Introduction
Formaldehyde is a widely used chemical, with global production of over 20 million tons per year (International Agency for Research on Cancer, 2012). It is utilized in industrial and consumer products and is also produced as a byproduct of fires, cigarette smoke and automotive exhaust. Furthermore, it is released from products used in building materials such as particle board and carpet. It has long been used as a fixation product for pathology specimens and in embalming. However, what is often not realized is that formaldehyde is also one of the oldest chemicals in the world. Life started with one carbon chemistry and formaldehyde was one of the earliest chemicals formed and utilized in cells. There are numerous sources of endogenous formaldehyde, including the one carbon pool, amino acid metabolism, methanol metabolism, lipid peroxidation, and p450 dependent demethylation (O-, N-, and S-methyl).
In 1980, formaldehyde was shown to be a carcinogen of the nasal passages of rats exposed by inhalation (Swenberg et al., 1980). This finding was followed by an intense effort to understand the Mode of Action (MOA). More than thirty years of research has focused on confirmation of its carcinogenicity, MOA studies and vastly expanded epidemiologic studies. This paper will briefly cover salient features of these comprehensive studies to place the carcinogenicity, epidemiology and risks of formaldehyde in perspective.
Carcinogenicity Studies
Part of the mission of the newly formed Chemical Industry Institute of Toxicology (CIIT) was to conduct state-of-the-art toxicity and carcinogenicity bioassays on commodity chemicals. In 1978, a 2-year carcinogenicity study in F344 rats and B6C3F1 mice exposed by inhalation to 0, 2, 5.6 or 14.3 ppm formaldehyde was initiated at Battelle, Columbus. The 12-month interim report noted that two rats in the high exposure group had squamous cell carcinomas of the nasal passages. In the 13th month of the study, a third squamous cell carcinoma was diagnosed in a rat from the same treatment group. This was alarming, as nasal squamous cell carcinomas are very rare neoplasms in rats. The FDA, CPSC, EPA and CIIT sponsors were simultaneously alerted to a strong concern that formaldehyde was likely to be carcinogenic. The first report on formaldehyde's carcinogenicity in the peer-reviewed literature appeared in September, 1980 (Swenberg et al., 1980). At 18 months into the study, 36 rats exposed to 14.3 ppm formaldehyde had developed squamous cell carcinomas, but no nasal cancers were present in rats exposed to 2 or 5.6 ppm, or in mice exposed to 2, 5.6 or 14.3 ppm formaldehyde. This report was followed by a comprehensive publication of the Battelle study by William Kerns, the study pathologist (Kerns et al., 1983). The exposures for this study were conducted for up to 24 months (6 hrs/day, 5 days/week) for male and female rats and mice. Additional animals were held up to 6 months post exposure to follow progression and/or regression of lesions. Significant formaldehyde-induced lesions were confined to the nasal tissues and proximal trachea. The distribution and severity of the pathology were concentration-dependent. Rhinitis, squamous metaplasia and epithelial dysplasia were diagnosed in all formaldehyde-exposed groups of rats and in the 5.6 and 14.3 ppm groups of mice. With increasing post-exposure time, these non-malignant lesions regressed. In total, 103 rats and 2 mice exposed to 14.3 ppm formaldehyde developed squamous cell carcinomas of the nose, as did two rats exposed to 5.6 ppm.
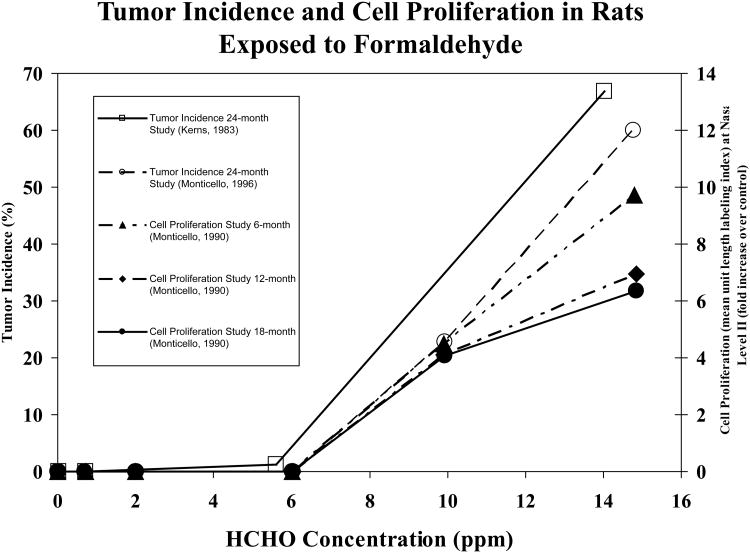
The initial carcinogenicity study was followed by a second inhalation study with F344 rats that incorporated two additional formaldehyde exposure concentrations, 0.7 and 10 ppm, as well as the original 0, 2, 6 and 15 ppm exposures (Monticello et al., 1990; Monticello et al., 1996). This study contained detailed evaluations of cell proliferation at 6, 12, and 18 months of exposure. Figure 1 illustrates the exposure-response relationships for squamous cell carcinoma in the Kerns' and Monticello studies, as well as the exposure and time relationships for cell proliferation. It clearly demonstrates the persistent and strong concentration-dependent increases in cell proliferation that are key events in the Mode of Action (MOA) for formaldehyde carcinogenesis.
Figure 1.
Incidence of squamous cell carcinoma of the nasal passages and cell proliferation in rats exposed to formaldehyde. □ shows tumor incidence (%) from the 24 month study versus formaldehyde concentration (ppm) (Kerns et al., 1983). ○ shows tumor incidence from the 24-month study (Monticello et al., 1996). ▲ shows cell proliferation (mean unit length labeling index) from the 6 month study as fold increase over control (Monticello et al., 1990). ◆ shows cell proliferation (mean unit length labeling index) from the 12 month study as fold increase over control (Monticello et al., 1990). ● shows cell proliferation (mean unit length labeling index) as fold increase over control (Monticello, Morgan, and Hurtt, 1990).
The findings of the CIIT inhalation studies have been confirmed by several other laboratories (International Agency for Research on Cancer, 2006). In addition, several oral studies were conducted that were also reviewed by IARC.
