Abstract
Purpose: Mucosal melanoma is a rare but aggressive subtype of melanoma with unique clinicopathologic features. We hypothesize that mucosal melanoma shows predilection for separate and unique metastatic pathways. Materials and methods: This was a retrospective analysis of 19 patients (5 men and 14 women; median age 60 years, range 38–76 years) with metastatic mucosal melanoma presenting to a tertiary oncology center between 2005 and 2010. We performed a review of medical records and histologic and imaging studies to evaluate the natural history, metastatic patterns and the role of imaging in the management of patients with advanced mucosal melanoma. Results: At presentation, disease was confined to the primary site (58%, n = 11) or to the regional lymph nodes (32%, n = 6) in most patients. The most common site of metastasis was the lungs (89%, n = 16), followed by the liver (67%, n = 12) and peritoneum (44%, n = 8). Sinonasal melanoma preferentially spread to the liver (100%, n = 4), vaginal melanoma to the lungs (100%, n = 7) and anal melanoma to the inguinal lymph nodes (100%, n = 4). Conclusion: Pathways of metastatic spread in mucosal melanoma may differ from other forms of melanoma and between different primary sites of mucosal origin.
Keywords: Melanoma, mucous membrane, diagnostic imaging, tumor metastasis, integrated PET/CT
Introduction
Mucosal melanoma encompasses a group of malignant that arise from melanocytes located in the epithelia of the nasal cavity, oropharynx, gastrointestinal tract, and genitorurinary tract. Mucosal melanoma is a rare entity, accounting for only 1.3% of all cases of melanoma[1]. However, these tumors are more aggressive than their cutaneous counterpart and approximately one-third of patients with mucosal melanoma present with advanced disease[2,3]. Management of mucosal melanoma also presents unique challenges and despite aggressive surgical intervention and adjuvant therapies, the overall 5-year survival remains at only 25%[2].
The biological behavior of mucosal melanoma is not well understood and explanations for its more aggressive behavior have been proposed, including delays in clinical presentation and diagnosis, the rich lymphatic and vascular supply of the mucosal surfaces, and an inherent more aggressive biology[3,4]. In contrast to cutaneous melanoma, there are no known risk factors for mucosal melanoma and there is no known premalignant phase[5], although it has recently been shown that cutaneous melanoma and mucosal melanoma may occur in the same patient and mucosal melanoma may develop in a familial setting[6]. The incidence of primary mucosal melanoma is greater in females, African American populations and in an older age group of patients than cutaneous melanoma[7]. Cellular morphologic diversity has been described in mucosal melanoma and the lesions frequently lack melanin pigment, particularly in oral mucosal melanoma, which can make the histologic diagnosis challenging[8]. Recent advances in gene sequencing have shown an increased prevalence of c-KIT mutation in mucosal melanoma, and a significantly lower expression of mutations in the BRAF and NRAS oncogenes compared with cutaneous melanoma[9–12].
The diagnosis of mucosal melanoma is usually made on the basis of histology and immunohistochemistry[2]. Radiologic evaluation is important for the purposes of staging, operative planning and monitoring of patients with metastatic disease undergoing systemic treatment. Computed tomography (CT) and positron emission tomography (PET)/CT are of relatively limited value in local disease evaluation but are primarily used to detect clinically unsuspected metastatic disease[2]. The role of [18F]fluorodeoxyglucose (FDG)-PET/CT in cutaneous melanoma management is well established[13,14]. Its role in mucosal melanoma is less well investigated, but given the high metabolic activity of these tumors, it is likely to provide similar staging information but this requires validation in large-scale trials.
The natural course and metastatic pathways for other types of melanoma have been described previously[15–17]. Because of the rarity of mucosal melanoma, little is known about its natural progression. We hypothesize that mucosal melanoma arising from different primary sites shows a predilection for separate and unique metastatic pathways. The aim of this study is to present our clinical experience with multimodality imaging of this rare tumor at a tertiary referral oncology center.
