Abstract
Adrenal collision tumors (ACTs) refer to coexistence of two adjacent, but histologically distinct neoplasms involving the adrenal gland without histologic admixture at interface. ACTs include adenoma with myelolipoma, adenoma with metastases, hemangioma with adenoma, and adrenocortical carcinoma with myelolipoma. In addition, hemorrhage into a pre-existing adrenal mass can mimic an ACT, and it is important to differentiate these two pathologies. Accurate characterization of ACTs is difficult, but critical, for correct staging of patients with malignancies and to guide percutaneous biopsy. Magnetic resonance imaging (MRI) and multidetector computed tomography imaging techniques may depict different tumor components separately; however, biopsy may be required in selected patients for confirmation. [18F]Fluorodeoxyglucose-positron emission tomography (PET) shows increased uptake in the malignant component of ACTs, and guides percutaneous biopsy. Even in patients requiring percutaneous biopsy for a definite diagnosis, imaging findings can help in guiding the appropriate component to be biopsied. Knowledge of imaging findings of different ACTs and their mimics on MRI, computed tomography, and PET help in optimal patient management.
Keywords: Adrenal collision tumors, magnetic resonance imaging, multidetector computed tomography, positron emission tomography/computed tomography
Introduction
The extensive use of cross-sectional imaging studies has led to the increased detection of unexpected adrenal lesions in asymptomatic patients. Benign adenomas are the most common adrenal tumors, with a reported incidence of 2% to 8% in patients at autopsy, followed by metastatic disease[1,2]. Adrenal collision tumor (ACT) is an infrequently described tumor entity, comprising two different neoplasms that coexist adjacent to one another within a single adrenal mass[3,4]. ACTs are rare tumors whose actual prevalence is unknown, despite the relatively high incidence of both benign and metastatic lesions of adrenal glands; however, many ACTs may go undetected because of the small size of one component and/or sampling error[4–6]. With the detection of an ACT, the major diagnostic problem is to exclude the possibility of malignancy in one or both of the components. Further characterizing these lesions is particularly important in patients with a known extra-adrenal primary malignancy for proper staging, management, and prognosis[4,7].
The distinction between benign and malignant neoplasms is generally based on the imaging characteristics demonstrated on cross-sectional imaging modalities, including multidetector computed tomography (MDCT), magnetic resonance imaging (MRI), and hybrid nuclear medicine techniques such as positron emission tomography (PET)/computed tomography (CT). Percutaneous biopsy may still be required in some of the patients for definitive diagnosis, even after extensive work-up with imaging studies. Occasionally, intralesional hemorrhage within an adrenal neoplasm may mimic an ACT, and it is important to differentiate this entity from ACT to avoid aggressive management.
In this article we discuss the cross-sectional imaging findings of different types of ACTs, and the role of the wide array of imaging techniques available for definitive diagnosis. In addition, differentiating features between the ACTs and their mimics are discussed.
Classification
ACTs are defined as the coexistence of two adjacent, but histologically distinct tumors of the adrenal gland without a substantial histologic admixture at the interface[3]. By contrast, composite tumors are neoplasms with an intimate admixture of two different cell types[3,6]. The components of an ACT may consist of two benign or two malignant tumors, or a benign lesion in contiguity with a malignant lesion. Each ACT is given a name based on the cell types within that particular tumor. The most commonly reported ACT comprises an adenoma and a myelolipoma[4]. However, ACTs composed of adenoma and metastases (typically from lung and breast carcinomas and melanoma) are the most problematic in terms of diagnosis and appropriate patient management[4]. In addition, various other ACTs, such as adenoma and pheochromocytoma or hemangioma, adrenocortical carcinoma and metastases or myelolipoma, adrenal carcinosarcoma and metastases, and myelolipoma and Hodgkin lymphoma, have been described in the literature, mostly as individual case reports[5,8–11]. Adrenal carcinosarcoma is one of the classic examples of composite tumor; in this tumor, both components may develop simultaneously, or from a sarcomatous change in an existing adrenal carcinoma[12]. Similarly, metastasis in a pre-existing adenoma may be the cause of a composite tumor with admixture of both components.
Pathogenesis and pathologic findings
The pathogenesis of ACTs has been debated, as the limited number of cases reported precludes detailed analysis of their etiology. Two theories have been postulated to describe their pathogenesis. The first and the simplest explanation is that two different primary tumors merely occur together by chance. A second hypothesis supposes that a single carcinogenic stimulus alters a particular region in the adrenal gland, allowing two separate tumors to occur in contiguity, or the presence of one tumor may alter the local environment, providing a fertile ground for the development of a second tumor[4,5]. Schwartz et al.[4] hypothesized that given the high prevalence of adrenal adenomas and the incidence of metastatic disease to the adrenal gland, the development of ipsilateral metastases adjacent to an existing adenoma would not be uncommon. Moreover, as the adrenal gland is small in size, two adrenal lesions are more likely to be in contiguity with one another. On gross pathologic examination of ACTs, the presence of yellow nodules indicates a lipomatous and typically benign component, while necrotic and hemorrhagic areas are suggestive of malignancy[13]. Histologically, ACTs demonstrate two different cell types with no significant admixture at the interface.
Role of cross-sectional imaging techniques
Cross-sectional imaging studies such as MDCT, MRI, and PET/CT play an important role in the detection, characterization, and follow-up of ACTs. In patients with potential ACTs, the goal of imaging is to differentiate the components with high specificity so that a metastatic focus is not mistaken for a benign lesion, thus potentially altering the treatment course and increasing patient morbidity[2,14]. MDCT is often the first modality utilized in detecting adrenal masses. Attenuation values on unenhanced and contrast-enhanced scans along with washout characteristics on delayed images are useful in differentiating potential benign and malignant components of an ACT[1] (Fig. 1). MRI is indicated for characterization of adrenal masses that show atypical findings on MDCT; images obtained with chemical shift imaging and gadolinium-enhanced techniques are most useful in delineating various tumor components[4,15,16]. If ACTs remain indeterminate on CT and MRI, [18F]fluorodeoxyglucose (FDG)-PET/CT can be performed to identify the metabolically active foci within these tumors, which are generally considered to be malignant[17]. Although each imaging modality has its strengths and weaknesses, the features demonstrated are cumulative in the accurate characterization of adrenal lesions.