Epidemiology of Formaldehyde and Cancer
The epidemiology of formaldehyde-induced cancer has been studied extensively. The literature was reviewed by IARC in 2004 (International Agency for Research on Cancer, 2006) and formaldehyde was classified as a Human Carcinogen, based on an increase in nasopharyngeal cancer (NPC) in the National Cancer Institute (NCI) cohort of >25,000 formaldehyde workers in 10 plants (Hauptmann et al., 2004). NPC was significantly elevated in one of the 10 plants, with 6/10 NPC being diagnosed in the Wallingford, CT plant workers, while workers at the other 9 plants had a 35% deficit in NPC. The other two largest cohort studies did not find increases in NPC cancer (Coggon et al., 2003; Pinkerton et al., 2004). Marsh et al., conducted an independent study of the Wallingford, CT plant workers and confirmed the increase in NPC (Marsh et al., 2007). However, they also showed that there was little evidence of a dosedependent relationship between NPC and formaldehyde exposure, and that 5/6 workers had a work history of silver smithing, brass plating and metal work, which involves acid mists and has been associated with human NPC (Marsh et al., 2007). Thus, confounding by exposure to other chemicals could not be excluded as the cause of elevated NPC in the Wallingford, CT plant. Four recent reviews/meta analyses concluded that there was little support for formaldehyde causing NPC (Bachand et al., 2010; Bosetti et al., 2008; Collins et al., 1997; Duhayon et al., 2008). In contrast, IARC reiterated its conclusion that “Formaldehyde causes cancer of the nasopharynx” in 2012. Clearly, there is biologic plausibility for formaldehyde causing NPC, as it is the initial site of contact for inhalation exposures. However, the overall data for human exposures appear to be limited at best.
Hauptmann et al., (Hauptmann et al., 2003) also examined the NCI cohort of formaldehyde workers for associations of formaldehyde exposure with hematolymphopoietic cancers. The association was strongest with peak exposures, but not with the more traditional cumulative exposure dose metric. This study was considered to provide limited evidence in humans by IARC (International Agency for Research on Cancer, 2006). In contrast, the UK cohort had a deficit in hematolymphopoietic cancers, yet had the highest exposures of the three large industry cohorts (Coggon et al., 2003). The NCI cohort was updated (Beane Freeman et al., 2009) to include workers who died between 1994 and 2004. It was also noted that 1006 deaths from the Hauptmann et al., (Hauptmann et al., 2003) study had been left out of that paper's analyses. When these workers, plus the additional 10 years of follow-up were analyzed, evidence for leukemia was weakened and no relationship between cumulative formaldehyde exposure and hematolymphopoietic malignancies was found. A third study reported an increase in myeloid leukemia in embalmers exposed to formaldehyde that was associated with the number of years of embalming (Hauptmann et al., 2009), however, no measurements of formaldehyde exposure were conducted as part of this study.
There have not been any reports that demonstrate if or how inhaled formaldehyde reaches sites distant to the site of initial contact following inhalation exposure. Thus, the limited evidence for formaldehyde causing hematolymphopoietic cancers and the biological implausibility of the hypothesis that inhaled formaldehyde causes leukemia has raised many questions (Committee to Review EPA's Draft IRIS Assessment of Formaldehyde and and National Research Council, 2011).
Early Mode of Action Studies
During the 1980-1990s, extensive research was conducted on cell proliferation, mutagenicity, and species differences in effects of formaldehyde exposure on respiratory minute volume (Chang et al., 1983). The latter effects provide important information that accounts for the marked species differences in carcinogenicity between rats and mice. When mice are exposed to 15 ppm formaldehyde, they reduce their respiratory minute volume so that they have the equivalent of a rat exposure to 6 ppm formaldehyde. Likewise, cell proliferation was concentration- and species-dependent, with rats exposed to 15 ppm for 5 days having > 20-fold increases in cell proliferation in the nasal respiratory epithelium. Mice also had increases in cell proliferation, but they were less pronounced. The most extensive study on formaldehyde-induced effects on cell proliferation was the mechanistic carcinogenesis bioassay by Monticello et al.,(Monticello et al., 1996). It determined the number of cells in each region of the nasal respiratory epithelium and the extent of cell proliferation in each region. As discussed above, exposure-related increases in cell proliferation were determined at 6, 12, and 18 months of exposure (Fig. 1).
Species differences in airflow were shown using upper respiratory castings of the rat, nonhuman primate and human (Kimbell et al., 2001b). This study demonstrated differences in the distribution and quantity of formaldehyde exposure across species.
Careful physical chemistry-based studies of formaldehyde-induced DNA-protein cross-links were conducted by Heck and Casanova (Casanova-Schmitz et al., 1984; Heck et al., 1990). These demonstrated a nonlinear relationship between cross-links and airborne formaldehyde concentration, with disproportionately greater amounts of DNA-protein cross-links (DPC) per ppm formaldehyde at exposures of 6 ppm and greater, reflecting saturation of the glutathione detoxication pathways. This is illustrated in Figure 2. They also found no evidence for accumulation of DPC in multiple day exposures (Casanova et al., 1994). However, the methods could not distinguish between loss, repair and protease degradation of the cross-links to small peptides. Furthermore, while the methods could distinguish DPC arising from inhaled formaldehyde when radiolabeled formaldehyde was used, the methods could not quantify DPC arising from endogenously formed formaldehyde.
Figure 2.
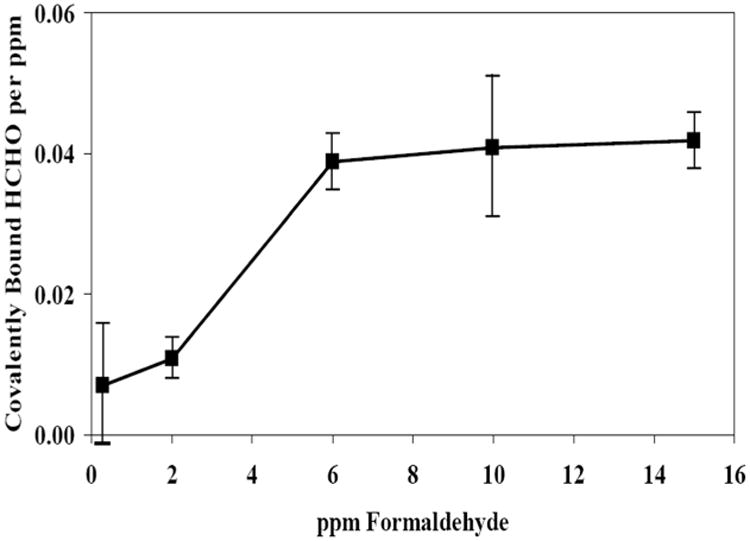
Nonlinear formation of formaldehyde DNA-protein cross-links following inhalation exposure of rats and normalization per ppm. Adapted from Casanova et al.,1984.