Materials and methods
Patient population
Institutional Review Board (IRB) approval was obtained for this study. A retrospective case-based analysis was performed of all patients with metastatic primary mucosal melanoma (19 patients; 5 men and 14 women; median age 60 years, age range 38–76 years) who attended a single tertiary referral cancer center over a 5-year period from January 2005 to February 2010. In all cases, the site of primary melanoma was confirmed histologically and primary sites included the anus, rectum, vagina and sinonasal cavity. Clinical data were obtained from a review of medical records, including demographics, date of diagnosis, site of primary tumor, size and depth of primary tumor and disease stage at presentation. A review of all imaging studies and pathology reports for each patient was performed and the number, dates and types of studies were documented.
Regional lymph node metastasis
Regional lymph node metastasis was defined as histologically proven disease in the primary draining lymph node basin for each primary site. These sites included cervical nodes (sinonasal primary), pelvic nodes (vaginal and rectal primary) and inguinal nodes (vaginal and anal primary). Histologic diagnosis was confirmed in all cases by means of fine-needle aspirate, sentinel node biopsy, and/or surgical lymph node resection. In patients without regional lymph node metastasis at presentation, the time to development of regional lymph node involvement was recorded.
Distant metastasis
Sites of distant metastatic disease detected on imaging studies were confirmed by biopsy with histologic confirmation in some cases or by clinical or radiologic follow-up. The presence and locations of distant metastatic disease at presentation was recorded. In patients without distant metastasis at presentation, the time to development of metastatic disease was recorded. Sites of subsequent sites of metastatic disease involvement were also evaluated for each case.
Treatment and outcomes
For each patient, treatment with surgical resection of the primary site and/ or local radiation therapy was recorded in the database. Whether or not systemic therapy was administered was also recorded. Clinical follow-up was defined as the interval from diagnosis to patient death, end of the study period, or until the patient was lost to follow-up.
Statistics
Standard descriptive statistics, tabulation and univariate analysis were used to evaluate the patient characteristics, tumor stage at presentation, site of metastases and time to detection of metastases.
Results
Patient population
The total study population included 19 patients with metastatic mucosal melanoma with a mean clinical follow-up period of 22.3 months (range 8–47 months). Patient demographics are presented in Table 1. The most common primary site was the vagina (n = 7), followed by the anus (n = 4), rectum (n = 4) and sinonasal cavity (n = 4). The median age was younger for patients with anal and sinonasal melanoma (50 years and 51 years respectively), than for patients with rectal or vaginal melanoma (66.5 years and 61 years respectively). A female predominance was seen for rectal melanoma (4 women, 0 men), whereas there was an equal gender distribution for anal melanoma (2 women, 2 men).
Table 1.
Patient characteristics, size of primary tumor, tumor stage at presentation and treatment types in a cohort of patients with advanced mucosal melanoma
| Anal (n = 4) | Rectal (n = 4) | Vaginal (n = 7) | Sinonasal (n = 4) | Total (N = 19) | |
|---|---|---|---|---|---|
| Sex (M/F) | 2/2 | 0/4 | 0/7 | 3/1 | 5/14 |
| Age at diagnosis | |||||
| Median (years) | 50 | 66.5 | 61 | 51 | 60 |
| Range (years) | 40–62 | 60–76 | 38–73 | 50–64 | 38–76 |
| Size of primary tumor | |||||
| Median (mm) | 13.5 | 38 | 27.5 | N/A | 25 |
| Range (mm) | 4–20 | 30–40 | 11–35 | 4–40 | |
| Disease stage at presentation | |||||
| Localized disease (n) | 2 | 1 | 5 | 3 | 11 |
| Regional lymph node involvement (n) | 2 | 1 | 2 | 1 | 6 |
| Distant metastases (n) | 0 | 2 | 0 | 0 | 2 |
| Surgical resection of primary tumor (n) | 4 | 3 | 7 | 3 | 17 |
| Radiation to primary site (n) | 2 | 1 | 4 | 3 | 10 |
Initial tumor staging and characteristics of the primary tumor
The initial tumor staging for each primary site is shown in Table 1. Most cases at presentation were confined to the local primary site (58%, n = 11) or to the regional lymph nodes (32%, n = 6). Only 2 of 19 patients had clinical or radiologic evidence of distant metastatic disease at presentation. Median size of the primary tumor, where available (n = 14) was 25 mm (range 4–40 mm). The largest primary tumors arose in the rectum, with a median size of 38 mm (range 30–40 mm).
