Summary
Infection is an important cause of stillbirths worldwide: in low-income and middle-income countries, 50% of stillbirths or more are probably caused by infection. By contrast, in high-income countries only 10–25% of stillbirths are caused by infection. Syphilis, where prevalent, causes most infectious stillbirths, and is the infection most amenable to screening and treatment. Ascending bacterial infection is a common cause of stillbirths, but prevention has proven elusive. Many viral infections cause stillbirths but aside from vaccination for common childhood diseases, we do not have a clear prevention strategy. Malaria, because of its high prevalence and extensive placental damage, accounts for large numbers of stillbirths. Intermittent malarial prophylaxis and insecticide-treated bednets should decrease stillbirths. Many infections borne by animals and vectors cause stillbirths, and these types of infections occur frequently in low-income countries. Research that better defines the relation between these infections and stillbirths, and develops strategies to reduce associated adverse outcomes, should play an important part in reduction of stillbirths in low-income countries.
Introduction
Stillbirth, defined as no sign of life in a neonate at delivery, is one of the most common adverse outcomes of pregnancy. 3·2 million stillbirths or more occur worldwide every year, with 98% or more in countries of low and middle income.1, 2, 3 In such countries, the rate of stillbirths is generally 20–40 per 1000 births, increasing to 100 per 1000 births in some areas, compared with 3–5 per 1000 births in most high-income countries.1, 2, 3 Maternal or fetal infections account for 50% of stillbirths or more in low-income and middle-income countries, but only 10–25% in high-income countries.4, 5, 6 In a review of stillbirth in countries of low and middle income, the population attributable fraction was greater than 50% for five risk factors associated with stillbirth, of which two factors—syphilis and chorioamnionitis—were infection-related.7 Important non-infectious causes of stillbirth include congenital anomalies, placental insufficiency, placental abruption, and asphyxia.8, 9
The lower limits of gestational age (20–28 weeks) and birthweight (350–1000 g) that are used to define stillbirth vary across geographical areas.3 Infection is more clearly associated with early (<28 weeks) compared with late stillbirth (≥28 weeks), and with reduced birthweight.5, 6, 10 Consequently, studies that assess only late fetal deaths will miss the large contribution of infection to stillbirths.
For many reasons, the relation between infection and stillbirth is often unclear.5, 6 Most importantly, infection is seldom apparent from the case history or physical examination of the mother or fetus. Histological assessments of the placenta, placental cultures, and fetal autopsies could miss important infections. Additionally, even with evidence of infection, the precise reasons for specific stillbirths are often difficult to establish. Neither positive serological tests nor organisms in the placenta or fetus prove causality.4, 5, 6, 7, 10 Furthermore, infection can initiate a chain of events leading to stillbirth, and its contribution to fetal death might not be appreciated (eg, rubella infections cause congenital anomalies). Which stillbirths are attributed to infection depends partly on the extent of the investigation and the classification system used.11
In countries of low and middle income, placental histological examination, placental cultures, and fetal autopsies are usually unavailable. In high-income countries, such tests are generally available but are not done routinely. Since confirmation of the infectious aetiology of stillbirth requires use of these techniques at a minimum, we believe that the contribution of infections to stillbirth is substantially underdiagnosed in settings of low, middle, and high income. Furthermore, if routine bacterial and viral cultures were supplanted by advanced molecular techniques, the apparent contribution of infection to stillbirth would probably rise still further. For example, in this Review we will discuss the coxsackie virus as a cause of unexplained stillbirths.12
Mechanisms
Infection can cause stillbirth by several mechanisms. Maternal infection might lead to systemic illness with the mother becoming severely ill (eg, severe influenza), and the fetus might die because of high maternal fever, respiratory distress, or other systemic reactions, without organisms transmitted to the placenta or fetus.13, 14 Alternatively, the placenta might be directly infected, resulting in reduced blood flow to the fetus (eg, malaria),15 or the fetus might be directly infected with damage to a vital organ.16 If infection occurs early in gestation, the fetus might not die immediately but development of a congenital anomaly could lead to fetal death at a later stage of pregnancy.17 Last, a maternal infection of the genital tract or elsewhere might precipitate preterm labour that the fetus is unable to tolerate.18
We identified at least 40 organisms with sufficient evidence to be implicated as a cause of fetal death,6 including many different bacteria, viruses, parasites, and fungi. Although we will not examine every organism in this Review, we have drawn attention to those that are important numerically or those for which evidence suggests that further research could be beneficial. Of note, many infectious causes of stillbirth are borne by animals or vectors (table 1 ).19, 20
Table 1.
Maternal infections borne by vectors and animals and associated with stillbirth
| Organism | Reservoir | |
|---|---|---|
| Vector-borne infections | ||
| Malaria | Plasmodium falciparum | Mosquito |
| Lyme disease | Borrelia burgdorferi | Tick |
| Relapsing fever | Borrelia duttonnii | Tick |
| Tick-borne relapsing fever | Borrelia recurrentis | Tick |
| African sleeping sickness | Trypanosoma brucei | Tsetse fly |
| Chagas' disease | Trypanosoma cruzi | Triatomine (kissing bug) |
| Dengue fever | Dengue virus | Mosquito |
| Tularaemia | Francisella tularensis | Tick or deerfly, animal carcasses, contaminated food or water |
| Animal-borne infections | ||
| Listeriosis | Listeria monocytogenes | Domesticated animal products |
| Anthrax | Bacillus anthracis | Domesticated animals |
| Q fever | Coxiella burnetti | Domesticated animals |
| Brucellosis | Brucella melitenses | Domesticated animals |
| Leptospirosis | Leptospira interrogans | Dogs, livestock, wild animals |
| Toxoplasmosis | Toxoplasma gondii | Warm-blooded animals |
| Lymphocytic choriomeningitis | Lymphocytic choriomeningitis virus | House mouse |
| Ljungan virus | Ljungan virus | Wild rodents (bank voles) |
| Streptococcus porcinus | Streptococcus porcinus | Swine |
Bacterial infections
More than 130 different bacteria might participate in intrauterine infections, and many of these have been associated with stillbirth.4, 6 The types of organisms and the mechanisms by which they cause fetal death are similar across geographical areas. However, the proportion of pregnancies affected by bacterial infection is much higher in countries of low and middle income than in those of high income.3, 7 Bacterial infections leading to stillbirth can be divided into those that reach the fetal compartment through the placenta, and those that ascend from the vagina through the cervix. In transplacental infections (eg, syphilis), the placental villi often show evidence of infection and since the organisms enter the fetus through the umbilical vein, the liver is the organ most frequently infected.