The carcinogenicity and mechanistic studies of formaldehyde have been incorporated into detailed biologically-based models for predicting the exposure-response for use in risk assessment (Conolly et al., 2003; Conolly et al., 2004). The data are consistent with high concentrations of formaldehyde being causal for nasal cancer, with disproportionately lower risks as exposures go below 2 ppm formaldehyde. A recent review of the Draft IRIS Risk Assessment on Formaldehyde (US Environmental Protection Agency, 2010) by the National Academy of Sciences strongly endorsed the use of the biologically-based models for formaldehyde's risk assessment (Committee to Review EPA's Draft IRIS Assessment of Formaldehyde and and National Research Council, 2011).
Recent Mode of Action Studies
Studies on Endogenous vs Exogenous Formaldehyde DNA Adducts
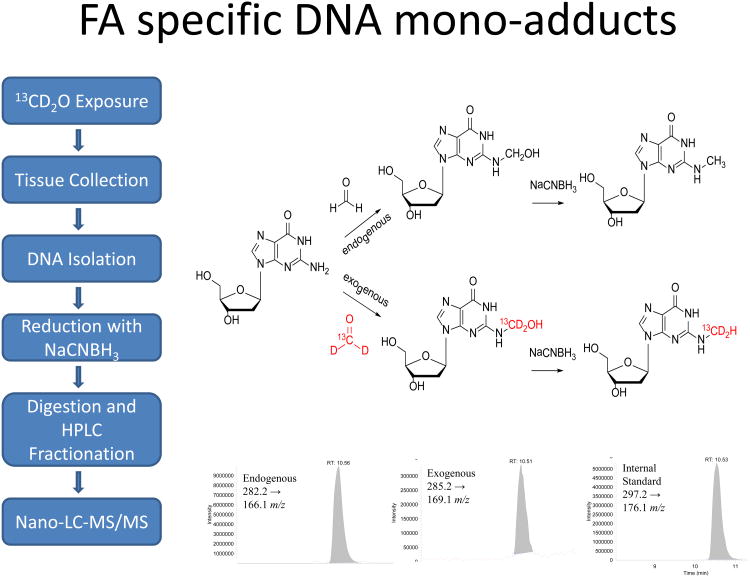
While it has long been known that formaldehyde is formed endogenously in all living cells, accurate measurements of its binding to DNA and proteins had never been made. Making use of mass spectrometry as a major tool for quantifying DNA damage and repair, we hypothesized that we could expose cells and intact animals to [13CD2]-formaldehyde, and thereby differentiate between the DNA adducts that arose from inhaled [13CD2]-formaldehyde and those that arose from endogenously formed formaldehyde. Lu et al., (Lu et al., 2010b) analyzed the in vitro reactions between formaldehyde and all of the amino acids and deoxynucleosides, and their oligomers. This study demonstrated that formaldehyde reacted predominantly with deoxyguanosine (dG). It also readily formed cross-links between lysine and dG, but these cross-links rapidly disintegrated, with a half-life of minutes. In contrast, dG cross-links with cysteine were much more stable. We knew that formaldehyde formed N2-hydroxy-methyl-dG adducts, so we exposed rats by nose-only inhalation to 10 ppm [13CD2]-formaldehyde for 6 hr/day for 1 or 5 days (Lu et al., 2010a), and rat tissues were analyzed for [13CD2]-N2-hydroxy-methyl-dG adducts resulting from the exposure, as well as [12CH2]-N2-hydroxy-methyl-dG adducts that were of endogenous origin. Since N2-hydroxy-methyl-dG adducts are not stable, they were reduced to N2-methyl-dG adducts using cyanoborohydride. Figure 3 illustrates the approach used for these experiments. The first study using stable isotope formaldehyde exposures provided clear evidence that inhaled formaldehyde reached the initial site of contact, the nasal respiratory epithelium. However, no [13CD2]-N2-methyl-dG adducts were detectable in other more distant tissues, including lung, liver, spleen, mononuclear white blood cells or bone marrow (Table 1). In contrast, all tissues had endogenous N2-methyl-dG adducts. Of additional interest, endogenous N6-deoxyadenosine-hydroxy-methyl adducts were also found in all tissues, but no [13CD2]-N6-hydroxy-methyl-dA adducts were found in any tissues. Finally, dG-[13CD2]-dG cross-links were quantified and found to be formed at ∼10% of the [13CD2]-N2-hydroxy-methyl-dG monoadducts. Lu et al., (Lu et al., 2010a) also showed that these cross-links form artifactually during sample preparation, suggesting that they are not a reliable biomarker of exposure. This study provided compelling information that inhaled formaldehyde does not reach tissues distant to the site of initial contact, raising major questions about the biological plausibility of hypotheses that inhaled formaldehyde causes leukemia.
Figure 3.
Analytical approach for the quantitation of endogenous and exogenous formaldehyde DNA adducts. The left panel outlines the methodology for the determination of the DNA adducts. The upper right panel shows the formation of the endogenous (upper route) and exogenous (lower route) N2-OHMedG adducts. The lower right panel shows the representative LC-MS/MS chromatograms of endogenous, exogenous and isotope-labeled internal standard.
Table 1.