FDG-PET/CT was used for staging in 18 of 19 patients (95%) and was also performed for follow-up in a number of cases. Magnetic resonance imaging was used for localization and staging of the primary site in all cases of sinonasal lymphoma (n = 4) and most cases of vaginal melanoma (n = 6). The most common staging examination performed overall was CT of the chest, abdomen and pelvis (total 100 studies, mean 5.3 studies per patient, range 0–13), followed by PET/CT (total 69 studies, mean 3.6 studies per patient, range 0–9).
Regional lymph node metastasis
Distribution and timing of regional lymph node involvement is shown in Table 2. Regional lymph node involvement was commonest with anal mucosal melanoma (100%, n = 4). Vaginal melanoma rarely involved the iliac (n = 1) or inguinal (n = 1) nodal regions. Overall, 6 of 19 (32%) patients had regional nodal disease at presentation with a further 6 patients (32%) developing regional lymph node metastasis during subsequent follow-up. In cases where regional lymph node metastasis developed during follow-up, the median time to occurrence was 7 months (range 3–18 months).
Table 2.
Presence or absence of regional nodal or distant metastases in a cohort of patients with mucosal melanoma at initial presentation and during follow-up
| Anal (n = 4) | Rectal (n = 4) | Vaginal (n = 7) | Sinonasal (n = 4) | Total (N = 19) | |
|---|---|---|---|---|---|
| Metastasis to regional lymph nodes | |||||
| At presentation (n) | 2 | 2 | 1 | 1 | 6 |
| During follow-up (n) | 2 | 1 | 1 | 2 | 6 |
| Interval from initial presentation to lymph node metastasis | |||||
| Median (months) | N/A | N/A | N/A | N/A | 7 |
| Range (months) | 3–5 | 18 | 4 | 9–16 | 3–18 |
| Distant metastases | |||||
| At presentation (n) | 0 | 2 | 0 | 0 | 2 |
| During follow-up (n) | 3 | 2 | 7 | 4 | 16 |
| Interval from initial presentation to distant metastases | |||||
| Median (months) | 5 | N/A | 19 | 11 | 16 |
| Range (months) | 3–20 | 4–18 | 9–36 | 1–25 | 1–36 |
In patients who did not initially present with advanced disease, the time interval (in months) is shown between presentation and the development of metastatic disease. Most patients (2 of 19) did not have distant metastatic disease at presentation.
Distant metastasis
Eighteen of 19 patients (95%) had evidence of distant metastatic disease either at presentation or during the course of their disease (Table 2). Of these cases, 16 developed during follow-up. In patients without distant metastases at presentation, median time interval to occurrence was 15 months (range 1–36 months). The first sites of distant metastases are shown in Table 3. The commonest initial site of distant metastasis was the lungs (66%, n = 12), followed by the liver (50%, n = 9) and peritoneum (33%, n = 6). Peritoneal metastasis was more common in patients with vaginal melanoma than other primary sites and was associated with local recurrence in all these cases. In addition, lung metastases were seen as an initial metastatic site in all patients with vaginal melanoma (n = 7).
Table 3.
First sites of radiologically detected metastases in patients with metastatic mucosal melanoma (N = 18)
| Site | Anal (n = 3) | Rectal (n = 4) | Vaginal (n = 7) | Sinonasal (n = 4) | Total (N = 18) |
|---|---|---|---|---|---|
| Lung, n (%) | 2 (67) | 2 (50) | 7 (100) | 1 (25) | 12 (67) |
| Liver, n (%) | 1 (33) | 3 (75) | 4 (57) | 1 (25) | 9 (50) |
| Peritoneum, n (%) | 1 (33) | 1 (25) | 3 (43) | 1 (25) | 6 (33) |
| Skin and muscular, n (%) | 1 (33) | 1 (25) | 0 (0) | 1 (25) | 3 (16) |
| Bone, n (%) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
Overall patterns of distant metastases during the course of disease are shown in Table 4. Anal melanoma spread to the inguinal lymph nodes in all cases but rarely spread to the liver (n = 1) and peritoneum (n = 1). Conversely, rectal melanoma showed a predilection for liver (n = 3) and peritoneal (n = 2) involvement. Sinonasal melanoma metastasized to the liver in all cases (n = 4) and peritoneal disease was seen in 50% (n = 2) of patients. Metastases to the central nervous system (CNS) occurred infrequently (n = 5 of 19) and late in the course of disease. No CNS metastasis was seen in patients with sinonasal primaries. Metastasis to the musculoskeletal soft tissues (e.g. skin and muscle) was also infrequent (16%, n = 3).
