Abstract
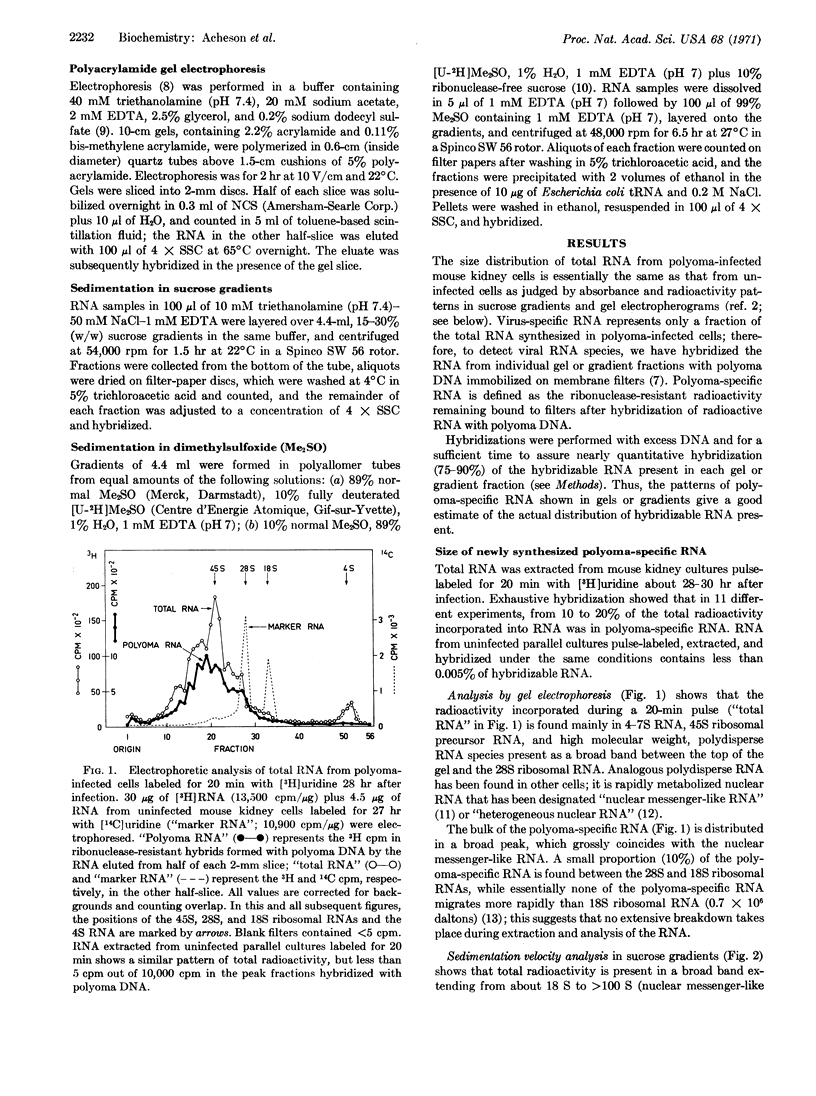
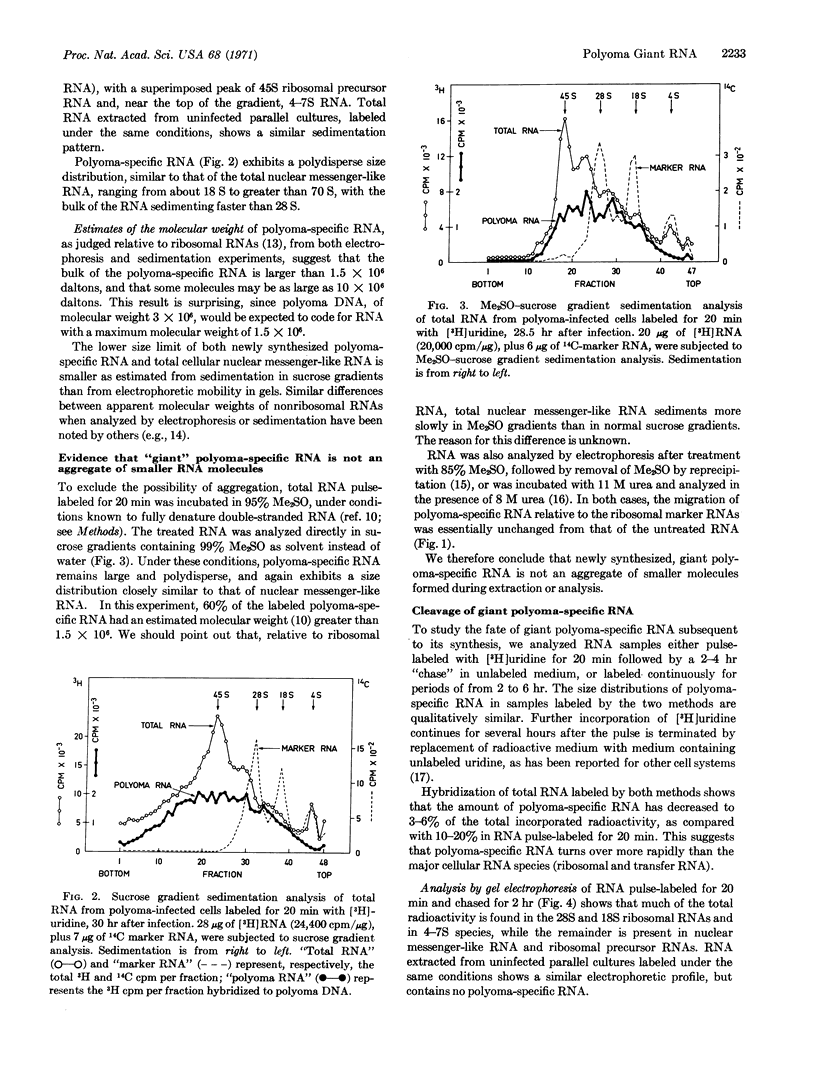
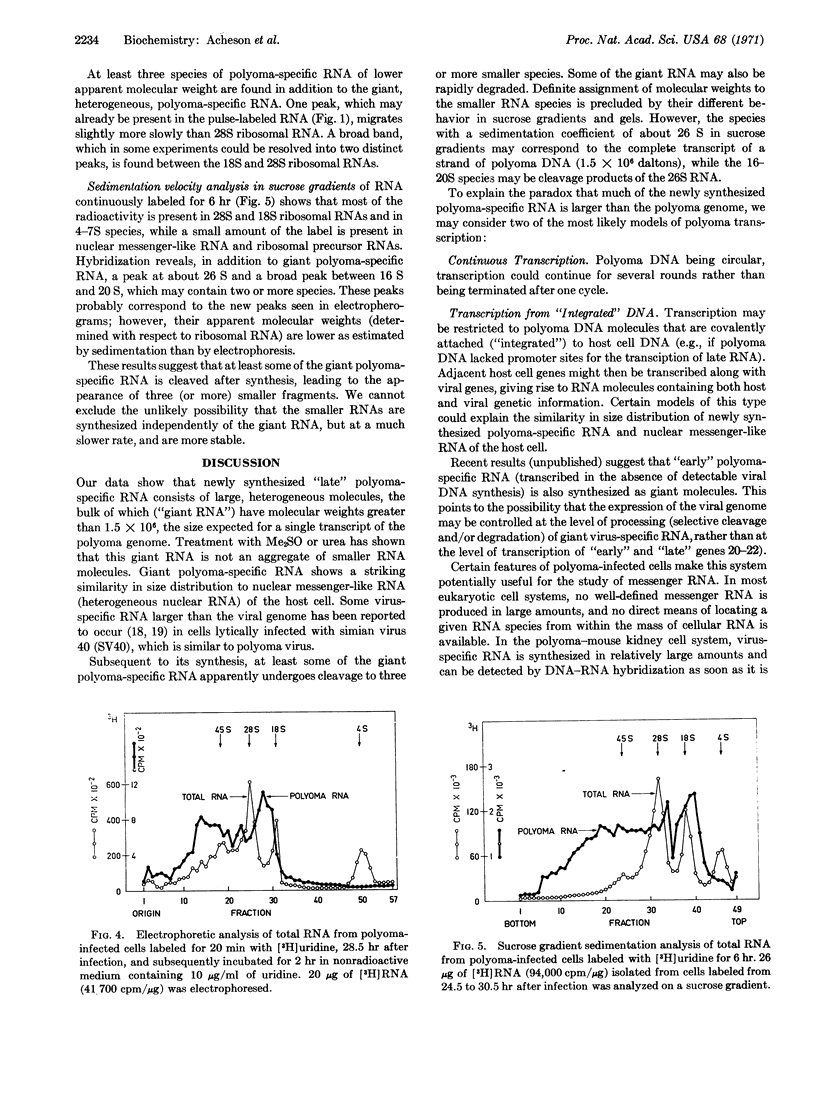
The size of virus-specific RNA synthesized in cultured mouse kidney cells infected with polyoma virus was estimated by electrophoresis and sedimentation analysis of RNA extracts from whole cells. Newly synthesized “late” polyoma-specific RNA appears as “giant” molecules of heterogeneous size, up to several times larger than a strand of polyoma DNA (1.5 × 106 daltons). Treatment with dimethylsulfoxide or urea showed that the large size of these molecules is not due to aggregation. Giant polyoma-specific RNA is strikingly similar in size distribution to “nuclear messenger-like” RNA (“heterogeneous nuclear” RNA) of the host cell. Subsequent to its synthesis, some of the giant polyoma-specific RNA appears to be cleaved to at least three smaller species.
Keywords: gel electrophoresis, density gradient centrifugation, RNA-DNA hybridization, tumor virus