Transplacental infections: syphilis and other spirochaetes
Of all potential infectious causes of stillbirth worldwide, syphilis stands out because the disease causes a large number of stillbirths but is highly preventable. Treponema pallidum is the spirochaete responsible for syphilis. Occurrence of infection in women of reproductive age varies from as high as 20% in some African populations to about 0·02% in high-income countries.21, 22, 23, 24, 25 Spirochaetes can cross the placenta and infect the fetus, with risk of fetal infection related to the stage of maternal syphilis. If the mother is infected but untreated, about 40% of fetuses will die in utero and another 30–40% will be born alive but have congenital syphilis. More than 1 million cases of congenital syphilis occur worldwide every year.26, 27 The most common cause of fetal death seems to be placental infection with decreasing blood flow to the fetus, although direct fetal infection also has a role.28 Most studies report syphilis to have a relative risk of stillbirth of 2–5; however, in a Tanzanian study, the relative risk was 18 for women with active syphilis.29 In some areas of sub-Saharan Africa, 25–50% of all stillbirths are associated with syphilis.24 Syphilis also contributes to stillbirths in other areas of the world including Russia, Asia, and South America.30, 31, 32
Stillbirths due to syphilis should be easy to eliminate. Within a functioning health system, screening of pregnant women for syphilis is feasible, and once disease is diagnosed, treatment is easy and inexpensive. Women who have been treated for syphilis have a similar or slightly greater stillbirth risk than do women who are not infected.33, 34 The reasons for the present failure to eliminate congenital syphilis, especially in sub-Saharan Africa, include poor access to prenatal care or use of such services, and failure to provide appropriate treatment for syphilis because of lack of resources, poorly functioning supply systems, and other priorities such as HIV screening and treatment. Consequently, point-of-care rapid testing and treatment could be the most cost-effective method to reduce adverse pregnancy outcomes associated with syphilis.35 Schmid and colleagues26, 27 converted the fetal or neonatal consequences of maternal syphilis into disability-adjusted life-years (DALYs). They suggested that when stillbirth was included, the cost to save 1 DALY was about US$10 and was 25 times less expensive than that for infants born to women infected with HIV.26, 27 Based on these and other data, WHO has launched a programme to eliminate congenital syphilis worldwide.36
Several other spirochaetal diseases are associated with stillbirth. Lyme disease, a systemic illness caused by the tick-borne Borrelia burgdorferi, was first associated with stillbirth in 1987.37 Small series of stillbirths associated with maternal Lyme disease have been reported, with most fetal deaths occurring in the midtrimester. Spirochaetes have been found in fetal liver, spleen, kidney, and brain. However, large-scale serological studies have shown that few stillbirths are associated with Lyme disease except in highly endemic areas.38 In Tanzania, more than 30% of adults are seropositive for B burgdorferi 39 compared with 2% in the USA and Norway, but whether Lyme disease is an important cause of stillbirths in African settings is unknown. Another spirochaetal disease associated with adverse pregnancy outcomes, mainly in sub-Saharan Africa, is tick-borne relapsing fever, caused by Borrelia duttonii. Findings from one study showed perinatal mortality of 30%.40 In the Democratic Republic of the Congo, 6·4% of pregnant women admitted to a maternity ward had relapsing fever.41 As with Lyme disease, in most African settings the prevalence of tick-borne relapsing fever and its contribution to stillbirths is unknown.42, 43
Other bacterial causes of transplacental infections
Listeria monocytogenes is another example of a haematogenously transmitted organism that causes fetal death.44 The mother acquires this infection by eating contaminated food such as unpasteurised soft cheese or undercooked meat. During bacteraemia, the organisms are transmitted to the placenta and can cause villous necrosis and microabscesses. The organisms can be transmitted to the fetus, and occurrence of stillbirth is attributed to both placental dysfunction and fetal infection. Transplacental infections have also been reported in association with maternal tularaemia, clostridia, anthrax, typhoid fever, brucellosis, Haemophilus influenzae, Pseudomonas pyocyanea, the plant bacterium Agrobacterium radiobacter, and Mycobacterium tuberculosis.6 Additionally, maternal cachexia associated with late diagnosis of tuberculosis and advanced disease seems to be a risk factor for stillbirth.45 Although tuberculosis is generally rare in pregnancy, occurrence is increasing in many locations, especially in sub-Saharan African countries where HIV is prevalent. Whether co-infection increases the risk of stillbirth compared with the risk from HIV alone is unclear.46
With the exception of syphilis, the occurrence of stillbirths related to bacterial transplacental infection in countries of low and middle income is largely unstudied. However, because of high prevalence of maternal infectious disease in such countries, transplacental infections with several bacterial species are probably frequent.
Ascending bacterial infections
Organisms that ascend from the vagina into the uterus enter the amniotic fluid either through intact choriodecidual membranes or after membrane rupture.47 The most common organ infected is the fetal lung, associated with fetal breathing of contaminated amniotic fluid. Consequently, a common autopsy finding in stillbirths is pneumonitis. Whether the fetus is stillborn with pneumonitis or is born alive with pneumonia depends on factors such as organism virulence, the fetal response, and time between infection and delivery. Blackwell48 and Romero49 and their colleagues postulated that a preterm infection usually elicits a fetal inflammatory response and ultimately preterm labour, but if the fetus cannot initiate an adequate inflammatory response, the outcome will probably be stillbirth. Stillbirths before 28 weeks seem to be strongly associated with intrauterine bacterial infection, whereas later preterm stillbirths are less likely to be caused by such infection.5, 10 Therefore, the apparent prevalence of this condition will depend on whether calculations include stillbirths occurring from 20 weeks onwards, or only those occurring at a later gestational age.
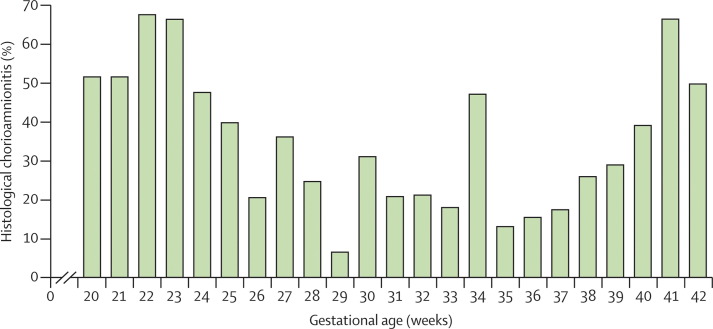
In virtually all studies investigating chorioamnionitis, the frequency of histological chorioamnionitis in stillbirths is at least several times greater than in controls; whether this finding implies causation is unknown.7, 50, 51 The occurrence of histological chorioamnionitis in stillbirths has varied substantially between studies, but results of about half of studies showed presence of the disorder in 70% or more stillbirths.48 In an Australian study, Lahra and colleagues50 reported that 37% of all stillbirths were associated with histological chorioamnionitis. Moreover, very early preterm and post-term stillbirths had substantially increased rates of histological chorioamnionitis (figure 1 ).
Figure 1.
Histological chorioamnionitis in stillborn babies by gestational age
Reproduced from Lahra and colleagues,50 with permission from Elsevier.
In several autopsy studies, organisms such as group B streptococcus, Escherichia coli, and Klebsiella spp have been cultured from fetal heart blood, liver, lung, or brain.52, 53, 54, 55 For example, E coli was identified in 25% of samples from the heart blood of stillborn babies in Zimbabwe. Naeye and co-workers56 studied stillbirths in Ethiopia and compared these results with findings from the US Collaborative Perinatal Study. The types of organisms associated with ascending infections were similar in Ethiopia and the USA, but the frequency of stillbirth associated with infection was several times greater in Ethiopia. The authors speculated that the difference was caused by increased exposure to infectious organisms and a decreased immune response resulting from widespread malnutrition in African populations.