Table 4.
Common sites of metastatic mucosal melanoma detected by imaging studies during the course of disease from diagnosis to death or end of follow-up period compared with cutaneous melanoma
| Site | Anal (n = 3) | Rectal (n = 4) | Vaginal (n = 7) | Sinonasal (n = 4) | Total (n = 18) | Cutaneous melanoma, %a |
|---|---|---|---|---|---|---|
| Lung, n (%) | 2 (66) | 4 (100) | 7 (100) | 3 (75) | 16 (89) | 18–36 |
| Liver, n (%) | 1 (33) | 3 (75) | 4 (57) | 4 (100) | 12 (67) | 14–20 |
| Peritoneum, n (%) | 1 (33) | 2 (50) | 3 (43) | 2 (50) | 8 (44) | N/A |
| CNS, n (%) | 1 (33) | 2 (50) | 2 (29) | 0 (0) | 5 (28) | 2–20 |
| Bone, n (%) | 1 (33) | 1 (25) | 0 (0) | 1 (25) | 3 (16) | 4–17 |
| Skin and subcutaneous, n (%) | 1 (33) | 1 (25) | 0 (0) | 1 (25) | 3 (16) | 37–59 |
| Gastrointestinal tract, n (%) | 0 (0) | 1 (25) | 0 (0) | 1 (25) | 2 (11) | 1–8 |
aFrom Leiter et al.[15]. These figures represent sites of metastatic cutaneous melanoma detected by clinical and imaging techniques. Autopsy-detected metastatic sites are not included.
Regional lymph node metastasis preceded distant metastatic disease in 33% (n = 6) of patients, with a median time interval from lymph node involvement to distant metastasis of 13 months (range 4–36 months). In 28% (n = 5) of cases, distant disease presented synchronously with regional nodal disease. In 39% (n = 7), metastasis to distant organs was seen in the absence of regional lymph node disease. Of these patients, most (n = 5) had vaginal primary melanoma.
Treatments and outcomes
Seventeen of 19 patients underwent surgical resection of the primary tumor site. Two patients did not undergo resection due to metastatic disease at presentation. Radiation therapy to the primary site was administered in 10 of 19 patients. Sixteen of 19 patients received systemic therapy.
Local disease recurrence developed in 71% (n = 5) of patients with vaginal melanoma with a median time to recurrence of 11 months (range 5–33 months) and in 75% (n = 3) of patients with rectal melanoma with a median time to recurrence of 3 months (range 2–30 months). There were no cases of locally recurrent disease with anal or sinonasal melanoma.
Of the initial 19 patients, 7 are still alive with a median follow-up period of 16 months (range 8–25 months). Three patients were lost to follow-up. Nine of 19 patients are deceased with a median survival of 33 months (range 28–47 months). After a diagnosis of distant metastatic disease, median survival time was 15 months (range 4–30 months).
Discussion
The results of this study illustrate the unique natural history of mucosal melanoma and important features of its biological behavior. In addition, although the study is limited by small numbers, important trends are shown that suggest different metastatic pathways for mucosal melanoma arising from anatomically separate primary sites.
The aggressiveness of mucosal melanoma may be explained by its late presentation and delayed diagnosis, the vascularity of the mucous membranes, which promotes hematogenous metastases, or by cellular and molecular differences that have been shown to exist between cutaneous and mucosal melanoma[2–5,18]. The results of this study show that in addition to these differences, the metastatic pathways of mucosal melanoma may also be quite unique. Dermal and subcutaneous metastases were rare in our patient cohort, in contrast to cutaneous melanoma where they are among the most frequent metastatic sites[15]. Mucosal melanoma, and in particular vaginal melanoma, shows a predilection for early peritoneal tumor spread, which was found in almost one-third of cases and from all primary sites. In vaginal melanoma, peritoneal metastasis was associated with local recurrence in all cases. This finding is most likely explained by the close anatomic relationship of the vagina to the peritoneal surface. Peritoneal tumor spread is rare in cutaneous melanoma and usually seen in the later stages of disseminated metastatic disease[19].