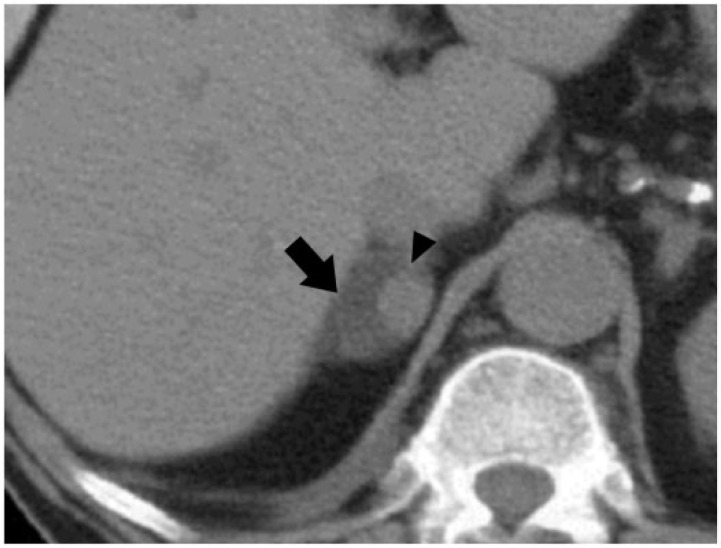
Figure 1.
A 52-year-old woman with a right adrenal collision tumor (ACT) composed of adenoma and metastases from breast carcinoma. Axial noncontrast computed tomography (CT) image shows low attenuating right adrenal mass (arrow) with a soft-tissue density nodule in the medial aspect (arrowhead). This lesion was proved to be an ACT on percutaneous CT-guided biopsy.
MDCT
On MDCT, ACTs demonstrate imaging findings of two different neoplasms that show distinct pathologic features (Fig. 2). An ACT should be considered if a heterogeneous adrenal lesion has appreciable differences in attenuation, or a previously known benign neoplasm changes in appearance or increases in size on follow-up imaging (Figs. 3, 4). These findings are particularly important in patients with known malignancies, and should raise a high suspicion for a potential ACT[4,11]. Intratumoral lipid content and washout characteristics after intravenous contrast administration can be exploited using MDCT to differentiate adenomatous components from malignant foci within the ACTs. It is generally accepted that an adrenal mass with an attenuation value of 10 HU or less is diagnostic of an adrenal adenoma, with a specificity of 96–98% and sensitivity of 71%[1,18]. The lower sensitivity is due to the fact that approximately 30% of adenomas are lipid poor, and contrast enhancement and washout characteristics are useful in differentiating these lesions from malignancy. Lipid-poor adenomas typically show relative percentage enhancement washout greater than 40% and absolute percentage washout greater than 60%[19,20]. This phenomenon is due to the increased capillary permeability in malignant tumors, unlike adenomas with normal capillaries showing rapid contrast washout[16].
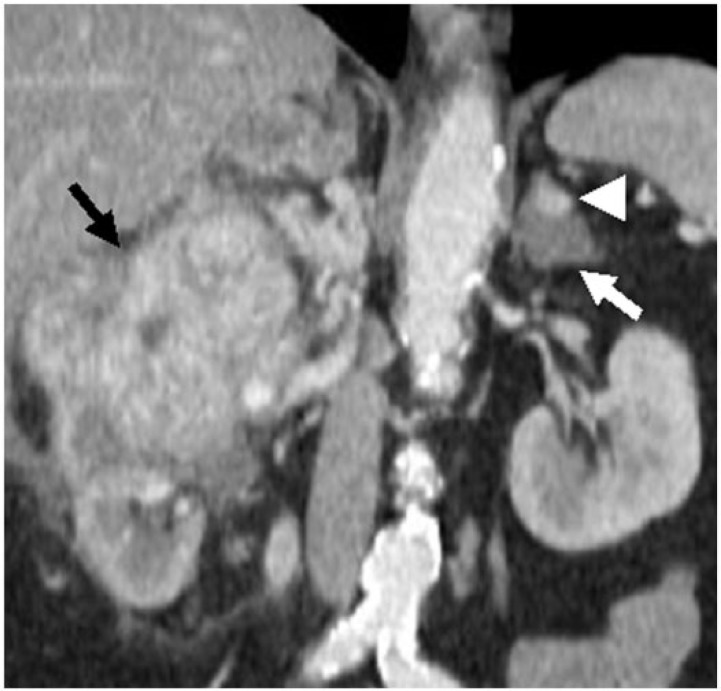
Figure 2.
A 49-year-old man with left ACT composed of an adenoma and renal cell carcinoma metastases. Coronal reconstructed contrast-enhanced CT images demonstrate hypervascular mass in the right kidney, consistent with clear-cell renal cell carcinoma (black arrow); in addition, there is a heterogeneously hypodense left adrenal mass (white arrow) with peripheral enhancing nodule (arrowhead), consistent with ACT that was pathologically proved.