Formaldehyde-induced N2-hydroxy-methyl-dG monoadducts in rats exposed to 10 ppm formaldehyde for 1 day or 5 days.
| Exposure Period | Tissues | N2-HOCH2-dG (adducts/107 dG) | N2-HOCH2-dA (adducts/107 dA) | ||
|---|---|---|---|---|---|
| Exogenous | Endogenous | Exogenous | Endogenous | ||
| 1 day | Nose | 1.28 ± 0.49 | 2.63 ± 0.73 | nd | 3.95 ± 0.26 |
| Lung | nd* | 2.39 ± 0.16 | nd | 2.62 ± 0.24 | |
| Liver | nd | 2.66 ± 0.53 | nd | 2.62 ± 0.46 | |
| Spleen | nd | 2.35 ± 0.31 | nd | 1.85 ± 0.19 | |
| Bone Marrow | nd | 1.05 ± 0.14 | nd | 2.95 ± 1.32 | |
| Thymus | nd | 2.19 ± 0.36 | nd | 2.98 ± 1.11 | |
| Mononuclear WBC | nd | 1.28 ± 0.38 | nd | 3.80 ± 0.29 | |
| 5 day | Nose | 2.43 ± 0.78 | 2.84 ± 1.13 | nd | 3.61 ± 0.95 |
| Lung | nd | 2.61 ± 0.35 | nd | 2.47 ± 0.55 | |
| Liver | nd | 3.24 ± 0.42 | nd | 2.87 ± 0.65 | |
| Spleen | nd | 2.35 ± 0.59 | nd | 2.23 ± 0.89 | |
| Bone Marrow | nd | 1.17 ± 0.35 | nd | 2.99 ± 0.08 | |
| Thymus | nd | 1.99 ± 0.30 | nd | 2.48 ± 0.11 | |
| Mononuclear WBC | nd | 1.10 ± 0.28 | nd | 3.66 ± 0.78 | |
Adapted from Lu, Collins, et al. (2010)
nd: not detected
Our methods for analyzing formaldehyde DNA adducts have recently been refined by switching from capillary liquid chromatography to a nano-UPLC system, providing a 10-fold lower detection limit (20 amol). This improved methodology was applied in three additional formaldehyde inhalation studies. Lu et al., (Lu et al., 2011) examined the molecular dosimetry of rat nose-only exposures to 0, 0.7, 2.0, 6, 10, or 15 ppm [13CD2]-formaldehyde for 6 hours. This study demonstrated striking differences in the ratio of endogenous to exogenous N2-hydroxy-methyl dG adducts. At 0.7 ppm, there were approximately 100 endogenous formaldehyde adducts for each exogenous formaldehyde DNA adduct present in rat nasal DNA. Table 2 shows the abundance of each adduct type as functions of exposure concentration. Only exposure to 15 ppm [13CD2]-formaldehyde for 6 hours induced exogenous adducts in greater numbers than the endogenous adducts that were always present. It should be pointed out that the endogenous formaldehyde DNA adducts were at steady-state, whereas a single 6 hour exposure does not produce steady-state amounts. Experiments that will establish the number of daily exposures needed to achieve near-steady-state exogenous formaldehyde adduct concentrations are currently underway.
Table 2.
Formaldehyde-induced N2-OHMe-dG adducts in the nasal epithelium of rats exposed to [13CD2]-formaldehyde for 6 hr.
| exposure (ppm) | endogenous dG adduct (adducts/107 dG) | exogenous dG adducts (adducts/107 dG) |
|---|---|---|
| 0.7±0.2 | 3.62±1.33a | 0.039±0.019 |
| 2.0±0.1 | 6.09 ±3.03b | 0.19±0.08 |
| 5.8± 0.5 | 5.51 ±1.06c | 1.04 ±0.24 |
| 9.1±2.2 | 3.41 ±0.46 | 2.03 ±0.43 |
| 15.2±2.1 | 4.24 ±0.92 | 11.15±3.01 |
Adapted from Lu et al. (2011).
Four to six rat samples were combined for each mass spectrometry measurement; n = 3.
Two rat samples were combined for each mass spectrometry measurement; n = 4.
Rat samples were not combined for 5.8, 9.1, and 15 ppm groups; typically n = 5.
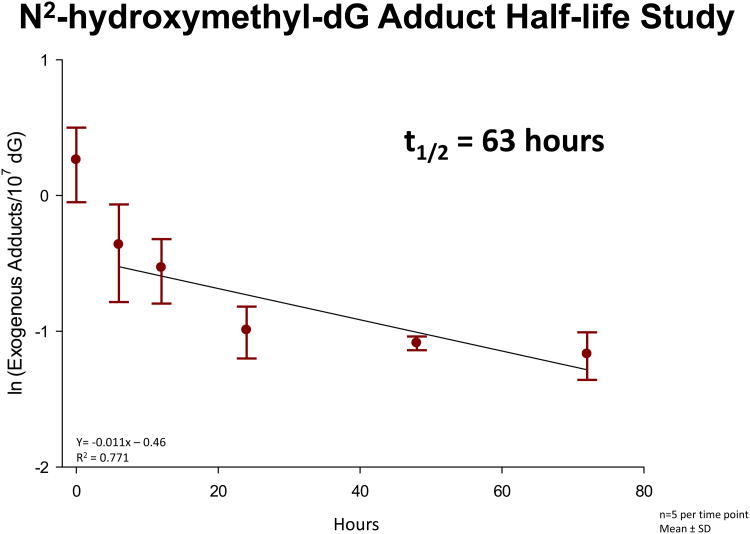
The same inhalation study (Lu et al., 2011) utilized additional rats exposed to a single 6 hour nose-only exposure to 10 ppm [13CD2]-formaldehyde. The additional animals were used to estimate the half-life of the [13CD2]-N2-hydroxy-methyl-dG adducts in rat nasal mucosa following a high concentration exposure. The study was designed to compare the number of exogenous DNA adducts at the end of a 6 hour, 10 ppm exposure with those for 6, 12, 24, 48 and 72 hours post-exposure (n= 4-5 rats per time point). The data are shown in Fig. 4. The loss of nearly half of the adducts in the first 6 hours post-exposure was surprising. This rapid initial falloff was followed by a relatively constant decrease in adducts with a T1/2 of 63 hours (R2 = 0.771). The rapid loss during the first 6 hours post-exposure is thought to be the result of cell death, not DNA repair, whereas the subsequent decreases over three days are believed to reflect DNA repair and/or spontaneous decomposition. The T1/2 for the repair/loss of [13CD2]-N2-hydroxy-methyl-dG adducts will be further elucidated in a 28 day study using exposures to 2 ppm [13CD2]-formaldehyde (6 hr/day, 7 days/week), with post-exposure times of 0, 6, 24, 72 and 168 hours. The exposures for this additional study have been completed, but the DNA adducts have not yet been analyzed. This study is not expected to show the rapid loss by 6 hours post-exposure, as 2 ppm inhalation exposures to formaldehyde have not resulted in prominent cytotoxicity or increased cell repair. The exogenous DNA adducts will likely have approached steady-state concentrations during this longer study, and this extended study should provide the rate of loss of the [13CD2]-N2-hydroxy-methyl-dG adducts under conditions more akin to past human occupational exposures.