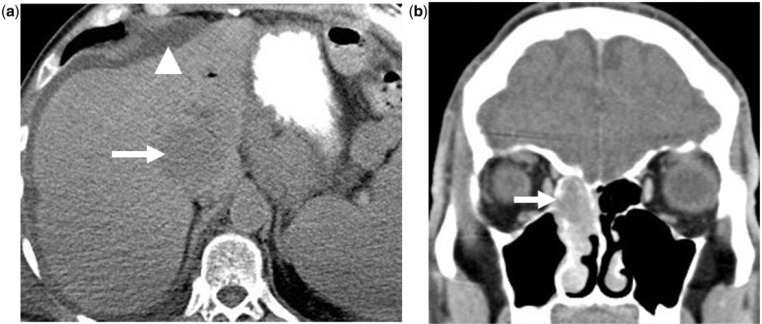
Figure 1.
Mucosal melanoma of the nasal cavity: a 52-year-old man who presented with epistaxis and nasal stuffiness. (a) Coronal unenhanced CT of the nasal sinuses shows a soft tissue mass in the right nasal cavity (arrow); (b) 9-month follow-up axial unenhanced CT of the upper abdomen shows new liver metastasis (arrow) and ascites (arrowhead).
The most frequent site of distant metastatic disease overall was the pulmonary parenchyma. All patients with vaginal melanoma developed lung metastases and this was the initial site of hematogenous spread in each of these cases. This highlights the importance of close attention to the lung parenchyma during radiologic surveillance of patients with vaginal melanoma. Mucosal melanoma originating from the sinonasal mucosa spread to the liver in all cases. The reason for this finding is unclear although a similar pattern has previously been described with uveal and oral mucosal primary melanoma[20–22].
Imaging studies in our patient cohort played an important role during all stages of disease, including diagnosis, treatment planning, staging, surveillance and monitoring of treatment response. Although contrast-enhanced CT was the most frequently used staging examination, FDG-PET/CT was used in almost all patients for staging and surveillance purposes.
Our results confirm those of previous studies that have indicated poor outcomes despite surgical resection of the primary tumor. Although surgical excision was performed in almost all patients (17 of 19 patients), local disease recurrence developed in almost half of these patients and metastatic disease subsequently occurred in most cases (16 of 17 patients). The overall median survival in our study was 33 months, decreasing to 15 months after the onset of distant metastatic disease. These findings may be due to the late presentation of these tumors, or the rich vascular and lymphatic supply of the mucous membranes resulting in micrometastases at the time of diagnosis. Wide surgical margins are often difficult to achieve in these cases, particularly in the case of sinonasal or vaginal melanoma, without resulting in significant disfigurement or morbidity[23,24]. In our series, both local disease recurrence and direct hematogenous metastases were most frequently seen with vaginal primary melanoma, each occurring in over 70% of cases despite aggressive local surgical management.
A number of limitations for this study exist. First, this study is limited by the retrospective study design. However, prospective studies of mucosal melanoma would require years of accrual and multicenter involvement due to its rare incidence. Second, the study numbers are small, which limits the ability to draw statistically significant conclusions. However, potentially important trends are highlighted and suggest the need for large-scale investigative studies into the biological behavior of this condition. Third, there is selection bias in that these patients were treated at a large, tertiary referral oncology center, and therefore, it is possible that patients with the most aggressive and refractory tumors were selected.