Prevention of stillbirths associated with ascending bacterial infection in the presence of intact membranes has proven elusive. No strategies—including antibiotic prophylaxis—seem to prevent intrauterine infection or associated stillbirths,57 although antibiotic treatment targeting bacterial vaginosis could be beneficial in some women.58 In women with preterm premature rupture of membranes, prophylactic antibiotics reduce histological chorioamnionitis, but have not yet shown a similar reduction in occurrence of stillbirths.59
Viral infections
Although viruses clearly cause stillbirths, the overall nature of this relation is not well studied, especially in countries of low and middle income. The complexity and expense of culturing of most viruses, and the technical difficulty and expense associated with PCR for identification of viral DNA or RNA have hampered research. Few of these tests are routinely available in high-income countries and almost never in countries of low or middle income. Consequently, the importance of maternal viral infection as a cause of stillbirth in most areas of the world is unknown.
Despite variations in data, many studies show a small and often insignificant increase in stillbirth associated with maternal HIV-1 infection.34, 60, 61 One meta-analysis, done before general use of antiretroviral treatments, suggested a significant increase by three times (odds ratio 3·91, 95% CI 2·65–5·77).62 In women with HIV-1 infection, risk of stillbirth seems to increase as maternal HIV disease worsens and CD4 cell counts drop.60 Since the virus rarely crosses the placenta or infects the fetus until labour, this increase in stillbirths is probably not directly caused by fetal HIV-1 infection. Instead, the stillbirth risk is probably due to maternal comorbidities and overall poor maternal health status, especially in countries of low and middle income. However, since the prevalence of maternal HIV-1 infection reaches more than 20% in sub-Saharan countries, the contribution of maternal HIV-1 infection to stillbirths is probably important in such areas. Concerns that combination antiretroviral treatment might contribute to an increase in stillbirth in women with HIV-1 infection have not been realised.63
Maternal infections with common childhood viruses (rubella, measles, mumps, and chickenpox) have been implicated in stillbirths.64, 65, 66, 67, 68, 69 Maternal rubella was associated with congenital cataracts by Gregg67 in 1941. Subsequently, other abnormalities such as major cardiac defects have been recorded, some of which result in stillbirth later in pregnancy. Rubella also infects the placenta, increasing the risk of stillbirth, and can seemingly do so without fetal spread.17 Rubella outbreaks are routinely reported from many low-income and middle-income countries; however, their contribution to stillbirths in such areas is unknown.68 Maternal infections with mumps and rubeola (measles) have both been implicated as causes of stillbirth,66 and both viruses have been isolated from fetal tissues. In Guinea-Bissau, occurrence of stillbirth increased by four to nine times if the mother was infected with measles during her pregnancy.69 Varicella (chickenpox) infections during pregnancy can cause maternal pneumonia, placing infected women at risk for death as well as stillbirth. The virus also occasionally crosses the placenta and attacks the fetus directly.65
Such findings confirm the association between maternal infection with common childhood viruses and increased risk of stillbirth. Because of widespread immunisation in high-income countries, maternal infection with these organisms is extremely rare and few stillbirths due to these infections are reported. However, with vaccination rates at 50% or less in many countries of low or middle income, stillbirths caused by such infections probably occur. Universal vaccination for childhood diseases should eliminate these stillbirths.
Historically, maternal influenza infection has been associated with increased maternal and fetal deaths, but little data is available from low-income and middle-income countries.70 In the 2003 epidemic, severe acute respiratory syndrome was shown to be associated with placental and fetal pathology, but the association with pregnancy outcome needs to be more clearly defined.71 Although reports of influenza A H1N1 (2009 pandemic) infections in pregnancy are few, maternal deaths and stillbirths associated with this infection have been recorded.72 The American College of Obstetricians and Gynecologists (ACOG) recommends immunisation for all women who will be pregnant during the influenza season.73 Influenza infections are common in low-income and middle-income countries, and since immunisation for these viruses rarely occurs, the potential for an increase in stillbirths associated with various types of influenza infection is strong.
Though the relation has not been studied in countries of low and middle income, in high-income countries, one of the best described relations of a virus with stillbirth is for parvovirus B19.74, 75, 76, 77, 78 Parvovirus causes the common childhood rash erythema infectiosum, and also causes aplastic anaemia in children with sickle cell disease. Parvovirus can cross the placenta and preferentially attacks erythropoietic tissue, causing severe fetal anaemia, non-immune hydrops, and fetal death.76, 77 Parvovirus can also cause stillbirth by directly attacking fetal cardiac tissue, resulting in cardiac damage without associated hydrops. Previous parvovirus infection elicits an antibody response that protects against subsequent maternal and fetal infection. However, even with a new maternal parvovirus infection, the risk of stillbirth is small.78, 79 In the USA, less than 1% of all stillbirths result from parvovirus infection.80 However, findings of a study in Sweden that used PCR for viral DNA to confirm parvovirus infection, showed that 15% of all stillbirths were attributed to parvovirus.77 In another Swedish study, 8% of stillbirths were caused by parvovirus, and similar rates have been reported in Germany.78, 79
The enterovirus family includes enterovirus, echovirus, coxsackie virus, and polio, all of which can cross the placenta and cause fetal death. However, the overall effect of such viruses on stillbirths is unknown, especially in countries of low and middle income. In a detailed investigation of unexplained perinatal deaths, Nuovo and colleagues12 identified coxsackie virus in 48% of cases. The authors emphasised that the histological findings in the placenta were often non-specific and underscored the need for molecular testing to define this relation. In a study from Sweden, of 21 women with stillborn babies, 52% were infected with coxsackie virus compared with only 22% of the control group.81 Echovirus and enteroviruses have also been cultured from stillborn babies. In most studies, unless the virus was specifically sought at autopsy with advanced molecular techniques, an infectious cause of stillbirth would have been missed. Similarly, cytomegalovirus has rarely been sought in cases of stillbirth.82, 83 Findings from an Australian study showed that 9% of blood samples taken from stillbirths by cardiac puncture and analysed by PCR were positive for cytomegalovirus.84 Furthermore, Syridou and colleagues85 showed significantly increased concentration of cytomegalovirus from PCR in the placentas of stillbirths (16%) compared with controls (3%; p=0·047). Since cytomegalovirus is the most common cause of congenital infection, and because it can infect the fetus, leading to fetal-growth restriction and CNS damage, the relation between cytomegalovirus and stillbirth warrants further research. Hepatitis and herpes simplex infections have also been described as a cause of fetal death.86
Newly described viruses are also being associated with stillbirth. For example, Ljungan virus, a picornavirus of bank voles, was originally isolated in the Ljungan Valley in Sweden and has since been reported in Denmark and the USA. Infection with the virus was recorded in 40% of stillborn babies in a small study of pregnant women, but not in any tissues from normal pregnancies. The overall importance of this infection as a cause of stillbirth in any location is unknown.19
Protozoal infections
Several protozoal intrauterine infections have been described, of which malaria seems to have the strongest association with stillbirth.