Figure 4.
The half-life of [13CD2]-N2-OHMedG adducts in rat nasal mucosa. Data was transformed using natural log and a linear regression was accomplished using the last 5 time points.
A third inhalation study with stable isotope formaldehyde (Moeller et al., 2011) was conducted in nonhuman primates (NHP) with the 20 amol detection limit. This study is important because rats are obligatory nose breathers, while humans and NHP breathe both nasally and orally. This study was conducted using whole body exposures to 1.9 or 6.1 ppm of [13CD2]-formaldehyde, 6 hr/day for 2 consecutive days. As in the rat studies, exogenous DNA adducts were only detected in nasal DNA. No exogenous adducts were detected in bone marrow, where a minimum of 300 μg of DNA was analyzed, using HPLC fraction collection of the endogenous and exogenous N2-methyl-dG adducts. The entire fraction was then applied to the nano-UPLC-MS/MS. This provided us with the ability to detect as few as one [13CD2]-N2-methyl-dG adduct in 10 billion dGs. No exogenous adducts were found, despite exquisite sensitivity. This study provides additional compelling data that inhaled formaldehyde does not reach the bone marrow of primates. In further analysis of the primate tissues, no exogenous DNA adducts were detected in multiple regions of the brain, several sections of the lungs, and nasopharynx. Exogenous adducts were detected in the dorsal nasal cavity from the 6 ppm exposures with exogenous adducts at ∼ 15% of the maxilloturbinate levels. Together, the data support the argument for formaldehyde being a nasal carcinogen and shed additional light on the contribution of differences in nasal anatomy and breathing patterns between species on the distribution of inhaled formaldehyde. Given that inhaled formaldehyde was again not detectable in distant tissues, the plausibility that inhaled formaldehyde can cause leukemia must be seriously questioned.
Epigenetic Effects of Inhaled Formaldehyde
In order to further investigate molecular mechanisms underlying formaldehyde-induced health effects, our research team has investigated additional epigenetic changes caused by formaldehyde exposure. Epigenetic changes are not mutations, but include altered DNA methylation, histone methylation, and changes in microRNA (miRNA) expression. Using in vitro exposures, we have demonstrated that formaldehyde binds to the lysine molecules in histone 4. Furthermore, when a histone lysine is adducted to formaldehyde, it cannot be acetylated, and if it was acetylated, it could not bind formaldehyde (Lu et al., 2008).
Our team has investigated whether formaldehyde exposure disrupts miRNA expression levels within cultured lung cells (Rager et al., 2010). It is important to investigate potential effects of formaldehyde on miRNAs because they regulate gene expression by binding to mRNA, causing 1) rapid decay of the message, (2) translational repression of the mRNA signals, and (3) inducing cleavage of newly translated polypeptides (Filipowicz et al., 2008). If miRNA expression is increased by formaldehyde exposure, the targeted mRNA and proteins can be reduced. Conversely, if the miRNA has decreased expression, one expects increased mRNA and translation of proteins. In our study, human A549 lung epithelial cells were exposed to formaldehyde (1 ppm) using an in vitro exposure system that physically simulates in vivo human lung exposures. Upon exposure to formaldehyde, the lung epithelial cells showed decreased expression in 89 of 534 miRNAs that we measured using human miRNA microarrays. All of the modulated miRNAs were down-regulated by formaldehyde exposure. This general trend of miRNA down-regulation has been observed in rat lung cells exposed to cigarette smoke (Izzotti et al., 2009), as well as in multiple tumor cell types, including lung cancer, breast cancer, and leukemia (Lu et al., 2008).
More recently, we have examined the effects of formaldehyde exposures on microRNA expression in the maxilloturbinate of Cynomolgus monkeys exposed to 2 or 6 ppm formaldehyde. If miRNA expression is increased by formaldehyde exposure, the targeted mRNA and proteins are reduced, while if the miRNA has decreased expression, one expects increased mRNA and translation of proteins. In the primate study, when comparing miRNAs in tissue from unexposed controls, we identified 3 miRNAs with altered expression in the maxilloturbinate of monkeys exposed to 2 ppm formaldehyde and 13 miRNAs with altered expression when exposures were to 6 ppm formaldehyde. MiRNA-125b had the greatest increase in expression and this was confirmed with RT-PCR. We then predicted transcriptional targets of miR-125b and used a systems biology approach to identify associated signaling pathways. This systems-level analysis revealed that apoptosis signaling was likely modified by formaldehyde's effect on miRNA expression. This prediction was confirmed at the gene expression level, where all apoptosis-related targets of miR-125b, specifically BAK1, CASP2, MAP2K7 and MCL1, were decreased in formaldehyde-exposed samples. Of additional interest, MAP2K7 and MCL1 had previously been shown to have altered gene expression in rat nasal tissue (Andersen et al., 2010).
We are currently examining miRNA expression in tissues from rats exposed to 2 ppm [13CD2]-formaldehyde for 4 weeks (6 hrs/day, 7 days/wk). To date, we have identified 59 miRNAs with altered expression in the nasal epithelium, 8 miRNAs with altered expression in mononuclear WBCs, and no miRNAs with altered expression in bone marrow. The greatest decrease in expression occurred in miRNA-203, in the nasal tissue of both rats and nonhuman primates. Using a systems biology approach, we predicted transcriptional targets for miRNA-203 and found Rap1 and RaP1A, members of the ras oncogene family, to be affected. Andersen et al., (Andersen et al., 2010) also observed altered expression in Rap1 and RAP1A in formaldehyde-exposed rat nasal epithelium. This study will also include exposures to 2 ppm [13CD2]-formaldehyde for 1 week, as well as 1 week post-exposure after the 4 weeks of exposure. These additional time points will allow us to determine temporal relationships for altered miRNAs. In addition, we will examine methylation of DNA for the same exposure groups.