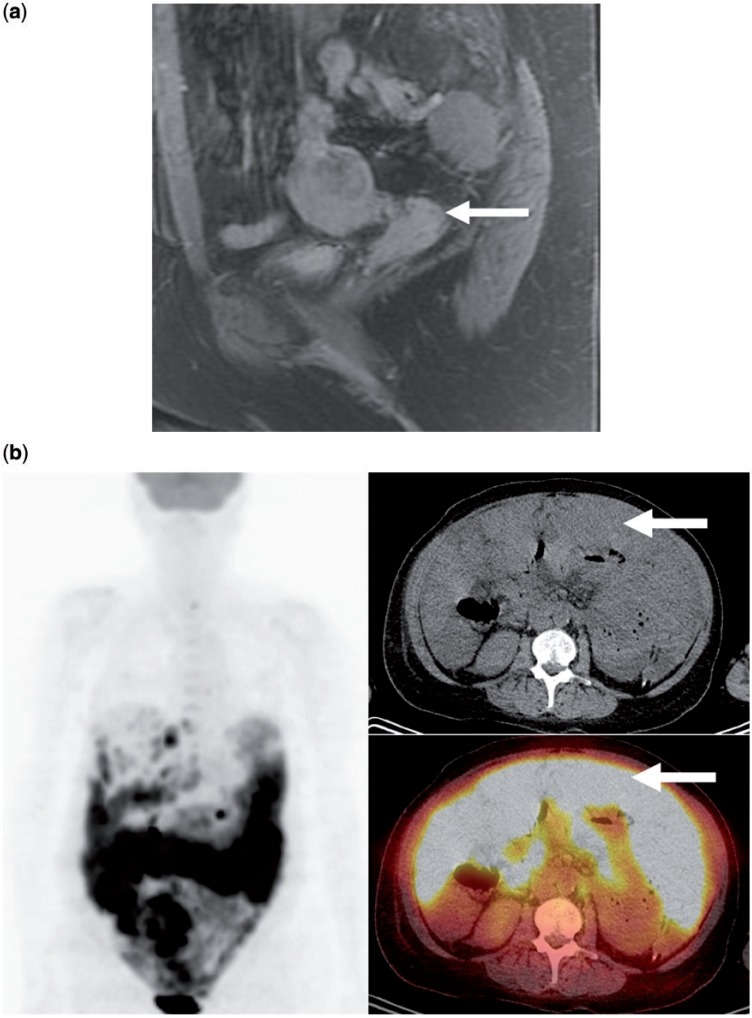
Figure 2.
Mucosal melanoma of the vagina: a 61-year-old woman who presented with vaginal bleeding. (a) Sagittal fat-suppressed T1-weighted magnetic resonance image of the pelvis with contrast enhancement demonstrates an enhancing mass involving the vaginal vault (arrow), which at biopsy proved to be melanoma; (b) FDG-PET/CT with coronal and axial reformats performed 19 months later for new abdominal pain and bloating shows diffuse peritoneal tumor involvement that is intensely FDG avid (arrows).
In conclusion, mucosal melanoma is a rare but highly aggressive form of melanoma for which no proven effective treatment strategies currently exist. Mucosal melanoma can arise from different anatomic sites and the pathways of metastatic spread may differ from other forms of melanoma and between different primary sites of mucosal origin. In particular, vaginal melanoma shows a predilection for local recurrence, lung and peritoneal metastasis; anal melanoma preferentially involves the inguinal lymph nodes; and sinonasal melanoma most commonly spreads to the liver and lungs. This knowledge may assist in future treatment planning and disease surveillance protocols. Because of the high FDG avidity of this tumor, FDG-PET/CT may play an important role in the staging of mucosal melanoma but this requires validation in large-scale studies. In the future, with recent advances in targeted treatments for melanoma, FDG-PET/CT may also provide vital information in the evaluation of novel therapeutic agents. There is a pressing need for large-scale clinical trials looking at the staging, management and follow-up of mucosal melanoma. However, due to the rarity of this tumor, this will most likely require collaboration between multiple institutions.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Chang A, Karnell L, Menck H. The National Cancer Data Base report on cutaneous and noncutaneous melanoma. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Patrick R, Fenske N, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol. 2007;56:828–834. doi: 10.1016/j.jaad.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Mihaljlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol. 2012;5:739–753. [PMC free article] [PubMed] [Google Scholar]
- 4.Stone M. Initial surgical management of melanoma of the skin and unusual sites. Uptodate, version 21.6, updated April 2013. Available from: http://www.uptodate.com/contents/initial-surgical-management-of-melanoma-of-the-skin-and-unusual-sites. [Google Scholar]
- 5.Tomicic J, Wanebo H. Mucosal melanomas. Surg Clin North Am. 2003;83:237–252. doi: 10.1016/S0039-6109(02)00100-7. [DOI] [PubMed] [Google Scholar]
- 6.Cazenave H, Maubec E, Mohamdi H, et al. Anorectal and genital mucosal melanoma is associated with cutaneous melanoma in patients and in families. Br J Dermatol. 2013;169:594–599. doi: 10.1111/bjd.12421. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland CM, Chmiel JS, Henson DE, Winchester DP. Patient characteristics, methods of diagnosis and treatment of mucous membrane melanoma in the United States of America. J Am Coll Surg. 1994;179:561–566. [PubMed] [Google Scholar]