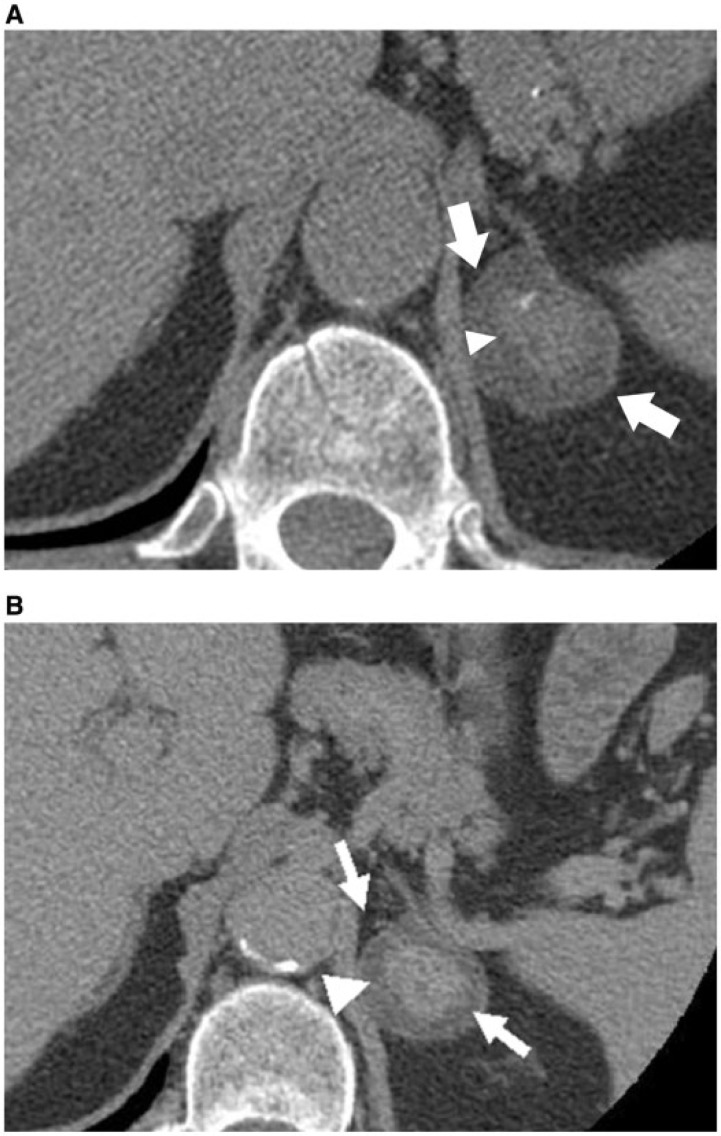
Figure 3.
A 72-year-old man with heterogeneously attenuating left adrenal mass. Axial unenhanced (A) and axial, delayed-phase, contrast-enhanced CT (B) images show a mixed attenuating left adrenal mass containing peripheral low density (arrows) and central hyperdense component (arrowhead), which demonstrates enhancement on delayed imaging. This mass was pathologically proved to be an ACT composed of an adenoma and metastases from laryngeal carcinoma.
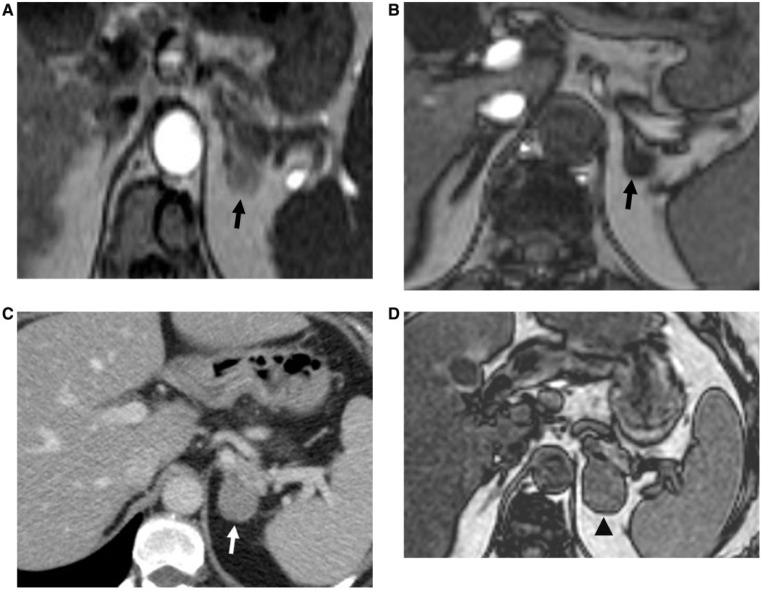
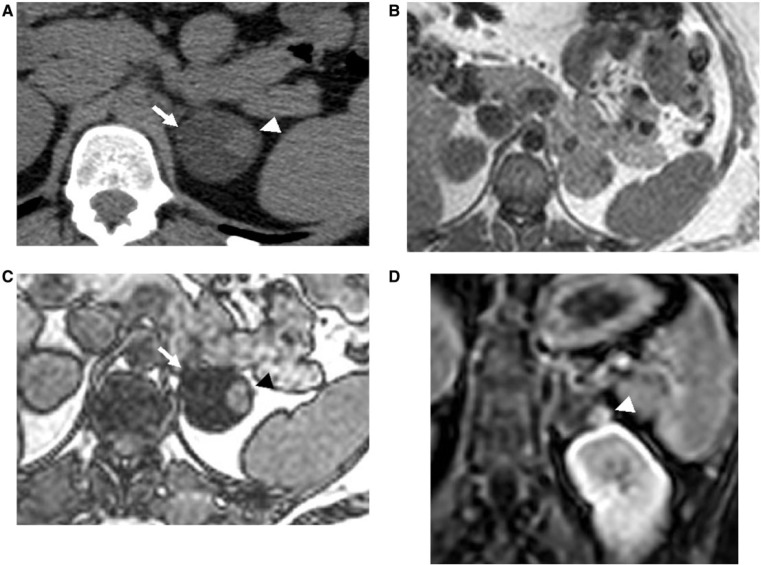
Figure 4.