Malaria
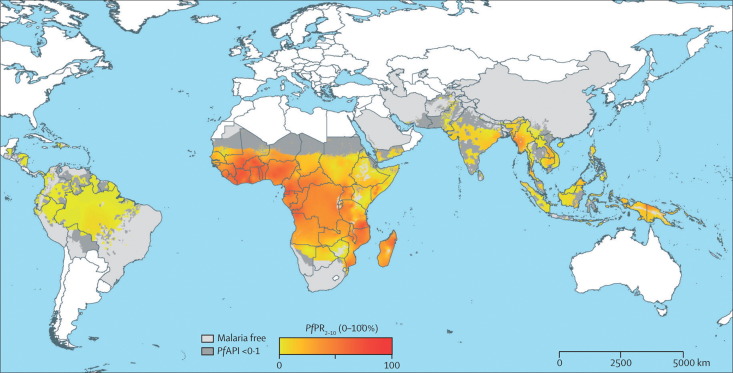
40% of births worldwide occur in areas in which malaria is endemic (figure 2 ); 30 million of these pregnancies occur yearly in Africa alone.88 Malaria is caused by four types of intracellular parasites (especially Plasmodium falciparum) transmitted by several mosquito species.89 Primiparous women who are infected with P falciparum, and especially those who have been previously unexposed to malaria, generally have the worst outcomes: maternal death, fetal-growth restriction, preterm birth, and stillbirth.88, 89, 90
Figure 2.
Global distribution of infection with Plasmodium falciparum malaria
Reproduced from Hay and Snow,87 and licensed to the Malaria Atlas Project (http://www.map.ox.ac.uk). PfAPI=P falciparum annual parasite incidence per 1000 people per year. PfPR2–10=P falciparum parasite rate age-standardised to 2–10 years.
Pregnancy outcome is directly related to the extent of placental malaria, which occurs in 13–63% of maternal infections.15, 91 Placental insufficiency results from lymphocyte and macrophage accumulation, and thickening of the basement membrane, impeding blood flow through the placenta and restricting transport of oxygen and nutrients to the fetus. Severe maternal anaemia associated with malaria could also contribute to stillbirth. Malaria organisms can cross the placenta, causing congenital malaria, but the importance of these infections for stillbirth is not clear. Results from a review of studies, mostly from endemic areas, showed that placental malaria was associated with twice the risk of stillbirth (odds ratio 2·19, 95% CI 1·49–3·22).92
Newman and colleagues93 reported that an Ethiopian population newly infected with malaria had increased risk by seven times, and the population attributable fraction of malaria for stillbirth is 32% according to findings from a Tanzanian study.94 Although most studies of malaria and stillbirth are from Africa, similar results have been recorded in other endemic areas.95 Since a large number of women live in endemic areas, of whom a high percentage have placental malaria and, consequently, double the risk of stillbirth, malaria is probably the most important preventable infectious cause of stillbirth in countries of low and middle income. Evidence suggests that intermittent antimalarial prophylaxis and use of insecticide-treated bednets could reduce the risk of stillbirth and other adverse outcomes associated with malaria.96, 97
Other protozoal infections
The parasite Toxoplasma gondii normally spends its lifecycle in animals, but can be transmitted to humans. Human infection, toxoplasmosis, is generally asymptomatic or causes only mild disease, but has been identified as a cause of stillbirth in case reports.98 The prevalence of toxoplasma antibodies is more than 80% for pregnant women in Nigeria, compared with 15% in the USA.99 In Zimbabwe, serological tests for toxoplasmosis were four times more common in mothers who had stillbirths than in the control group, and in Jordan, the presence of T gondii was significantly higher in women with adverse pregnancy outcomes than in the control group (54% vs 12%, p<0·02).100 However, the importance of T gondii as a cause of stillbirth in low-income and middle-income countries is unknown. Many other protozoal infections that are common in tropical regions—such as trypanosomiasis (African sleeping sickness transmitted by the tsetse fly) and Chagas' disease (caused by Trypanosoma cruzi in South and Central America)—have been associated with stillbirth,101, 102 but the extent of the association is unknown.
Other infectious causes of stillbirths
Little research has been done on the relation between various fungal infections and stillbirth, but case reports indicate that candida infections cause stillbirth.6 Additionally, findings from an observational study in Sri Lanka showed significant reductions in occurrence of stillbirth associated with anthelmintic treatment, implicating worm infestations as a possible cause of stillbirth.103
Prevention of infection-related stillbirths
Low-income and middle-income countries
The infectious disease burden during pregnancy is extremely high in many countries, and the stillbirth rate is probably high as a consequence.7, 56 Therefore, programmes that screen for and treat syphilis should substantially reduce stillbirths (table 2 ). Antibiotic treatment for women with syphilis seems to effectively reduce the stillbirth risk to almost that of women without infection.32 Reduction of maternal malaria infections in endemic areas should also reduce the stillbirth rate. Antimalarial strategies that have been assessed specifically for implementation during pregnancy95, 96 include malaria prophylaxis, especially intermittent therapy, and use of insecticide-treated bednets. Although these strategies clearly reduce malaria and outcomes such as severe anaemia, most studies have not been powered to show improvements in rare outcomes such as stillbirth.
Table 2.
Treatment priorities to reduce infection-related stillbirths
| Intervention | Rationale | |
|---|---|---|
| Syphilis | Screening and treatment | Intervention is effective; scale-up strategies are needed, especially in areas of high prevalence |
| Malaria | Chemoprophylaxis (directed or intermittent); insecticide-treated bednets against malaria | Neither strategy has been tested for targeting of stillbirth specifically, but these strategies are effective against other malaria-related pregnancy outcomes |
| Measles, mumps, rubella, polio, varicella, influenza | Maternal vaccination | Effective for prevention of maternal disease and will probably prevent stillbirths associated with maternal infection in pregnancy; the contribution of these maternal infections to stillbirth in developing countries is unknown |
| Worms | Deworming | Associated with a decrease in stillbirths, but cause and effect has not been proven and the contribution to the burden of stillbirth is unknown |
A reduction in bacterial intrauterine infections, if achievable, could also have a substantial effect on stillbirth rates. Potential strategies (table 3 ) that should be tested are: nutritional supplementation with calories or vitamin and mineral preparations, or both; reduced exposure of the genital tract to bacteria by use of antibiotics or vaginal washes with chlorhexidine; and effective management of both preterm and term premature rupture of membranes.58, 59, 104 Achievement of high rates of antiviral vaccination (rubella, rubeola, varicella, polio) should reduce the number of associated stillbirths (table 2). Increased understanding of the association between stillbirth and infections transmitted by animals and vectors is also required.
Table 3.
Research priorities for reduction of infection-related stillbirths
| Research direction | Rationale | |
|---|---|---|
| Maternal infections | Establish burden of infectious causes of stillbirth in developing countries with molecular biological techniques | Proportions of stillbirths in developing countries that are associated with Lyme disease, relapsing fever, Chagas' disease, parvovirus, enterovirus, and many other maternal infections are unknown |
| Chorioamnionitis | Develop effective prevention and treatment strategies | Chorioamnionitis is probably the most common cause of stillbirth worldwide, and research aimed at reduction of such infection is crucial |
| Viral infections | Develop vaccines for viral causes of stillbirth (eg, parvovirus, coxsackie viruses A and B, cytomegalovirus), and test effectiveness for prevention of stillbirth | Elimination of these infections during pregnancy should reduce stillbirths and other adverse pregnancy outcomes |
| Bacterial infections | Establish clean delivery practices, maternal vaginal antisepsis (eg, chlorhexidine), and nutrition supplementation | Effects of such strategies for reduction of stillbirth needs to be ascertained |
High-income countries
Since the occurrence of infection-related stillbirths is reduced in high-income countries, and the range of organisms that could be responsible is broad, further decreases in occurrence might be difficult. However, continued attention to screening and treatment of sexually transmitted infections, especially syphilis, and to maintenance of high vaccination rates for common childhood diseases should keep associated stillbirths to a minimum (table 2). Although ACOG and other organisations recommend an influenza vaccine for pregnant women during the influenza season, only about 25% of pregnant women have the vaccine in the USA.