Cancer Risk Assessment of Formaldehyde
The demonstration that formaldehyde is an animal carcinogen that induced a very high incidence of nasal cancer in rats provoked widespread concerns regarding potential human health effects resulting from formaldehyde exposure. Quantitative risk assessment methods were still being developed, with emphasis on linearized multistage models that utilized high administered dose cancer incidence data collected in laboratory animal bioassays (Cohn, 1981; U.S.Environmental Protection Agency, 1984; US Consumer Product Safety Commission, 1982). These early risk assessments did not utilize any data from the early mode of action studies discussed above. Starr and Gibson pointed out that such assessments needed to take into account the differences between administered dose and delivered dose, as this distinction could make enormous differences in estimated risks (Starr and Gibson, 1985). In spite of this, most quantitative risk assessments, even to the present day, continue to use administered doses with the linearized multistage approach to low dose human cancer risk assessment. Using such approaches is, in essence, equivalent to stating that none of the mode of action data inform our understanding of the potential low-dose risks for cancer associated with formaldehyde. Ironically, just using the delivered dose formaldehyde data developed in the early 1980s lowered the multistage maximum likelihood risk estimates by a factor of 53 (Starr and Gibson, 1985).
Today, our knowledge regarding the mode of action of formaldehyde-induced carcinogenesis is much greater, yet most of this new knowledge has not been applied in assessments of formaldehyde risk (International Agency for Research on Cancer, 2012; National Toxicology Program, 2011; US Environmental Protection Agency, 2010). The NRC (Committee to Review EPA's Draft IRIS Assessment of Formaldehyde and and National Research Council, 2011) review of the EPA report (US Environmental Protection Agency, 2010) was critical of its lack of use of mode of action data, as well as its lack of use of the biologically-based models. We now have much greater knowledge of the distribution of endogenous and inhaled formaldehyde at the molecular level. Two such findings are of great importance: (1) every living cell contains formaldehyde and measurable formaldehyde N2-hydroxy-methyl-dG adducts; and (2) there is no evidence that inhaled formaldehyde reaches sites distant to the initial site of contact. Indeed, there is strong evidence that inhaled formaldehyde does not reach any distant tissues. Likewise, no consideration has been given to confounding for NPC in the Wallingford, CT plant workers, even though the other 9 plants had a 35% decrement in the expected number of NPC cases.
Data regarding the molecular dose of formaldehyde reaching various tissues has recently been employed in a new “bottom-up” risk assessment approach that places useful upper bounds on low-dose cancer risks that do not depend upon high dose data for humans or animals. Thus, this novel approach provides an independent “reality check” on low dose risk estimates that are derived with models fit to high dose cancer data (Starr and Swenberg, 2012). This approach 1) is consistent with the “additivity to background” concept, 2) yields central and upper-bound risk estimates that are linear at all doses, and 3) only requires information regarding background risk and background exposures to be implemented. Using the molecular data for endogenous and exogenous DNA adducts from the primate study of Moeller et al., (Moeller et al., 2011) and the detection limit of 20 × 10-18 mol of N2-hydroxy-methyl-dG, central and lower 95% confidence bound estimates of steady-state exogenous adducts expected to arise in nasal mucosa and bone marrow from continuous 24 hrs/day, 7 days/week exposure to 2 ppm formaldehyde were developed. Background human risk estimates for nasopharyngeal cancer and leukemia were taken from the 2010 EPA draft,(US Environmental Protection Agency, 2010)and SEER Cancer Statistics Review, 1975-2007 (Altekruse et al., 2010), respectively. The upper bound estimate of NPC risk obtained with the “bottom-up” approach was nearly 29-fold lower than EPA's plausible upper bound estimate derived from high dose epidemiology data. For the risk of leukemia, the “bottom-up” upper bound risk estimate was more than 14,000-fold lower than EPA's corresponding estimate, again as it was derived from high dose epidemiology data. These results strongly support a conclusion that the much larger risk estimates derived by the EPA (US Environmental Protection Agency, 2010), are overly conservative. It is thus imperative that high quality mode of action data be brought into the risk assessment process and that the traditional risk assessment assumptions that lack such strong scientific bases are tested rigorously.
When USEPA developed lifetime human nasal cancer risk estimates from tumor data for rats, the Agency made adjustments for human-rat differences in nasal anatomy, breathing rates, and exposure duration (US Environmental Protection Agency, 2010). Results from Computational Fluid Dynamics (CFD) modeling simulations conducted by Kimbell et al. (Kimbell et al., 2001a; Kimbell et al., 2001b) were utilized to compute the average flux of formaldehyde into rat nasal tissues (excluding those from the olfactory region) that results from exposure, at resting breathing rates, to a given airborne formaldehyde concentration. A similar calculation was undertaken for human nasal tissues (including those from the olfactory region) exposed to the same airborne formaldehyde concentration at breathing rates corresponding to equal durations (8 hours) of resting, light, and moderate activity levels.
The rat-to-human ratio of these average flux rates under the specified conditions was determined to be 0.46 (US Environmental Protection Agency, 2010). This implies that approximately twice (1/0.46) the amount of formaldehyde is expected to be deposited in the nasal tissues of humans as would be deposited in the non-olfactory nasal tissues of rats when both species are exposed to the same airborne formaldehyde concentration. Alternatively, it implies that the “human equivalent” exposure concentration that corresponds to a given rat exposure concentration is approximately one-half (a factor of 0.46) as large. Thus, at least according to this Agency computation, humans are expected to be about twice as sensitive as rats to the carcinogenic effects of airborne formaldehyde in nasal tissues.