A 49-year-old woman with a known left adrenal adenoma, who developed right breast carcinoma that subsequently, metastasized to the left adrenal gland, resulting in the development of an ACT. Axial T1-weighted in-phase (A) and opposed-phase (B) magnetic resonance (MR) images show a nodule in the left adrenal gland, which is isointense on in-phase image and demonstrates signal drop on opposed-phase image (black arrow), consistent with an adrenal adenoma. (C) Axial contrast-enhanced CT image after 5 years demonstrates interval enlargement and heterogeneous enhancement of the left adrenal mass (white arrow), concerning for metastases from patient’s known breast carcinoma. This lesion was proved to be an ACT on histopathologic examination. (D) Axial T1-weighted opposed-phase MR image depicts heterogeneous left adrenal mass with foci of mixed high and low signal intensity, consistent with ACT (black arrowhead), which was proved subsequently on histopathologic examination.
There are some limitations in using MDCT for the evaluation of ACTs. Subtle thickening of one of the limbs of adrenal gland, signifying the presence of metastatic disease, may not be detectable on CT imaging. In addition, while stability in tumor size is frequently thought to indicate benignity, hemorrhage in the mass may result in abrupt enlargement that may be considered as an indicator of malignancy[7,18]. MRI and PET/CT are helpful in these patients for further characterization of ACTs.
MRI
MRI is able to characterize different components of an ACT. Chemical shift MRI with T1-weighted in-phase and opposed-phase gradient-echo pulse sequences and gadolinium-enhanced images are the most useful sequences in the evaluation of adrenal lesions[21]. Chemical shift MRI allows differentiation of an adenoma from a malignant lesion by taking advantage of the presence of intracellular lipid within adenoma cells; benign adenomatous components show signal loss on opposed-phase images, whereas metastatic components fail to show signal drop (Fig. 5). This MR technique thus generally helps in distinguishing benign from malignant lesions, with a reported sensitivity and specificity of 81–100% and 94–100%, respectively[4,7,22]. However, volume averaging on opposed-phase images has the potential to artificially decrease signal intensity, which is problematic in characterizing small adrenal lesions with chemical shift MRI. In addition, this MR technique is also limited in characterizing lipid-poor adenomas and metastatic lesions with cytoplasmic lipid, such as from clear-cell renal cell carcinoma[21,23].
Figure 5.
A 76-year-old man with left ACT consisting of adenoma and metastases from lung carcinoma. (A) Unenhanced CT image shows a hypodense left adrenal mass (arrow) with a hyperdense nodule in the periphery (arrowhead). (B, C) Axial T1-weighted in-phase (B) and opposed-phase MR (C) images show signal drop-out of hypodense component on opposed-phase image consistent with adenoma (arrow), while hyperdense focus remains hyperintense (black arrow). (D) Gadolinium-enhanced T1-weighted fat-saturated image demonstrates enhancement of eccentric hyperdense component (white arrowhead), consistent with metastatic focus. CT-guided biopsy from different tumor components demonstrated separate adenomatous and metastatic cells on pathologic examination.
Adenomas may have varying amounts of cytoplasmic fat, leading to a heterogeneous appearance on T2-weighted imaging. Adrenal cortical carcinoma and lymphoma can also have a heterogeneous appearance on T2-weighted imaging secondary to hemorrhage and necrosis. Pheochromocytomas typically show high signal intensity on T2-weighted images, described as the “light bulb sign.” Furthermore, metastatic lesions have an increased water content, which is manifested as increased T2-weighted signal. Metastases and pheochromocytomas have varying amounts of fat, however, leading to a more heterogeneous appearance. Given this overlap in histologic characteristics and their manifestations in T2-weighted MRI, early reliance on calculated T2 measurements to differentiate lesions did not prove satisfactory[7,24]. Although MRI contrast enhancement and washout characteristics have been used for the characterization of adrenal masses, recent studies have demonstrated no significant difference in enhancement characteristics between benign and malignant adrenal lesions[24–26]. More commonly, heterogeneity within an adenoma obviates the ability of contrast-enhanced MRI from definitely differentiating benign and malignant masses[15]. In addition, macroscopic fat of the myelolipomatous component of ACTs can be accurately depicted on fat-saturated sequences. Gadolinium-enhanced sequences, however, can be useful in the detection of hemangiomatous components of ACTs, which show hyperintensity on T2-weighted images, with peripheral nodular enhancement on arterial phase and gradual fill-in of contrast during portal venous and delayed phases.
PET/CT
Although CT and MRI techniques can provide useful information on the anatomic details of ACTs, FDG-PET has the added advantage of providing a noninvasive technique of yielding functional characteristics of these tumors[27]. A focus of increased FDG uptake in an ACT typically indicates the presence of malignancy, and allows the differentiation of malignant and benign components in ACTs (Fig. 6A, B)[5]. While adrenal inflammatory conditions, endothelial cysts, some adenomas, and periadrenal abnormalities can lead to a 5% false-positive interpretation, FDG uptake in adrenal lesions is generally considered malignant when uptake is greater than liver uptake[7,17]. The use of coregistered PET/CT offers a hybrid technique that integrates the attenuation and morphologic detail of CT with the metabolic information from PET, allowing easier location and analysis of increased FDG activity. This synergism, in turn, enables confident location of malignant foci within an ACT (Fig. 6C)[17,28,29]. Occasionally subtle thickening of the adrenal gland may harbor metastases, which can be depicted on PET/CT and also helps in guiding percutaneous biopsy. Multiple studies have reported high sensitivity (94–100%) and specificity (80–100%) of FDG-PET in differentiating benign and malignant adrenal lesions[29,30]. Metser et al.[31] reported increased sensitivity from 99% to 100%, specificity from 92% to 98%, and accuracy from 94% to 99% in differentiating benign and malignant adrenal lesions when CT findings were combined with PET findings. However, mild to moderately metabolically active adenomas may show increased FDG uptake and thus mimic malignancy, and hemorrhage and necrosis within a malignant lesion can be interpreted as benign[7]. PET/CT is also not recommended for adrenal lesions smaller than 1 cm, as uptake in smaller lesions is typically less and cannot be accurately distinguished from normal tissue[14].