Increased attention to prevention and treatment of conditions—eg, Lyme disease—could reduce associated stillbirths. Stillbirths linked to toxoplasmosis occur rarely, but educational attention to efforts such as hand washing could have a small effect. Similarly, a small reduction in stillbirths associated with listeriosis might be achieved by encouraging pregnant women to avoid soft cheeses and various meat and seafood products.
The largest potential area for progress seems to be for stillbirths associated with intrauterine bacterial infections preceding membrane rupture. Reduction of these infections would decrease the frequency of both stillbirths and preterm births. Thus far, no effective strategies are available to reduce such infections, and research to develop strategies is crucial.
From a research perspective, the development of vaccines for some of the viral causes of stillbirth (parvovirus, coxsackie viruses A and B, cytomegalovirus, group B streptococcus105, 106) should eliminate some stillbirths (table 3). However, because of the rarity of these deaths in high-income countries, such an approach will probably have a small effect on stillbirth rates.
Conclusions
High-quality research is crucial to establish causality of infection for stillbirths. The best evidence of an infectious aetiology for a stillbirth is obtained from a careful autopsy and placental examination with appropriate serological studies, cultures, and DNA or RNA specimens taken to identify infectious organisms. New organisms associated with stillbirth should be sought.107 However, even if an autopsy is not done, a histological study of the placenta, membranes, and umbilical cord, with appropriate serological studies, cultures, and DNA or RNA specimens, will often provide evidence for an infectious aetiology.
One of the most compelling findings related to our Review of infection and stillbirths is the many unknowns regarding this relation, especially in low-income and middle-income countries. For example, we do not know whether the high seroprevalences of Lyme disease or toxoplasmosis in African settings are associated with increased risk of stillbirth, or whether African tick-borne relapsing fever is an important cause of stillbirth. Further, potential causes of stillbirth such as worm infestations have rarely been considered.103 We believe that research in several settings in countries of low and middle income, using both traditional and the newest molecular and culture techniques, should be done to ascertain the full extent of the relation between stillbirths and infection. Only with this research can we hope to define the reasons for the excessive stillbirth burden in such countries and develop effective reduction strategies.108
Search strategy and selection criteria
We searched Medline, PubMed, and available Cochrane Reviews (January, 1990–July, 2009) using specific search terms including “stillbirth”, “perinatal death”, and “fetal death”, and several terms for infection and specific organisms, for articles on infection and stillbirth. We included studies published in the past 20 years, but we did not exclude older studies that we deemed relevant. We reviewed all studies identified by the search that were published in English, and we reviewed English language abstracts of papers that were published in other languages. We also selected appropriate articles from the reference lists of reviewed papers. We focused on sentinel studies addressing infections that had the potential to cause a substantial number of stillbirths worldwide, and were likely or potentially able to contribute to an important reduction in stillbirths through prevention, treatment programmes, and further research.
Acknowledgments
Acknowledgments
RLG, EMM, and SS are funded by grants from the National Institute of Child Health and Human Development Global Network.
Contributors
RLG is the corresponding author, has had full access to all the data in the Review, and had final responsibility for the decision to submit for publication. RLG outlined the structure of the report with help from EMM and SS, and UMR helped to conceptualise the report. RLG and EMM searched for and reviewed published work. RLG wrote the report, and EMM, SS, and UMR reviewed and participated in creating drafts of the report. All authors have seen and approved the final version.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet. 2006;367:1487–1494. doi: 10.1016/S0140-6736(06)68586-3. [DOI] [PubMed] [Google Scholar]
- 2.Lawn J, Shibuya K, Stein C. No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths 2005. Bull World Health Organ. 2005;83:409–417. [PMC free article] [PubMed] [Google Scholar]
- 3.McClure EM, Phiri M, Goldenberg RL. Stillbirth in developing countries: a review of the literature. Int J Gynaecol Obstet. 2006;94:82–90. doi: 10.1016/j.ijgo.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Rawlinson WD, Hall B, Jones CA. Viruses and other infections in stillbirth: what is the evidence and what should we be doing? Pathology. 2008;40:149–160. doi: 10.1080/00313020701813792. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs RS. The origins of stillbirth: infectious diseases. Semin Perinatol. 2002;26:75–78. doi: 10.1053/sper.2002.29839. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Thompson C. The infectious origin of stillbirth. Am J Obstet Gynecol. 2003;189:861–873. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 7.Di Mario S, Say L, Lincetto O. Risk factors for stillbirth in developing countries: a systematic review of the literature. Sex Transm Dis. 2007;34(suppl):S11–S21. doi: 10.1097/01.olq.0000258130.07476.e3. [DOI] [PubMed] [Google Scholar]
- 8.Fretts R. Etiology and prevention of stillbirths. Am J Obstet Gynecol. 2005;193:1923–1935. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 9.Cnattingius S, Stephansson O. The epidemiology of stillbirth. Semin Perinatol. 2002;26:25–30. doi: 10.1053/sper.2002.29841. [DOI] [PubMed] [Google Scholar]
- 10.Copper RL, Goldenberg RL, DuBard MB, Davis RO. Risk factors for fetal death in white, black, and Hispanic women. Obstet Gynecol. 1994;84:490–495. [PubMed] [Google Scholar]
- 11.Korteweg FJ, Gordijn SJ, Timmer A, Holm JP, Ravise JM, Erwich JJHM. A placental cause of intra-uterine fetal death depends on the perinatal mortality classification system used. Placenta. 2008;29:71–80. doi: 10.1016/j.placenta.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Nuovo GJ, Cooper LD, Bartholomew D. Histologic, infectious, and molecular correlates of idiopathic spontaneous abortion and perinatal mortality. Diagn Mol Pathol. 2005;14:152–158. doi: 10.1097/01.pas.0000176769.18423.37. [DOI] [PubMed] [Google Scholar]