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Virus-specific RNA in cells productively infected or transformed by polyoma virus. J Mol Biol. 1966 Apr;16(2):359–373. doi: 10.1016/s0022-2836(66)80179-1. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Sauer G., Sokol F. The effect of actinomycin D on the transcription and replication of simian virus 40 deoxyribonucleic acid. Virology. 1969 Feb;37(2):214–226. doi: 10.1016/0042-6822(69)90201-3. [DOI] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hudson J. B. An analysis of the polyoma virus DNA-RNA hybridization reaction in vitro. Eur J Biochem. 1969 May 1;9(1):112–117. doi: 10.1111/j.1432-1033.1969.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Hudson J., Goldstein D., Weil R. A study on the transcription of the polyoma viral genome. Proc Natl Acad Sci U S A. 1970 Jan;65(1):226–233. doi: 10.1073/pnas.65.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Byrne J. C. Sedimentation properties of simian virus 40-specific ribonucleic acid present in green monkey cells during productive infection and in mouse cells undergoing abortive infection. J Virol. 1970 Oct;6(4):463–469. doi: 10.1128/jvi.6.4.463-469.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Regulation of transcription of the SV40 DNA in productively infected and in transformed cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):525–532. doi: 10.1073/pnas.60.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L. Messenger RNA in avian erythroblasts at the transcriptional and translational levels and the problem of regulation in animal cells. J Cell Physiol. 1968 Oct;72(2 Suppl):181+–181+. doi: 10.1002/jcp.1040720413. [DOI] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L., Zajdela F., London I. M., Gros F. Patterns of RNA metabolism in a differentiated cell: a rapidly labeled, unstable 60S RNA with messenger properties in duck erythroblasts. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1571–1578. doi: 10.1073/pnas.56.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Birnboim H. C., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. II. Base composition and cellular localization of a heterogeneous RNA fraction. J Mol Biol. 1966 Aug;19(2):362–372. doi: 10.1016/s0022-2836(66)80010-4. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]