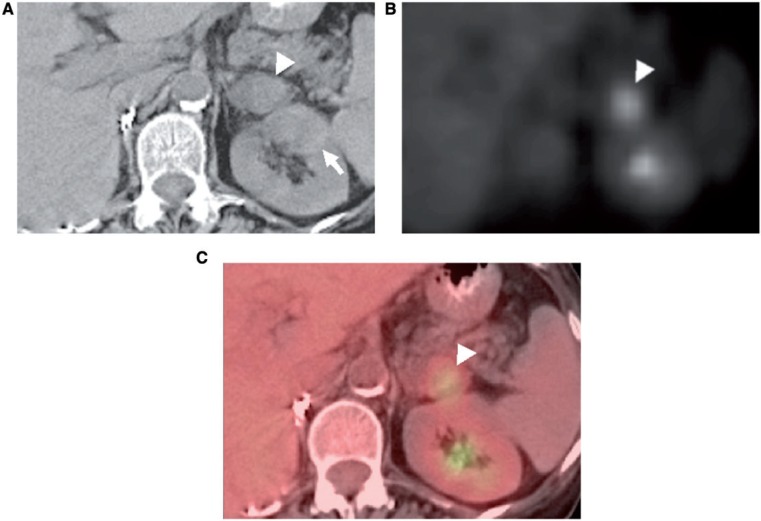
Figure 6.
A 66-year-old man with known right renal cell carcinoma, status post right nephrectomy, who developed metastases in the known adrenal adenoma consistent with ACT. (A) Axial unenhanced CT image shows a left adrenal mass with a hyperattenuating component (arrowhead). Note also a left renal mass consistent with renal cell carcinoma (arrow). (B, C) [18F]Fluorodeoxyglucose (FDG)-positron emission tomography (PET) (B) and PET/CT (C) images depict increased FDG activity in the hyperattenuating component (arrowheads), consistent with ACT (adenoma with metastases), which was subsequently proved on pathologic examination.
Percutaneous biopsy
Despite the success of noninvasive cross-sectional imaging techniques in accurately characterizing adrenal lesions, a small subset of tumors remains indeterminate and percutaneous biopsy is necessary. Image-guided percutaneous biopsy of the adrenal lesions has been performed to detect metastatic disease in patients with adrenal lesions and an extra-adrenal primary malignancy. The reported accuracy and sensitivity of percutaneous biopsy of adrenal metastases in patients with a known primary lesion is 83–96% and 90%, respectively[9,14]. However, the accuracy and sensitivity of biopsy in diagnosing the presence of a metastatic focus within ACTs has not yet been established, owing to the rarity of this entity. If the possibility of a collision tumor within a heterogeneous adrenal mass is not entertained, biopsy of only the benign component can lead to misdiagnosis and, thus, inappropriate patient management. Recognition of an ACT is necessary to ensure proper sampling of the suspected components based on imaging findings. CT guidance should be utilized during biopsy of ACTs to ensure samples from the most suspicious region or of each region with different imaging features, thus increasing the yield of the biopsy. An important consideration when planning a percutaneous biopsy of an ACT is the potential for pheochromocytoma to be a component of the lesion. There have been thus far 10 reported cases of ACTs with a pheochromocytoma component in the literature[32–34]. Biopsy of pheochromocytoma is relatively contraindicated, given the potential for catecholamine secretion and the subsequent risk of hemodynamic instability, hypertensive crisis, uncontrolled hemorrhage, and possible death following tumor manipulation. Screening for excess catecholamine secretion can obviate these potential adverse outcomes[14,35,36]. Furthermore, it is generally accepted that while a positive adrenal biopsy result “rules in” a malignant process, a negative result does not definitively exclude malignancy[37]. Given this uncertainty, an ACT may be surgically removed, either upon initial diagnosis or if the lesion has demonstrated increased growth on follow-up imaging[4,9].
Mimics of ACTs
Heterogeneous adrenal lesions with imaging characteristics of two separate pathologic processes do not necessarily represent collision and/or composite tumors. Intralesional hemorrhage, fibrosis, or fatty change in an adrenal neoplasm can mimic an ACT. Although malignant tumors have an increased propensity to bleed, any adrenal lesion is subject to intratumoral hemorrhage, particularly as they increase in size. Hemorrhage within adenomas is a very rare, and only a few cases of hemorrhagic degeneration within a hypervascular adrenal adenoma have been reported[38]. Adrenal masses with intratumoral hemorrhage typically present as heterogeneous masses with different attenuation values on MDCT, and mimic ACTs (Fig. 7). In addition, periadrenal fat stranding may also be identified. CT findings mainly depend on the age of the hemorrhage, presence of necrosis, and cystic degeneration. MRI and PET/CT are extremely helpful for further characterization and avoiding unnecessary percutaneous biopsy. Hemorrhagic components of an adrenal lesion may demonstrate heterogeneous signal intensities on MRI depending on the age of the hematoma, and typically show no enhancement after contrast administration (Fig. 7). FDG-PET/CT demonstrates no increased uptake within hemorrhagic components, which differentiates it from malignancy within an ACT (Fig. 8). Follow-up MRI is indicated in 3 months in cases of adrenal lesions suspicious for hemorrhage, prior to attempting percutaneous biopsy of such lesions. Indicators of intralesional hemorrhage on repeat imaging are interval decrease in tumor size and change in morphology (Fig. 7)[39].