This rat-to-human ratio can also be estimated from rat-to-monkey using molecular dosimetry data for DPC from Heck et al. (Heck et al., 1990) and exogenous DNA adducts derived from Lu et al., (Lu et al., 2011) and Moeller et al., (Moeller et al., 2011), since the anatomy and breathing patterns of humans and monkeys are similar (Kimbell et al., 2001b). Monitoring DPC formation in rats and primates showed that at 2 and 6 ppm the ratio of rat-to-primate DPC formation was approximately 6 at both concentrations. Thus, the molecular dose of formaldehyde DNA damage suggests that the data driven ratio for rats-to-humans is also likely to be ∼6, rather than 0.46. The more recent primate studies investigating exogenous DNA adduct formation can be compared in a similar manner by dividing the exogenous adducts by the length of exposure (6 hours for rats and 12 hours for primates) with the ratio of rat-to-primate formation being ∼1.5 and 5.1 at 2 and 6 ppm, respectively. The ratio of formation of exogenous DPCs and adducts in the nasal tissues between the two species is very similar at the 6 ppm exposure concentration, while it is less similar at 2 ppm. While differences exist between the two biomarkers of exposure (DPC and adducts), it is remarkable that similar results were obtained using completely different methodologies nearly 20 years later. Since DNA damage is considered a key event in formaldehyde's MOA, these data provide an important check on the accuracy of the EPA estimated effects of inhalation exposure for humans.
In summary, over 30 years of intense research on formaldehyde has provided a path forward for improving the way we evaluate carcinogens, examine dose-response relationships, and conduct critical mode of action studies to better understand the processes that drive the biology of carcinogenesis in animals and humans. The results of such efforts should play major roles in science-based risk assessments that will more accurately predict human risks for inhaled exposures to humans and protect the public health.
References
- Altekruse SF, Kosary CL, Karcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review, 1975-2007. National Cancer Institute; Bethesda: 2010. [Google Scholar]
- Andersen ME, Clewell HJ, III, Bermudez E, Dodd DE, Willson GA, Campbell JL, Thomas RS. Formaldehyde: integrating dosimetry, cytotoxicity, and genomics to understand dose-dependent transitions for an endogenous compound. Toxicol Sci. 2010;118:716–731. doi: 10.1093/toxsci/kfq303. [DOI] [PubMed] [Google Scholar]
- Bachand AM, Mundt KA, Mundt DJ, Montgomery RR. Epidemiological studies of formaldehyde exposure and risk of leukemia and nasopharyngeal cancer: a meta-analysis. Crit Rev Toxicol. 2010;40:85–100. doi: 10.3109/10408440903341696. [DOI] [PubMed] [Google Scholar]
- Beane Freeman LE, Blair A, Lubin JH, Stewart PA, Hayes RB, Hoover RN, Hauptmann M. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: the National Cancer Institute cohort. J Nat Cancer Inst. 2009;101:751–761. doi: 10.1093/jnci/djp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti C, McLaughlin JK, Tarone RE, Pira E, La VC. Formaldehyde and cancer risk: a quantitative review of cohort studies through 2006. Ann Oncol. 2008;19:29–43. doi: 10.1093/annonc/mdm202. [DOI] [PubMed] [Google Scholar]
- Casanova M, Morgan KT, Gross EA, Moss OR, Heck HA. DNA-protein cross-links and cell replication at specific sites in the nose of F344 rats exposed subchronically to formaldehyde. Fundam Appl Toxicol. 1994;23:525–536. doi: 10.1006/faat.1994.1137. [DOI] [PubMed] [Google Scholar]
- Casanova-Schmitz M, Starr TB, Heck Hd. Differentiation between metabolic incorporation and covalent binding in the labeling of macromolecules in the rat nasal mucosa and bone marrow by inhaled [14C]- and [3H]formaldehyde. Toxicol Appl Pharmacol. 1984;76:26–44. doi: 10.1016/0041-008x(84)90026-7. [DOI] [PubMed] [Google Scholar]
- Chang JCF, Gross EA, Swenberg JA, Barrow CS. Nasal cavity deposition, histopathology, and cell proliferation after single or repeated formaldehyde exposures in B6C3F1 mice and F-344 rats. Toxicol Appl Pharmacol. 1983;68:161–176. doi: 10.1016/0041-008x(83)90001-7. [DOI] [PubMed] [Google Scholar]
- Coggon D, Harris EC, Poole J, Palmer KT. Extended follow-up of a cohort of British chemical workers exposed to formaldehyde. J Natl Cancer Inst. 2003;95:1608–1615. doi: 10.1093/jnci/djg046. [DOI] [PubMed] [Google Scholar]
- Cohn MS. Revised Carcinogenic Risk Assessment of Urea-Formaldehyde Foam Insulation: Estimates of Cancer Risk due to Inhalation of Formaldehyde Released by UFFI Oct 26. Washington, DC: US Cons Prod Safety Comm; 1981. [Google Scholar]
- Collins JJ, Acquavella JF, Esmen NA. An updated meta-analysis of formaldehyde exposure and upper respiratory tract cancers. J Occup Environ Med. 1997;39:639–651. doi: 10.1097/00043764-199707000-00009. [DOI] [PubMed] [Google Scholar]
- Committee to Review EPA's Draft IRIS Assessment of Formaldehyde and National Research Council. Review of the Environmental Protection Agency's Draft IRIS Assessment of Formaldehyde. The National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Conolly RB, Kimbell JS, Janszen D, Schlosser PM, Kalisak D, Preston J, Miller FJ. Biologically motivated computational modeling of formaldehyde carcinogenicity in the F344 rat. Toxicol Sci. 2003;75:432–447. doi: 10.1093/toxsci/kfg182. [DOI] [PubMed] [Google Scholar]
- Conolly RB, Kimbell JS, Janszen D, Schlosser PM, Kalisak D, Preston J, Miller FJ. Human respiratory tract cancer risks of inhaled formaldehyde: dose-response predictions derived from biologically-motivated computational modeling of a combined rodent and human dataset. Toxicol Sci. 2004;82:279–296. doi: 10.1093/toxsci/kfh223. [DOI] [PubMed] [Google Scholar]
- Duhayon S, Hoet P, Van Maele-Fabry G, Lison D. Carcinogenic potential of formaldehyde in occupational settings: a critical assessment and possible impact on occupational exposure levels. Int Arch Occup Environ Health. 2008;81:695–710. doi: 10.1007/s00420-007-0241-9. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Hauptmann M, Lubin JH, Stewart PA, Hayes RB, Blair A. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries. J Nat Cancer Inst. 2003;95:1615–1623. doi: 10.1093/jnci/djg083. [DOI] [PubMed] [Google Scholar]
