Abstract
Increased 4N (G2/tetraploid) cell populations have been postulated to be genetically unstable intermediates in the progression to many cancers, but the mechanism by which they develop and their relationship to instability have been difficult to investigate in humans in vivo. Barrett's esophagus is an excellent model system in which to investigate the order in which genetic and cell cycle abnormalities develop relative to each other during human neoplastic progression. Neoplastic progression in Barrett's esophagus is characterized by inactivation of the p53 gene, the development of increased 4N (G2/tetraploid) cell fractions, and the appearance of aneuploid cell populations. We investigated the hypothesis that patients whose biopsies have increased 4N (G2/tetraploid) cell fractions are predisposed to progression to aneuploidy and determined the relationship between inactivation of p53 and the development of 4N abnormalities in Barrett's epithelium. Our results indicate that increased 4N (G2/tetraploid) populations predict progression to aneuploidy and that the development of 4N abnormalities is interdependent with inactivation of the p53 gene in Barrett's esophagus in vivo.
Full text
PDF


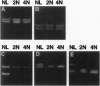
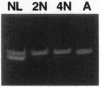
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlogie B., Drewinko B., Schumann J., Göhde W., Dosik G., Latreille J., Johnston D. A., Freireich E. J. Cellular DNA content as a marker of neoplasia in man. Am J Med. 1980 Aug;69(2):195–203. doi: 10.1016/0002-9343(80)90379-4. [DOI] [PubMed] [Google Scholar]
- Barrett M. T., Reid B. J., Joslyn G. Genotypic analysis of multiple loci in somatic cells by whole genome amplification. Nucleic Acids Res. 1995 Sep 11;23(17):3488–3492. doi: 10.1093/nar/23.17.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P. L., Galipeau P. C., Sanchez C. A., Neshat K., Levine D. S., Yin J., Suzuki H., Abraham J. M., Meltzer S. J., Reid B. J. 17p allelic losses in diploid cells of patients with Barrett's esophagus who develop aneuploidy. Cancer Res. 1994 May 1;54(9):2292–2295. [PubMed] [Google Scholar]
- Blount P. L., Meltzer S. J., Yin J., Huang Y., Krasna M. J., Reid B. J. Clonal ordering of 17p and 5q allelic losses in Barrett dysplasia and adenocarcinoma. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3221–3225. doi: 10.1073/pnas.90.8.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. J., Ott B. J., Payne W. S. The incidence of adenocarcinoma in columnar-lined (Barrett's) esophagus. N Engl J Med. 1985 Oct 3;313(14):857–859. doi: 10.1056/NEJM198510033131404. [DOI] [PubMed] [Google Scholar]
- Carder P., Wyllie A. H., Purdie C. A., Morris R. G., White S., Piris J., Bird C. C. Stabilised p53 facilitates aneuploid clonal divergence in colorectal cancer. Oncogene. 1993 May;8(5):1397–1401. [PubMed] [Google Scholar]
- Cross S. M., Sanchez C. A., Morgan C. A., Schimke M. K., Ramel S., Idzerda R. L., Raskind W. H., Reid B. J. A p53-dependent mouse spindle checkpoint. Science. 1995 Mar 3;267(5202):1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995 Aug 25;82(4):675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Hameeteman W., Tytgat G. N., Houthoff H. J., van den Tweel J. G. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989 May;96(5 Pt 1):1249–1256. doi: 10.1016/s0016-5085(89)80011-3. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Harvey M., Sands A. T., Weiss R. S., Hegi M. E., Wiseman R. W., Pantazis P., Giovanella B. C., Tainsky M. A., Bradley A., Donehower L. A. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene. 1993 Sep;8(9):2457–2467. [PubMed] [Google Scholar]
- Hereford L. M., Hartwell L. H. Sequential gene function in the initiation of Saccharomyces cerevisiae DNA synthesis. J Mol Biol. 1974 Apr 15;84(3):445–461. doi: 10.1016/0022-2836(74)90451-3. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Huang Y., Boynton R. F., Blount P. L., Silverstein R. J., Yin J., Tong Y., McDaniel T. K., Newkirk C., Resau J. H., Sridhara R. Loss of heterozygosity involves multiple tumor suppressor genes in human esophageal cancers. Cancer Res. 1992 Dec 1;52(23):6525–6530. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Levine D. S., Sanchez C. A., Rabinovitch P. S., Reid B. J. Formation of the tetraploid intermediate is associated with the development of cells with more than four centrioles in the elastase-simian virus 40 tumor antigen transgenic mouse model of pancreatic cancer. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6427–6431. doi: 10.1073/pnas.88.15.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Neshat K., Sanchez C. A., Galipeau P. C., Blount P. L., Levine D. S., Joslyn G., Reid B. J. p53 mutations in Barrett's adenocarcinoma and high-grade dysplasia. Gastroenterology. 1994 Jun;106(6):1589–1595. doi: 10.1016/0016-5085(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Neshat K., Sanchez C. A., Galipeau P. C., Cowan D. S., Ramel S., Levine D. S., Reid B. J. Barrett's esophagus: a model of human neoplastic progression. Cold Spring Harb Symp Quant Biol. 1994;59:577–583. doi: 10.1101/sqb.1994.059.01.065. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Powell S. N., DeFrank J. S., Connell P., Eogan M., Preffer F., Dombkowski D., Tang W., Friend S. Differential sensitivity of p53(-) and p53(+) cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Res. 1995 Apr 15;55(8):1643–1648. [PubMed] [Google Scholar]
- Rabinovitch P. S. DNA content histogram and cell-cycle analysis. Methods Cell Biol. 1994;41:263–296. doi: 10.1016/s0091-679x(08)61723-9. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., Reid B. J., Haggitt R. C., Norwood T. H., Rubin C. E. Progression to cancer in Barrett's esophagus is associated with genomic instability. Lab Invest. 1989 Jan;60(1):65–71. [PubMed] [Google Scholar]
- Reid B. J. Barrett's esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am. 1991 Dec;20(4):817–834. [PubMed] [Google Scholar]
- Reid B. J., Blount P. L., Rubin C. E., Levine D. S., Haggitt R. C., Rabinovitch P. S. Flow-cytometric and histological progression to malignancy in Barrett's esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992 Apr;102(4 Pt 1):1212–1219. [PubMed] [Google Scholar]
- Reid B. J., Sanchez C. A., Blount P. L., Levine D. S. Barrett's esophagus: cell cycle abnormalities in advancing stages of neoplastic progression. Gastroenterology. 1993 Jul;105(1):119–129. doi: 10.1016/0016-5085(93)90017-7. [DOI] [PubMed] [Google Scholar]
- Sandell L. L., Zakian V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993 Nov 19;75(4):729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Shackney S. E., Smith C. A., Miller B. W., Burholt D. R., Murtha K., Giles H. R., Ketterer D. M., Pollice A. A. Model for the genetic evolution of human solid tumors. Cancer Res. 1989 Jun 15;49(12):3344–3354. [PubMed] [Google Scholar]
- Spechler S. J. Endoscopic surveillance for patients with Barrett esophagus: does the cancer risk justify the practice? Ann Intern Med. 1987 Jun;106(6):902–904. doi: 10.7326/0003-4819-106-6-902. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988 Jul 15;241(4863):317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cui X., Schmitt K., Hubert R., Navidi W., Arnheim N. Whole genome amplification from a single cell: implications for genetic analysis. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5847–5851. doi: 10.1073/pnas.89.13.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]