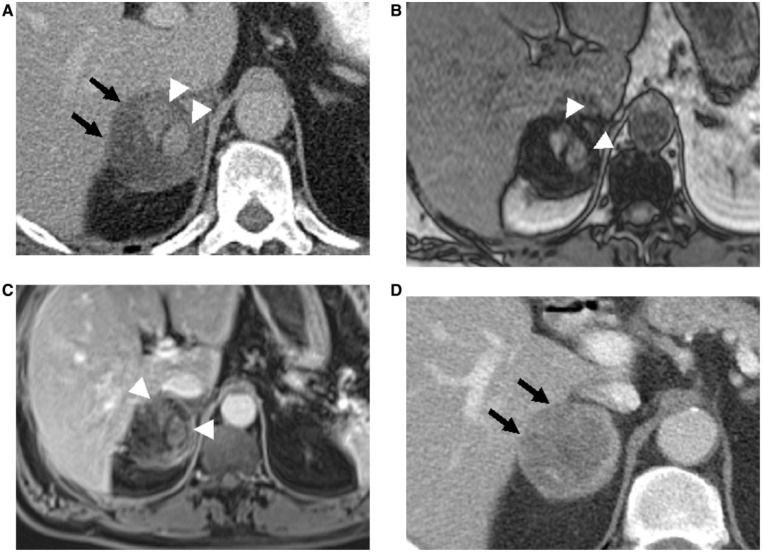
Figure 7.
Intralesional hemorrhage in the right adrenal adenoma mimicking ACT in a 47-year-old man, who came to the emergency room with severe right abdominal pain. (A) Axial contrast-enhanced CT image depicts a hypodense right adrenal mass (arrows) with hyperdense nodular components (arrowheads), concerning for ACT. (B) Axial T1-weighted opposed-phase MR image demonstrates diffuse signal loss in the adenomatous component, whereas hyperdense components show no signal loss (arrowheads). (C) Contrast-enhanced MR image depicts no enhancement of these hyperdensities, consistent with hemorrhage (arrowheads). No biopsy was attempted and a follow-up CT was recommended. (D) Follow-up contrast-enhanced CT demonstrates interval decrease in size, resolution of hyperdensities, and overall change in attenuation of the right adrenal mass (arrows), consistent with hemorrhage.
Figure 8.
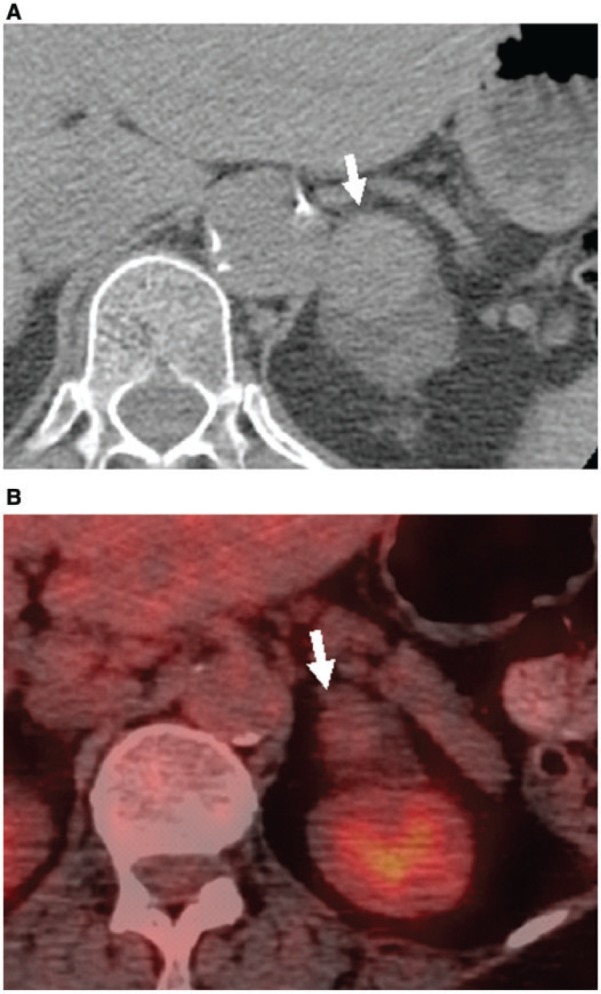
Hemorrhage complicating left adrenal adenoma in a 56-year-old man. (A) Unenhanced CT image shows a hyperattenuating focus in the left adrenal adenoma (arrow). (B) FDG-PET/CT image demonstrates no increased activity within this hyperdensity, consistent with hemorrhage (arrow).
Fibrosis within an adrenal adenoma can also mimic ACTs. Large adenomas can undergo fibrotic degeneration, which typically appears as a hypoattenuating mass with a focus of high density[40]. The fibrotic region should not enhance after contrast administration on CT or MRI, nor be associated with uptake of FDG. Intralesional fatty degeneration within adrenal masses may also mimic ACTs; lesions treated with percutaneous radiofrequency ablation may show macroscopic fat at a later time and may be confused with an ACT (Fig. 9). In addition, juxta-adrenal masses can mimic ACTs in the presence of an ipsilateral intra-adrenal mass. Three-dimensional reconstructions and reformatted images in multiple planes obtained with MDCT and MRI can help differentiate an adrenal mass from a juxta-adrenal lesion[40].
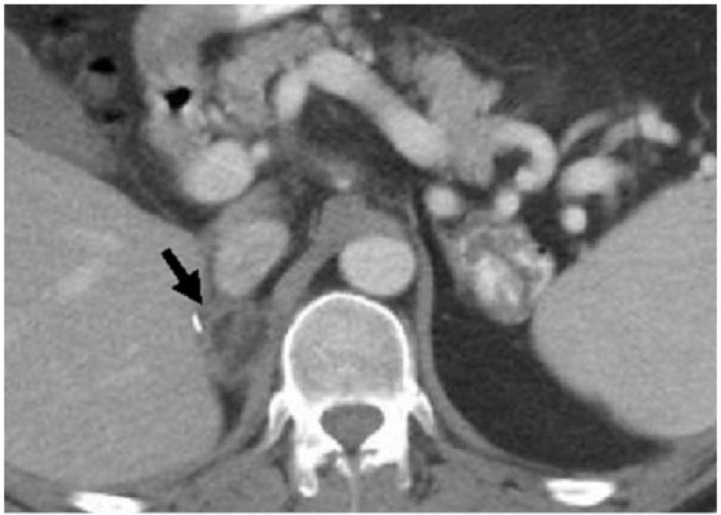
Figure 9.
