambien B., Yang S.X., Yamakuchi M., Bao C., Hara M.R., Quick R.A., Cao W., O'Rourke B., Lowenstein J.M., Pevsner J., Wagner D.D., and Lowenstein C.J. (2003). Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115, 139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan K., and Masters B.S.S. (1993). Optical difference spectrophotometry as a probe of rat brain nitric oxide synthase heme-substrate interaction. Biochemistry 32, 9875–9880 [DOI] [PubMed] [Google Scholar]
- Michel T., and Feron O. (1997). Nitric oxide synthases: which, where, how, and why? J Clin Invest 100, 2146–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R.M.J., and Higgs E.A. (1991). Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43, 109–142 [PubMed] [Google Scholar]
- Nathan C., and Xie Q.W. (1994). Regulation of biosynthesis of nitric oxide. J Biol Chem 269, 13725–13728 [PubMed] [Google Scholar]
- Nishimura J.S., Martasek P., McMillan K., and Salerno J.C. (1995). Modular structure of neuronal nitric oxide synthase: localization of the arginine binding site and modulation by pterin. Biochem Biophys Res Commun 210, 288–294 [DOI] [PubMed] [Google Scholar]
- Ravi K., Brennan L.A., Levic S., Ross P.A., and Black S.M. (2004). S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA 101, 2619–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J.P., Boucher J.L., Vadon S., Delafoge M., and Mansuy D. (1993). Particular ability of liver P450s3A to catalyze the oxidation of N omega-hydroxyarginine to citrulline and nitrogen oxides and occurrence in no synthases of a sequence very similar to the heme-binding sequence in P450s. Biochem Biophys Res Commun 192, 53–60 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Crespo I., and Ortizde Montellano P.R. (1996). Human endothelial nitric oxide synthase: expression in Escherichia coli, coexpression with calmodulin, and characterization. Arch Biochem Biophys 336, 151–156 [DOI] [PubMed] [Google Scholar]
- Rossig L., Fichtlscherer B., Breitschopf K., Haendeler J., Zeiher A.M., Mulsch A., and Dimmeler S. (1999). Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem 274, 6823–6826 [DOI] [PubMed] [Google Scholar]
- Schilling B., Yoo C.B., Collins C.J., and Gibson B.W. (2004). Determining cystein oxidation status using differential alkylation. Int J Mass Spectrom 236, 117–127 [Google Scholar]
- Schniable V., Wefing S., Bucker A., Wolf-Kummeth S., and Hoffmann D. (2002). Partial reduction and two step modification of proteins for identification of disulfide bonds. Anal Chem 74, 2386–2393 [DOI] [PubMed] [Google Scholar]
- Stamler J.S. (1994). Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78, 931–936 [DOI] [PubMed] [Google Scholar]
- Stamler J.S., Jaraki O., Osbourne J., Simon D.I., Keaney K., Vita J., Singel D., Valeri C.R., and Lascalzo J. (1992). Nitric oxide circulates in mammalian plasma primarily as a S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA 89, 7674–7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J.S., Lamas S., and Fang F.C. (2001). Nitrosylation. The prototypic redox-based signaling mechanism. Cell 106, 675–683 [DOI] [PubMed] [Google Scholar]
- Stamler J.S., Toone E.J., Lipton S.A., and Sucher N.J. (1997). (S)NO signals: translocation, regulation, and a consensus motif. Neuron 18, 691–696 [DOI] [PubMed] [Google Scholar]
- Stuehr D.J. (1999). Mammalian nitric oxide synthases. Biochim Biophys Acta 1411, 217–230 [DOI] [PubMed] [Google Scholar]
- Taldone F.S., Tummala M., Goldstien E.J., Ryzhov V., Ravi K., and Black S.M. (2005). Studying the S-nitrosylation of model peptides and eNOS protein by mass spectrometry. Nitric Oxide 13, 176–187 [DOI] [PubMed] [Google Scholar]
- Ueberheide B., Shabanowitz J., and Hunt D.F. (2004). Identification of S-nitrosylated Cysteines. In 52nd ASMS Conference on Mass Spectrometry and Allied Topics Nashville, TN [Google Scholar]
- Voss A.A., Lango J., Ernst-Russell M., Morin D., and Pessah I.N. (2004). Identification of hyperreactive cysteines within ryanodine receptor type 1 by mass spectrometry. J Biol Chem 279, 34514–34520 [DOI] [PubMed] [Google Scholar]
- Wedgewood S., Steinhorn R.H., Bunderson M., Wilham J., Lakshminrusimha S., Brennan L.A., and Black S.M. (2005). Increased hydrogen peroxide downregulates soluble guanylate cyclase in lungs of lambs with persistent hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 289, L660–L666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata A., Kristensen D.B., Takeda Y., Miyamoto Y., Okada K., Inamatsu M., and Yoshizato K. (2002). Mapping of phosphorylated proteins on two-dimensional polyacrylamide gels using protein phosphatase. Proteomics 2, 1267–1276 [DOI] [PubMed] [Google Scholar]
- Yang T., Horejsh D.R., Mhan K.J., Zaluzec E.J., Watson T.J., and Gage D.A. (1996). Mapping cross-linking sites in modified proteins with mass spectrometry: an application to cross linking hemoglobins. Anal Biochem 242, 55–63 [DOI] [PubMed] [Google Scholar]
- Yang Y., and Loscalzo J. (2005). S-nitrosoprotein formation and localization in endothelial cells. Proc Natl Acad Sci USA 102, 117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]