- 13.Hardy JB, Azarowicz EN, Mannini A, Medaris DN, Jr, Cooke RE. The effect of Asian influenza on the outcome of pregnancy, Baltimore, 1957–1958. Am J Public Health Nations Health. 1961;51:1182–1188. doi: 10.2105/ajph.51.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn P. Poliomyelitis in pregnancy: a twenty-year report from Los Angeles County, California. Obstet Gynecol. 1955;6:121–137. [PubMed] [Google Scholar]
- 15.Schwarz NG, Adegnika AA, Breitling LP. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis. 2008;47:1017–1025. doi: 10.1086/591968. [DOI] [PubMed] [Google Scholar]
- 16.Blanc W. Pathways of fetal and early neonatal infection. J Pediatr. 1961;59:473–496. doi: 10.1016/s0022-3476(61)80232-1. [DOI] [PubMed] [Google Scholar]
- 17.Cooper LZ, Alford CA. Rubella. In: Remington JS, Klein JO, Baker C, Wilson CB, editors. Infectious diseases of the fetus and newborn infant. WB Saunders; Philadelphia, PA: 2006. pp. 893–926. [Google Scholar]
- 18.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 19.Niklasson B, Samsioe A, Papadogiannakis N. Association of zoonotic Ljungan virus with intrauterine fetal deaths. Birth Defects Res A Clin Mol Teratol. 2007;79:488–493. doi: 10.1002/bdra.20359. [DOI] [PubMed] [Google Scholar]
- 20.Martin C, Fermeauz V, Eyraud JL, Aubard Y. Streptococcus porcinus as a cause of spontaneous preterm human stillbirth. J Clin Microbiol. 2004;42:4396–4398. doi: 10.1128/JCM.42.9.4396-4398.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumbiganon P, Piaggio G, Villar J. The epidemiology of syphilis in pregnancy. Int J STD AIDS. 2002;13:486–494. doi: 10.1258/09564620260079653. [DOI] [PubMed] [Google Scholar]
- 22.Genc M, Ledger WJ. Syphilis in pregnancy. Sex Transm Infect. 2000;76:73–79. doi: 10.1136/sti.76.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Congenital syphilis—United States, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:573–577. [PubMed] [Google Scholar]
- 24.Folgosa E, Osman NB, Gonzalez C, Hagerstrand I, Bergstrom S, Ljungh A. Syphilis seroprevalence among pregnant women and its role as a risk factor for stillbirth in Maputo, Mozambique. Genitourin Med. 1996;72:339–342. doi: 10.1136/sti.72.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter D, Goldenberg RL, Read JS. Correlates of syphilis seroreactivity among pregnant women: The HIVNET 024 trial in Malawi, Tanzania and Zambia. Sex Transm Dis. 2006;33:604–609. doi: 10.1097/01.olq.0000216029.00424.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid GP, Stoner BP, Hawkes S, Broutet N. The need and plan for global elimination of congenital syphilis. Sex Transm Dis. 2007;34(suppl):S5–S10. doi: 10.1097/01.olq.0000261456.09797.1b. [DOI] [PubMed] [Google Scholar]
- 27.Schmid GP. Economic and programmatic aspects of congenital syphilis elimination. Bull World Health Organ. 2004;82:402–409. [PMC free article] [PubMed] [Google Scholar]
- 28.Sheffield JS, Sanchez PJ, Wendel GD. Placental histopathology of congenital syphilis. Obstet Gynecol. 2002;100:126–133. doi: 10.1016/s0029-7844(02)02010-0. [DOI] [PubMed] [Google Scholar]
- 29.Watson-Jones D, Changalucha J, Gumadoka B. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J Infect Dis. 2002;186:940–947. doi: 10.1086/342952. [DOI] [PubMed] [Google Scholar]
- 30.Salakhov E, Tikhonova L, Southwick K, Shakarishvili A, Ryan C, Hillis C. Congenital syphilis in Russia: the value of counting epidemiologic cases and clinical cases. Sex Transm Dis. 2004;31:127–132. doi: 10.1097/01.OLQ.0000109516.47951.B8. [DOI] [PubMed] [Google Scholar]
- 31.Sethi S, Sharma K, Dhaliwal LK, Banga SS, Sharma M. Declining trends in syphilis prevalence among antenatal women in northern India: a 10-year analysis from a tertiary healthcare centre. Sex Transm Infect. 2007;83:592. doi: 10.1136/sti.2007.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vásquez-Manzanilla O, Dickson-Gonzalez SM, Salas JG, Rodriguez-Morales AJ, Arria M. Congenital syphilis in Valera, Venezuela. J Trop Pediatr. 2007;53:274–277. doi: 10.1093/tropej/fmm025. [DOI] [PubMed] [Google Scholar]
- 33.Watson-Jones D, Weiss HA, Changalucha JM. Adverse birth outcomes in United Republic of Tanzania—impact and prevention of maternal risk factors. Bull World Health Organ. 2007;85:9–17. doi: 10.2471/BLT.06.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chi BH, Wang L, Read JS. Stillbirth in sub-Saharan Africa. Obstet Gynecol. 2007;110:989–997. doi: 10.1097/01.AOG.0000281667.35113.a5. [DOI] [PubMed] [Google Scholar]
- 35.Rydzak CE, Goldie SJ. Cost-effectiveness of rapid point-of-care prenatal syphilis screening in sub-Saharan Africa. Sex Transm Dis. 2008;35:775–784. doi: 10.1097/OLQ.0b013e318176196d. [DOI] [PubMed] [Google Scholar]
- 36.Hossain M, Broutet N, Hawkes S. The elimination of congenital syphilis: a comparison of the proposed World Health Organization Action Plan for the elimination of congenital syphilis with existing national maternal and congenital syphilis policies. Sex Transm Dis. 2007;34:S22–S30. doi: 10.1097/01.olq.0000261049.84824.40. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald A, Benach J, Burgdorfer W. Stillbirth following maternal Lyme disease. N Y State J Med. 1987;87:615–616. [PubMed] [Google Scholar]
- 38.Strobino BA, Williams CL, Abid S, Chalson R, Spierling P. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol. 1993;169:367–374. doi: 10.1016/0002-9378(93)90088-z. [DOI] [PubMed] [Google Scholar]
- 39.Mhalu FS, Matre R. Serological evidence of Lyme borreliosis in Africa: results from studies in Dar Es Salaam, Tanzania. East Afr Med J. 1996;73:583–585. [PubMed] [Google Scholar]
- 40.Melkert PW. Relapsing fever in pregnancy: analysis of high-risk factors. Br J Obstet Gynaecol. 1988;95:1070–1072. doi: 10.1111/j.1471-0528.1988.tb06516.x. [DOI] [PubMed] [Google Scholar]
- 41.Dupont HT, La Scola B, Williams R, Raoult D. A focus of tick-borne relapsing fever in southern Zaire. Clin Infect Dis. 1997;25:139–144. doi: 10.1086/514496. [DOI] [PubMed] [Google Scholar]
- 42.McConnell J. Tick-borne relapsing fever under-reported. Lancet Infect Dis. 2003;3:604. doi: 10.1016/s1473-3099(03)00787-4. [DOI] [PubMed] [Google Scholar]
- 43.Jongen VH, van Roosmalen J, Tiems J, Van Holten J, Wetsteyn JC. Tick-borne relapsing fever and pregnancy outcome in rural Tanzania. Acta Obstet Gynecol Scand. 1997;76:834–838. doi: 10.3109/00016349709024361. [DOI] [PubMed] [Google Scholar]
- 44.Smith B, Kemp M, Ethelberg S. Listeria monocytogenes: maternal-foetal infections in Denmark 1994–2005. Scand J Infect Dis. 2009;41:21–25. doi: 10.1080/00365540802468094. [DOI] [PubMed] [Google Scholar]