Axial contrast-enhanced CT image in a 43-year-old man shows a right adrenal lesion containing fat and soft-tissue density rim mimicking ACT (arrow). This patient was a known case of adrenal metastases treated with radiofrequency ablation, resulting in the development of intralesional fat and a thick soft-tissue rim.
Conclusions
ACTs are rare. Advanced cross-sectional imaging techniques including MDCT, MRI, and PET/CT can help identify and characterize ACTs. MRI is especially useful in tumors with microscopic fat and intralesional hemorrhage. In some cases, percutaneous biopsy may still be necessary for definitive characterization. When planning for biopsy of a heterogeneous adrenal mass, the possibility of an ACT should be considered so as to appropriately sample the lesion, as proper tissue diagnosis is critical for staging and treatment. In addition, interval hemorrhage, fibrosis, or fatty change within a pre-existing adrenal mass may mimic ACTs on imaging. It is important to recognize this possibility and further characterize these lesions, as short-interval follow-up is appropriate, rather than percutaneous biopsy. Knowledge of imaging findings of different ACTs and their mimics is helpful in guiding optimal patient care.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Blake MA, Kalra MK, Sweeney AT, et al. Distinguishing benign from malignant adrenal masses: multi-detector row CT protocol with 10-minute delay. Radiology. 2006;238:578–585. doi: 10.1148/radiol.2382041514. [DOI] [PubMed] [Google Scholar]
- 2.Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol. 2002;179:559–568. doi: 10.2214/ajr.179.3.1790559. [DOI] [PubMed] [Google Scholar]
- 3.Meyer R. Beitrag zur verständigung über die namengebung in der geschwulstlehre. Zentralbl Allg Pathol. 1919;30:291–296. [Google Scholar]
- 4.Schwartz LH, Macari M, Huvos AG, Panicek DM. Collision tumors of the adrenal gland: demonstration and characterization at MR imaging. Radiology. 1996;201:757–760. doi: 10.1148/radiology.201.3.8939227. [DOI] [PubMed] [Google Scholar]
- 5.Anderson SB, Webb MD, Banks KP. Adrenal collision tumor diagnosed by F-18 fluorodeoxyglucose PET/CT. Clin Nucl Med. 2010;35:414–417. doi: 10.1097/RLU.0b013e3181db4df1. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqi AJ, Miller FH, Kasuganti D, Nikolaidis P. Adrenal hemangioma-adenoma: an exceedingly rare adrenal collision tumor. J Magn Reson Imaging. 2009;29:949–952. doi: 10.1002/jmri.21430. [DOI] [PubMed] [Google Scholar]
- 7.Blake MA, Cronin CG, Boland GW. Adrenal imaging. AJR Am J Roentgenol. 2010;194:1450–1460. doi: 10.2214/AJR.10.4547. [DOI] [PubMed] [Google Scholar]
- 8.Shifrin RY, Bechtold RE, Scharling ES. Metastatic adenocarcinoma within an adrenal adenoma: detection with chemical shift imaging. AJR Am J Roentgenol. 1996;167:891–892. doi: 10.2214/ajr.167.4.8819376. [DOI] [PubMed] [Google Scholar]
- 9.Thorin-Savoure A, Tissier-Rible F, Guignat L, et al. Collision/composite tumors of the adrenal gland: a pitfall of scintigraphy imaging and hormone assays in the detection of adrenal metastasis. J Clin Endocrinol Metab. 2005;90:4924–4929. doi: 10.1210/jc.2004-2572. [DOI] [PubMed] [Google Scholar]
- 10.Bertolini F, Rossi G, Fiocchi F, et al. Primary adrenal gland carcinosarcoma associated with metastatic rectal cancer: a hitherto unreported collision tumor. Tumori. 2011;97:27e–30e. doi: 10.1177/030089161109700526. [DOI] [PubMed] [Google Scholar]
- 11.Hagspiel KD. Manifestation of Hodgkin's lymphoma in an adrenal myelolipoma. Eur Radiol. 2005;15:1757–1759. doi: 10.1007/s00330-004-2523-x. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki K, Desimone M, Rao HR, Huang GJ, Seethala RR. Adrenocortical carcinosarcoma: a case report and review of the literature. Diagn Pathol. 2010;5:51. doi: 10.1186/1746-1596-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otal P, Escourrou G, Mazerolles C, et al. Imaging features of uncommon adrenal masses with histopathologic correlation. Radiographics. 1999;19:569–581. doi: 10.1148/radiographics.19.3.g99ma07569. [DOI] [PubMed] [Google Scholar]
- 14.Boland GW, Blake MA, Hahn PF, Mayo-Smith WW. Incidental adrenal lesions: principles, techniques, and algorithms for imaging characterization. Radiology. 2008;249:756–775. doi: 10.1148/radiol.2493070976. [DOI] [PubMed] [Google Scholar]
- 15.Elsayes KM, Mukundan G, Narra VR, et al. Adrenal masses: MR imaging features with pathologic correlation. Radiographics. 2004;24(Suppl 1):S73–S86. doi: 10.1148/rg.24si045514. [DOI] [PubMed] [Google Scholar]
- 16.Krestin GP, Freidmann G, Fishbach R, Neufang KF, Allolio B. Evaluation of adrenal masses in oncologic patients: dynamic contrast-enhanced MR vs CT. J Comput Assist Tomogr. 1991;15:104–110. doi: 10.1097/00004728-199101000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Blake MA, Sweeney AT, Kalra MK, Maher MM. Collision adrenal tumors on PET/CT. AJR Am J Roentgenol. 2004;183:864–865. doi: 10.2214/ajr.183.3.1830864. [DOI] [PubMed] [Google Scholar]