- 8.Prasad M, Busam K, Snehal G, et al. Clinicopathologic differences in malignant melanoma arising in oral squamous and sinonasal respiratory mucosa of the upper aerodigestive tract. Arch Pathol Lab Med. 2003;127:997–1002. doi: 10.5858/2003-127-997-CDIMMA. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Zhou J, Yuen N, et al. Imatinib targeting of KIT-Mutant oncoprotein in melanoma. Clin Cancer Res. 2008;14:7726–7732. doi: 10.1158/1078-0432.CCR-08-1144. [DOI] [PubMed] [Google Scholar]
- 10.Smalley K, Sondak V, Weber J. c-KIT signaling as the driving oncogenic event in sub-groups of melanomas. Histol Histopathol. 2009;24:643–650. doi: 10.14670/HH-24.643. [DOI] [PubMed] [Google Scholar]
- 11.Wong C, Fan Y, Chan T, et al. BRAF and NRAS mutations are uncommon in melanomas arising in diverse internal organs. J Clin Pathol. 2005;58:640–644. doi: 10.1136/jcp.2004.022509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombino M, Lissia A, Franco R, et al. Unexpected distribution of cKIT and BRAF mutations among southern Italian patients with sinonasal melanoma. Dermatology. 2013;226:279–284. doi: 10.1159/000350683. [DOI] [PubMed] [Google Scholar]
- 13.Holder W, White R, Zuger J, Easton E, Greene E. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg. 1998;227:764–771. doi: 10.1097/00000658-199805000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr P, Eggermont AM, Hauschild A, Buzaid A. Staging of cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi14–21. doi: 10.1093/annonc/mdp256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leitier U, Meier F, Schitek B, Garbe C. The hatural course of cutaneous melanoma. J Surg Oncol. 2004;86:172–178. doi: 10.1002/jso.20079. [DOI] [PubMed] [Google Scholar]
- 16.Meier F, Will S, Ellwanger U, et al. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002;147:62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- 17.Tuomaala S, Kivela T. Metastatic pattern and survival in disseminated conjunctival melanoma. Ophthalmology. 2004;111:816–821. doi: 10.1016/j.ophtha.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Hussein M. Extracutaneous malignant melanomas. Cancer Invest. 2008;26:516–534. doi: 10.1080/07357900701781762. [DOI] [PubMed] [Google Scholar]
- 19.Kawashima A, Fishman EK, Kuhlman JE, Schuchter LM. CT of malignant melanoma: patterns of small bowel and mesenteric involvement. J Comput Assist Tomogr. 1991;15:570–574. doi: 10.1097/00004728-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Bakalian S, Marshall JC, Logan P, et al. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res. 2008;14:951–956. doi: 10.1158/1078-0432.CCR-06-2630. [DOI] [PubMed] [Google Scholar]
- 21.Rapidis A, Apostolidis C, Valsamis S. Primary malignant melanoma of the oral mucosa. J Oral Maxillofac Surg. 2003;61:1132–1139. doi: 10.1016/s0278-2391(03)00670-0. [DOI] [PubMed] [Google Scholar]
- 22.Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev. 2012;38:549–553. doi: 10.1016/j.ctrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Roth T, Gengler C, Huber G, Holzmann D. Outcome of sinonasal melanoma: clinical experience and review of the literature. Head Neck. 2010;32:1385–1392. doi: 10.1002/hed.21340. [DOI] [PubMed] [Google Scholar]
- 24.Piura B. Management of primary melanoma of the female urogenital tract. Lancet Oncol. 2008;9:973–981. doi: 10.1016/S1470-2045(08)70254-7. [DOI] [PubMed] [Google Scholar]