- 45.Jana N, Vasishta K, Jindal SK, Khunnu B, Ghosh K. Perinatal outcome in pregnancies complicated by pulmonary tuberculosis. Int J Gynaecol Obstet. 1994;44:119–124. doi: 10.1016/0020-7292(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 46.Pillay T, Sturm AW, Khan M. Vertical transmission of Mycobacterium tuberculosis in KwaZulu Natal: impact of HIV-1 co-infection. Int J Tuberc Lung Dis. 2004;8:59–69. [PubMed] [Google Scholar]
- 47.Madan E, Meyer MP, Amortequi A. Chorioamnionitis: a study of organisms isolated in perinatal autopsies. Ann Clin Lab Sci. 1988;18:39–45. [PubMed] [Google Scholar]
- 48.Blackwell S, Romero R, Chaiworapongsa T. Maternal and fetal inflammatory responses in unexplained fetal death. J Matern Fetal Neonatal Med. 2003;14:151–157. doi: 10.1080/jmf.14.3.151.157. [DOI] [PubMed] [Google Scholar]
- 49.Romero R, Gomez R, Ghezzi F. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 50.Lahra MM, Gordon A, Jeffery HE. Chorioamnionitis and fetal response in stillbirth. Am J Obstet Gynecol. 2007;196:e1–e4. doi: 10.1016/j.ajog.2006.10.900. [DOI] [PubMed] [Google Scholar]
- 51.Folgosa E, Gonzalez C, Osman NB, Hagerstrand I, Bergstrom S, Ljungh A. A case control study of chorioamniotic infection and histological chorioamnionitis in stillbirth. APMIS. 1997;105:329–336. doi: 10.1111/j.1699-0463.1997.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 52.Tolockiene E, Morsing E, Holst E. Intrauterine infection may be a major cause of stillbirth in Sweden. Acta Obstet Gynecol Scand. 2001;80:511–518. [PubMed] [Google Scholar]
- 53.Moyo SR, Hagerstrand I, Nystrom L. Stillbirths and intrauterine infection, histologic chorioamnionitis and microbiological findings. Int J Gynaecol Obstet. 1996;54:115–123. doi: 10.1016/0020-7292(96)02705-1. [DOI] [PubMed] [Google Scholar]
- 54.Moyo SR, Tswana SA, Nystrom L, Bergstrom S, Blomberg J, Ljungh A. Intrauterine death and infections during pregnancy. Int J Gynaecol Obstet. 1995;51:211–218. doi: 10.1016/0020-7292(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 55.Axemo P, Ching C, Machungo F, Osman NB, Bergstrom S. Intrauterine infections and their association with stillbirth and preterm birth in Maputo, Mozambique. Gynecol Obstet Invest. 1993;35:108–113. doi: 10.1159/000292676. [DOI] [PubMed] [Google Scholar]
- 56.Naeye RL, Tafari N, Judge D, Gilmour D, Marboe C. Amniotic fluid infections in an African city. J Pediatr. 1977;90:965–970. doi: 10.1016/s0022-3476(77)80573-8. [DOI] [PubMed] [Google Scholar]
- 57.Goldenberg RL, Mudenda V, Read JS, for the HPTN 024 Study Team HPTN 024 study: histologic chorioamnionitis, antibiotics and adverse infant outcomes in a predominantly HIV-1-infected African population. Am J Obstet Gynecol. 2006;195:1065–1074. doi: 10.1016/j.ajog.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 58.Lamont R. Infection in the prediction and antibiotics in the prevention of spontaneous preterm labour and preterm birth. BJOG. 2003;110:71–75. doi: 10.1016/s1470-0328(03)00034-x. [DOI] [PubMed] [Google Scholar]
- 59.Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2003;2 doi: 10.1002/14651858.CD001058. CD001058. [DOI] [PubMed] [Google Scholar]
- 60.Tuomala RE, Shapiro DE, Mofenson LM. Antiretrival therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346:1863–1870. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- 61.Olagbuji BN, Ezeanochie MC, Ande AB, Oboro VO. Obstetric and perinatal outcome in HIV positive women receiving HAART in urban Nigeria. Arch Gynecol Obstet. 2009 doi: 10.1007/s00404-009-1186-x. published online July 24. [DOI] [PubMed] [Google Scholar]
- 62.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105:836–848. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 63.Cotter AM, Garcia AG, Duthely ML, Luke B, O'Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. 2006;193:1195–1201. doi: 10.1086/503045. [DOI] [PubMed] [Google Scholar]
- 64.Cutts FT, Robertson SE, Diaz-Ortega JL, Samuel R. Control of rubella and congenital rubella syndrome (CRS) in developing countries, 1: burden of disease from CRS. Bull World Health Organ. 1997;75:55–68. [PMC free article] [PubMed] [Google Scholar]
- 65.Gershon AA. Chickenpox, measles and mumps. In: Remington JS, Klein JO, Baker C, Wilson CB, editors. Infectious diseases of fetus and newborn infant. WB Saunders; Philadelphia, PA: 2006. pp. 693–738. [Google Scholar]
- 66.Ornoy A, Tenenbaum A. Pregnancy outcome following infections by coxsackie, echo, measles, mumps, hepatitis, polio and encephalitis viruses. Reprod Toxicol. 2006;21:446–457. doi: 10.1016/j.reprotox.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Gregg NM. Congenital cataract following German measles in the mother. Trans Ophthalmol Soc Aust. 1941;3:35–46. [PubMed] [Google Scholar]
- 68.Dayan GH, Zimmerman L, Shteinke L. Investigation of a rubella outbreak in Kyrgyzstan in 2001: implications for an integrated approach to measles elimination and prevention of congenital rubella syndrome. J Infect Dis. 2003;187(suppl 1):S235–S240. doi: 10.1086/368037. [DOI] [PubMed] [Google Scholar]
- 69.Aaby P, Lisse IM, Bukh J, Seim E, de Silva MC. Increased perinatal mortality among children of mothers exposed to measles during pregnancy. Lancet. 1988;331:516–519. doi: 10.1016/s0140-6736(88)91306-2. [DOI] [PubMed] [Google Scholar]
- 70.Stanwell-Smith R, Parker AM, Chakraverty P, Soltanpoor N, Simpson CN. Possible association of influenza A with fetal loss: investigation of a cluster of spontaneous abortions and stillbirths. Commun Dis Rep CDR Rev. 1994;4:R28–R32. [PubMed] [Google Scholar]
- 71.Ng WF, Wong SF, Lam A. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology. 2006;38:210–218. doi: 10.1080/00313020600696280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jamieson DJ, Honein MA, Rasmussen SA, the Novel Influenza A (H1N1) Pregnancy Working Group H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 73.ACOG Committee on Obstetric Practice ACOG committee opinion number 305, November 2004. Influenza vaccination and treatment during pregnancy. Obstet Gynecol. 2004;104:1125–1126. [PubMed] [Google Scholar]
- 74.Riipinen A, Väisänen E, Nuutila M. Parvovirus b19 infection in fetal deaths. Clin Infect Dis. 2008;47:1519–1525. doi: 10.1086/593190. [DOI] [PubMed] [Google Scholar]
- 75.Public Health Laboratory Service Working Party on Fifth Disease Prospective study of human parvovirus (B19) infection in pregnancy. BMJ. 1990;300:1166–1170. doi: 10.1136/bmj.300.6733.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller E, Fairley CK, Cohen BJ, Seng C. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. Br J Obstet Gynaecol. 1998;105:174–178. doi: 10.1111/j.1471-0528.1998.tb10048.x. [DOI] [PubMed] [Google Scholar]