- Hauptmann M, Lubin JH, Stewart PA, Hayes RB, Blair A. Mortality from solid cancers among workers in formaldehyde industries. Am J Epidemiol. 2004;159:1117–1130. doi: 10.1093/aje/kwh174. [DOI] [PubMed] [Google Scholar]
- Hauptmann M, Stewart PA, Lubin JH, Beane Freeman LE, Hornung RW, Herrick RF, Hoover RN, Fraumeni JF, Blair A, Hayes RB. Mortality from lymphohematopoietic malignancies and brain cancer among embalmers exposed to formaldehyde. J Nat Cancer Inst. 2009;101:1696–1708. doi: 10.1093/jnci/djp416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck HD, Casanova M, Starr TB. Formaldehyde toxicity--new understanding. Crit Rev Toxicol. 1990;20:397–426. doi: 10.3109/10408449009029329. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. IARC Monogr Eval Carcinog Risks Hum. 2006;88:1–287. [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Formaldehyde In A review of human carcinogens Part F: Chemical agents and related occupations. IARC; Lyon, France: 2012. pp. 401–435. [Google Scholar]
- Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. The FASEB Journal. 2009;23:806–812. doi: 10.1096/fj.08-121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns WD, Pavkov KL, Donofrio DJ, Gralla EJ, Swenberg JA. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 1983;43:4382–4392. [PubMed] [Google Scholar]
- Kimbell JS, Overton JH, Subramaniam RP, Schlosser PM, Morgan KT, Conolly RB, Miller FJ. Dosimetry modeling of inhaled formaldehyde: binning nasal flux predictions for quantitative risk assessment. Toxicol Sci. 2001a;64:111–121. doi: 10.1093/toxsci/64.1.111. [DOI] [PubMed] [Google Scholar]
- Kimbell JS, Subramaniam RP, Gross EA, Schlosser PM, Morgan KT. Dosimetry modeling of inhaled formaldehyde: comparisons of local flux predictions in the rat, monkey, and human nasal passages. Toxicol Sci. 2001b;64:100–110. doi: 10.1093/toxsci/64.1.100. [DOI] [PubMed] [Google Scholar]
- Lu K, Boysen G, Gao L, Collins LB, Swenberg JA. Formaldehyde-induced histone modifications in vitro. Chem Res Toxicol. 2008;21:1586–1593. doi: 10.1021/tx8000576. [DOI] [PubMed] [Google Scholar]
- Lu K, Collins LB, Ru H, Bermudez E, Swenberg JA. Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol Sci. 2010a;116:441–451. doi: 10.1093/toxsci/kfq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Moeller B, Doyle-Eisele M, McDonald J, Swenberg JA. Molecular dosimetry of N2-hydroxymethyl-dG DNA adducts in rats exposed to formaldehyde. Chem Res Toxicol. 2011;24:159–161. doi: 10.1021/tx1003886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Ye W, Zhou L, Collins LB, Chen X, Gold A, Ball LM, Swenberg JA. Structural characterization of formaldehyde-induced cross-links between amino acids and deoxynucleosides and their oligomers. J Am Chem Soc. 2010b;132:3388–3399. doi: 10.1021/ja908282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh GM, Youk AO, Buchanich JM, Erdal S, Esmen NA. Work in the metal industry and nasopharyngeal cancer mortality among formaldehyde-exposed workers. Regul Toxicol Pharmacol. 2007;48:308–319. doi: 10.1016/j.yrtph.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Moeller BC, Lu K, Doyle-Eisele M, McDonald J, Gigliotti A, Swenberg JA. Determination of N2-hydroxymethyl-dG adducts in nasal epithelium and bone marrow of non-human primates following 13CD2-formaldehyde inhalation exposure. Chem Res Toxicol. 2011;24:162–164. doi: 10.1021/tx1004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticello TM, Morgan KT, Hurtt ME. Unit length as the denominator for quantitation of cell proliferation in nasal epithelia. Toxicol Pathol. 1990;18:24–31. doi: 10.1177/019262339001800104. [DOI] [PubMed] [Google Scholar]
- Monticello TM, Swenberg JA, Gross EA, Leininger JR, Kimbell JS, Seilkop S, Starr TB, Gibson JE, Morgan KT. Correlation of regional and nonlinear formaldehyde-induced nasal cancer with proliferating populations of cells. Cancer Res. 1996;56:1012–1022. [PubMed] [Google Scholar]
- National Toxicology Program. U S Department of Health andx Human Services, Public Health Service. Twelfth. National Toxicology Program; Washington, DC: 2011. Report on Carcinogens. [Google Scholar]
- Pinkerton LE, Hein MJ, Stayner LT. Mortality among a cohort of garment workers exposed to formaldehyde: an update. Occup Environ Med. 2004;61:193–200. doi: 10.1136/oem.2003.007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager JE, Smeester L, Jaspers I, Sexton KG, Fry RC. Epigenetic changes induced by air toxics: formaldehyde xposure Alters miRNA Expression Profiles in Human Lung Cells. Environ Health Perspect. 2010;119:494–500. doi: 10.1289/ehp.1002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TB, Gibson JE. The mechanistic toxicology of formaldehyde and its implications for quantitative risk estimation. Annu Rev Pharmacol Toxicol. 1985;25:745–767. doi: 10.1146/annurev.pa.25.040185.003525. [DOI] [PubMed] [Google Scholar]
- Starr TB, Swenberg JA. A novel bottom-up approach to bounding low-dose human cancer risks from chemical exposures. Regul Toxicol Pharmacol. 2013;65:311–5. doi: 10.1016/j.yrtph.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Swenberg JA, Kerns WD, Mitchell RI, Gralla EJ, Pavkov KL. Induction of squamous cell carcinomas of the rat nasal cavity by inhalation exposure to formaldehyde vapor. Cancer Res. 1980;40:3398–3402. [PubMed] [Google Scholar]
- US Consumer Product Safety Commission. Part IV: Consumer Product Safety Commission ban of urea formaldehyde foam insulation, withdrawal of proposed labeling rule, and denial of petition to issue a standard. Fed Regist. 1982;47:14366–14419. [Google Scholar]
- US Environmental Protection Agency. Formaldehyde: Determination of significant risk. Fed Regist. 1984;49:21870–21898. [Google Scholar]
- US Environmental Protection Agency. IRIS Toxicological Review of Formaldehyde (Inhalation)(External Review Draft) EPA/635/R-10/002A 2010 [Google Scholar]