- 18.Johnson PT, Horton KM, Fishman EK. Adrenal imaging with multidetector CT: evidence-based protocol optimization and interpretative practice. Radiographics. 2009;29:1319–1331. doi: 10.1148/rg.295095026. [DOI] [PubMed] [Google Scholar]
- 19.Caoili EM, Korobkin M, Francis IR, Cohan RH, Dunnick NR. Delayed enhanced CT of lipid-poor adrenal adenomas. AJR Am J Roentgenol. 2000;175:1411–1415. doi: 10.2214/ajr.175.5.1751411. [DOI] [PubMed] [Google Scholar]
- 20.Caoili EM, Korobkin M, Francis IR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002;222:629–633. doi: 10.1148/radiol.2223010766. [DOI] [PubMed] [Google Scholar]
- 21.Bilbey JH, McLoughlin RF, Kurkjian PS, et al. MR imaging of adrenal masses: value of chemical-shift imaging for distinguishing adenomas from other tumors. AJR Am J Roentgenol. 1995;164:637–642. doi: 10.2214/ajr.164.3.7863885. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell DG, Crovello M, Matteucci T, Petersen RO, Miettinen MM. Benign adrenocortical masses: diagnosis with chemical shift MR imaging. Radiology. 1992;185:345–351. doi: 10.1148/radiology.185.2.1410337. [DOI] [PubMed] [Google Scholar]
- 23.Haider MA, Ghai S, Jhaveri K, Lockwood G. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711–716. doi: 10.1148/radiol.2313030676. [DOI] [PubMed] [Google Scholar]
- 24.Reinig JW, Stutley JE, Leonhardt CM, Spicer KM, Margolis M, Caldwell CB. Differentiation of adrenal masses with MR imaging: comparison of techniques. Radiology. 1994;192:41–46. doi: 10.1148/radiology.192.1.8208962. [DOI] [PubMed] [Google Scholar]
- 25.Krestin GP, Steinbrich W, Friedmann G. Adrenal masses: evaluation with fast gradient-echo MR imaging and Gd-DTPA-enhanced dynamic studies. Radiology. 1989;171:675–680. doi: 10.1148/radiology.171.3.2717737. [DOI] [PubMed] [Google Scholar]
- 26.Semelka RC, Shoenut JP, Lawrence PH, et al. Evaluation of adrenal masses with gadolinium enhancement and fat-suppressed MR imaging. J Magn Reson Imaging. 1993;3:337–343. doi: 10.1002/jmri.1880030208. [DOI] [PubMed] [Google Scholar]
- 27.Boland GW, Dwamena BA, Jagtiani Sangwaiya M, et al. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology. 2011;259:117–126. doi: 10.1148/radiol.11100569. [DOI] [PubMed] [Google Scholar]
- 28.Chong S, Lee KS, Kim HY, et al. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: diagnostic efficacy and interpretation pitfalls. Radiographics. 2006;26:1811–1824; discussion 1824–1826. doi: 10.1148/rg.266065057. [DOI] [PubMed] [Google Scholar]
- 29.Blake MA, Slattery JM, Kalra MK, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy—initial experience. Radiology. 2006;238:970–977. doi: 10.1148/radiol.2383042164. [DOI] [PubMed] [Google Scholar]
- 30.Yun M, Kim W, Alnafisi N, Lacorte L, Jang S, Alavi A. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med. 2001;42:1795–1799. [PubMed] [Google Scholar]
- 31.Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006;47:32–37. [PubMed] [Google Scholar]
- 32.Sato H, Igarashi H, Kishimoto Y, et al. Combined tumor consisting of non-functioning adrenocortical adenoma and pheochromocytoma in the same gland. Int J Urol. 2002;9:398–401. doi: 10.1046/j.1442-2042.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- 33.Cotesta D, Petramala L, Serra V, et al. Pheochromocytoma associated with adrenocortical tumor in the same gland. Two case reports and literature review. Minerva Endocrinol. 2006;31:183–189. [PubMed] [Google Scholar]
- 34.Sakamoto N, Tojo K, Saito T, et al. Coexistence of aldosterone-producing adrenocortical adenoma and pheochromocytoma in an ipsilateral adrenal gland. Endocr J. 2009;56:213–219. doi: 10.1507/endocrj.k08e-196. [DOI] [PubMed] [Google Scholar]
- 35.Paulsen SD, Nghiem HV, Korobkin M, Caoili EM, Higgins EJ. Changing role of imaging-guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033–1037. doi: 10.2214/ajr.182.4.1821033. [DOI] [PubMed] [Google Scholar]
- 36.Mazzaglia PJ, Monchik JM. Limited value of adrenal biopsy in the evaluation of adrenal neoplasm: a decade of experience. Arch Surg. 2009;144:465–470. doi: 10.1001/archsurg.2009.59. [DOI] [PubMed] [Google Scholar]
- 37.Harisinghani MG, Maher MM, Hahn PF, et al. Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin Radiol. 2002;57:898–901. doi: 10.1053/crad.2002.1054. [DOI] [PubMed] [Google Scholar]
- 38.Khati NJ, Javitt MC, Schwartz AM. Adrenal adenoma and hematoma mimicking a collision tumor at MR imaging. Radiographics. 1999;19:235–239. doi: 10.1148/radiographics.19.1.g99ja19235. [DOI] [PubMed] [Google Scholar]
- 39.Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19:949–963. doi: 10.1148/radiographics.19.4.g99jl13949. [DOI] [PubMed] [Google Scholar]
- 40.Newhouse JH, Heffess CS, Wagner BJ, Imray TJ, Adair CF, Davidson AJ. Large degenerated adrenal adenomas: radiologic-pathologic correlation. Radiology. 1999;210:385–391. doi: 10.1148/radiology.210.2.r99fe12385. [DOI] [PubMed] [Google Scholar]