- 77.Tolfvenstam T, Papadogiannakis N, Norbeck O, Petersson K, Broliden K. Frequency of human parvovirus B19 infection in intrauterine fetal death. Lancet. 2001;357:1494–1497. doi: 10.1016/S0140-6736(00)04647-X. [DOI] [PubMed] [Google Scholar]
- 78.Enders M, Weidner A, Zoellner I, Searle K, Enders G. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: a prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513–518. doi: 10.1002/pd.940. [DOI] [PubMed] [Google Scholar]
- 79.Wright C, Hinchliffe SA, Taylor C. Fetal pathology in intrauterine death due to parvovirus B19 infection. Br J Obstet Gynaecol. 1996;103:133–136. doi: 10.1111/j.1471-0528.1996.tb09664.x. [DOI] [PubMed] [Google Scholar]
- 80.Kinney JS, Anderson LT, Farrar J, Strikas RA, Kumar ML, Kliegman RM. Risk of adverse outcomes after parvovirus B19 infection. J Infect Dis. 1988;157:663–667. doi: 10.1093/infdis/157.4.663. [DOI] [PubMed] [Google Scholar]
- 81.Frisk G, Diderholm H. Increased frequency of Coxsackie B virus IgM in women with spontaneous abortion. J Infect. 1992;24:141–145. doi: 10.1016/0163-4453(92)92798-n. [DOI] [PubMed] [Google Scholar]
- 82.Maruyama Y, Sameshima H, Kamitomo M. Fetal manifestations and poor outcomes of congenital cytomegalovirus infections: possible candidates for intrauterine antiviral treatments. J Obstet Gynaecol Res. 2007;33:619–623. doi: 10.1111/j.1447-0756.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 83.Tongsong T, Sukpan K, Wanapirak C, Phadungkiatwattna P. Fetal cytomegalovirus infection associated with cerebral hemorrhage, hydrops fetalis, and echogenic bowel: case report. Fetal Diagn Ther. 2008;23:169–172. doi: 10.1159/000116737. [DOI] [PubMed] [Google Scholar]
- 84.Howard J, Hall B, Brennan LE. Utility of newborn screening cards for detecting CMV infection in cases of stillbirth. J Clin Virol. 2009;44:215–218. doi: 10.1016/j.jcv.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 85.Syridou G, Spanakis N, Konstantinidou A. Detection of cytomegalovirus, parvovirus B19 and herpes simplex viruses in cases of intrauterine fetal death: association with pathological findings. J Med Virol. 2008;80:1776–1782. doi: 10.1002/jmv.21293. [DOI] [PubMed] [Google Scholar]
- 86.Ahmed RE, Karsany MS, Adam I. Brief report: acute viral hepatitis and poor maternal and perinatal outcomes in pregnant Sudanese women. J Med Virol. 2008;80:1747–1748. doi: 10.1002/jmv.21284. [DOI] [PubMed] [Google Scholar]
- 87.Hay SI, Guerra CA, Gething PW. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Skeketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 89.Desai M, Oter Kuile F, Nosten F. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 90.McGregor IA, Wilson ME, Billewicz WZ. Malaria infection of the placenta in The Gambia, West Africa: its incidence and relationship to stillbirth, birth weight, and placenta weight. Trans R Soc Trop Med Hyg. 1983;77:232–244. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- 91.Dorman EK, Shulman CE, Kingdom J. Impaired uteroplacental blood flow in pregnancies complicated by falciparum malaria. Ultrasound Obstet Gynecol. 2002;19:165–170. doi: 10.1046/j.0960-7692.2001.00545.x. [DOI] [PubMed] [Google Scholar]
- 92.Van Geertruyden JP, Thomas F, Erhart A, D'Alessandro U. The contribution of malaria in pregnancy to perinatal mortality. Am J Trop Med Hyg. 2004;71(suppl):35–40. [PubMed] [Google Scholar]
- 93.Newman RD, Hailemariam A, Jimma D. Burden of malaria during pregnancy in areas of stable and unstable transmission in Ethiopia during a non-epidemic year. J Infect Dis. 2003;187:1765–1772. doi: 10.1086/374878. [DOI] [PubMed] [Google Scholar]
- 94.Oladapo OT, Adekanle DA, Durajaiye BO. Maternal risk factors associated with fetal death during antenatal care in low-resource tertiary hospitals. Aust NZJ Obstet Gynaecol. 2007;47:383–388. doi: 10.1111/j.1479-828X.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 95.Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77:69–78. [PubMed] [Google Scholar]
- 96.Coll O, Menedez C, Botet F, Dayal R, WAPM Perinatal Infections Working Group Treatment and prevention of malaria in pregnancy and newborn. J Perinat Med. 2008;36:15–29. doi: 10.1515/JPM.2008.002. [DOI] [PubMed] [Google Scholar]
- 97.Okie S. A new attack on malaria. N Engl J Med. 2008;358:2425–2428. doi: 10.1056/NEJMp0803483. [DOI] [PubMed] [Google Scholar]
- 98.Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R. Congenital toxoplasmosis: a review. Obstet Gynecol Surv. 2001;56:296–305. doi: 10.1097/00006254-200105000-00025. [DOI] [PubMed] [Google Scholar]
- 99.Onadeko MO, Joynson DH, Payne RA, Francis J. The prevalence of toxoplasma antibodies in pregnant Nigerian women and the occurrence of stillbirth and congenital malformation. Afr J Med Med Sci. 1996;25:331–334. [PubMed] [Google Scholar]
- 100.Nimri L, Pelloux H, Elkhatib L. Detection of Toxoplasma gondii DNA and specific antibodies in high-risk pregnant women. Am J Trop Med Hyg. 2004;71:831–835. [PubMed] [Google Scholar]
- 101.Lingam S, Marshall WC, Wilson J, Gould JM, Reinhardt MC, Evans DA. Congenital trypanosomiasis in a child born in London. Dev Med Child Neurol. 1985;27:664–674. doi: 10.1111/j.1469-8749.1985.tb14141.x. [DOI] [PubMed] [Google Scholar]
- 102.Buekens P, Almendares O, Carlier Y. Mother-to-child transmission of Chagas' disease in North America: why don't we do more? Matern Child Health J. 2008;12:283–286. doi: 10.1007/s10995-007-0246-8. [DOI] [PubMed] [Google Scholar]
- 103.de Silva NR, Sirisena JLGJ, Gunasekera DPS, Ismail MM, de Silva HJ. Effect of mebendazole therapy during pregnancy on birth outcome. Lancet. 1999;353:1145–1149. doi: 10.1016/s0140-6736(98)06308-9. [DOI] [PubMed] [Google Scholar]
- 104.Okun N, Gronau KA, Hannah ME. Antibiotics for bacterial vaginosis or Trichomonas vaginalis in pregnancy: a systematic review. Obstet Gynecol. 2005;105:857–868. doi: 10.1097/01.AOG.0000157108.32059.8f. [DOI] [PubMed] [Google Scholar]
- 105.Gibbs RS, Roberts DJ. Case records of the Massachusetts General Hospital. Case 27—2007: a 30-year-old pregnant woman with intrauterine fetal death. N Engl J Med. 2007;357:918–925. doi: 10.1056/NEJMcpc079021. [DOI] [PubMed] [Google Scholar]
- 106.Colbourn T, Asseburg C, Bojke L. Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses. Health Technol Assess. 2007;11:1–226. doi: 10.3310/hta11290. [DOI] [PubMed] [Google Scholar]
- 107.DiGiulio D, Romero R, Amogan HP. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:E3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(suppl):519–617. doi: 10.1542/peds.2004-1441. [DOI] [PubMed] [Google Scholar]