Abstract
Self-reactive B cells in BALB/c AM14 transgenic (AM14 Tg) rheumatoid factor (RF) mice are not subject to central or peripheral toleralization. Instead, they remain at a stage of “clonal ignorance”, i.e. they do not proliferate and differentiate into Ab-producing cells. However, the immunoregulatory mechanisms that prevent autoantibody production in these mice remain unclear. In this study, we show that crossing AM14 Tg mice to a mouse strain deficient in Act1, a molecule involved in the regulation of BAFF-R and CD40-signaling in B cells, results in spontaneous activation of AM14 Tg B cells and production of AM14-specific antibodies. Three to five-month old AM14 Tg Act1−/− mice showed significant expansion of AM14 Tg B cells, including a 2–3 fold increase in the spleen and cLNs compared to AM14 Tg Act1+/+ mice. Furthermore, in the presence of endogenous self-Ag (IgHa congenic background), AM14 Tg Act1−/− B cells were spontaneously activated and differentiated into antibody forming cells (AFC). In contrast with previous studies using AM14 Tg MLR.Faslpr mice, we found that a significant number of AM14 Tg cells AM14 Tg Act1−/− mice displayed phenotypic characteristics of GC B cells. Anti-CD40L treatment significantly limited the expansion and activation of AM14 Tg Act1−/− B cells, suggesting that CD40L-mediated signals are required for the retention of these cells. Our results support the important role of Act1 in the regulation of self-reactive B cells and reveal how Act1 functions to prevent the production of autoantibodies.
INTRODUCTION
Systemic autoimmunity is characterized by the loss of tolerance to self-antigens and the production of autoantibodies by activated B cells. The origins of pathogenic B cells in autoimmune diseases and the mechanisms involved in their regulation are not fully understood (1–3). B cells recognizing high avidity self-antigens are eliminated in the bone marrow through mechanisms of receptor editing, anergy, and clonal deletion (4–8). By contrast, autoreactive B cells that recognize soluble self-antigens with low-to-moderate affinity are not subject to central toleralization in the bone marrow, but instead colonize the peripheral lymphoid organs (5). An additional selection of autoreactive B cells occurs at the transitional checkpoint, as immature B cells develop into more mature follicular (Fo) or marginal zone (MZ) B cells (9, 10).
Signaling through the B cell antigen receptor (BCR) is necessary but not sufficient for the full activation of autoreactive B cells. Recent studies suggested that dual engagement of the B-cell receptor (BCR) and intracellular TLRs by endogenous nucleic acid-containing immune complexes can promote the activation of autoreactive B cells and drive the production of pathogenic antibodies (11–13). In addition, alterations of the strength of signaling via the CD40 and BAFF receptors were shown to disrupt the normal selection of autoreactive B cells and to promote their survival and activation (14–16).
Transgenic (Tg) mice expressing BCRs designed to specifically recognize self-antigens are valuable tools in identifying the critical checkpoints of B cell development involved in the regulation of self-reactive cells (5, 17–24). AM14 H chain Tg mice, originally derived from the MRL.Faslpr mouse strain, harbor an increased frequency of B cells with low to moderate affinity to self protein, in this case IgG2a (RF-specificity) (25, 26). RFs are a dominant class of autoantibodies commonly found in patients with autoimmune diseases, including Rheumatoid Arthritis (RA), Sjögren’s Syndrome (SjS), and Systemic Lupus Erythematosus (SLE) (27, 28). In the presence of self-Ag (IgHa-congenic background), AM14 Tg B cells are not subject to toleralization in the BM, and are not anergized in the periphery. Instead, they develop into B cells with phenotypic and functional characteristics of pre-immune follicular B cells (26). Under normal conditions, such cells remain at a stage of “clonal ignorance”, i.e. they do not proliferate and differentiate into plasmablasts. The mechanisms required for the maintenance of this “ignorant” state are still unclear.
When crossed onto the MLR.Faslpr lupus-prone background, AM14 Tg mice develop RF-secreting cells as they age (22). The activation of AM14 Tg cells in MLR.Faslpr mice takes place at extrafollicular sites in the spleen and, upon proper stimulation, can occur even in the absence of Ag-specific T cell help (29–31). The ability of AM14 Tg B cells to become spontaneously activated and to differentiate into AFC in other autoimmune-prone backgrounds has not been studied.
In previous work, we have identified the molecule Act1, an inhibitor that functions downstream of BAFF-R- and CD40-signaling pathways, as an important regulator of peripheral B cell homeostasis (32–34). Deficiency of Act1 results in B cell hyperplasia, and is associated with splenomegaly and lymphadenopathy. Act1−/− mice showed increased numbers of Fo, MZ and transitional (TR) B cell subsets in the spleen (32, 34). Histological analyses of the spleen and cervical lymph nodes from Act1−/− mice indicated increased GC formation and accumulation of large numbers of CD138+ plasma cells. Consistent with these findings, Act1−/− mice developed hypergammaglobulinemia, characterized by increased levels of serum IgG1, IgG2a/c, and IgG2b (34). The development of systemic autoimmune disease in the Act1−/− mice was also associated with increased titers of circulating RF and the appearance of anti-histone, anti-ssDNA and anti-dsDNA IgG autoantibodies (33, 34). As early as 8 weeks of age, Act1−/− BALB/c mice developed symptoms of Sjögren’s Syndrome (SjS), characterized by eye and mouth dryness, lymphoid infiltration of the lacrimal and salivary gland, and the production of anti-SS-A/Ro and anti-SS-B/La autoantibodies, which are two characteristic autoantibodies for SjS (33). Interestingly Act1−/− BAFF−/− double deficient mice produced detectable levels of anti-SS-A/Ro and anti-SS-B/La autoantibodies. In contrast, no autoantibody production was measurable in Act1−/− CD40−/− double knockout mice, suggesting that CD40-mediated activation plays a key role in the activation and differentiation of autoantigen-specific AFCs (33).
In the current study, we tested whether Act1 deficiency can promote the activation of RF-specific autoreactive B cells by generating AM14 Tg Act1−/− BALB/c mice.
MATHERIALS AND METHODS
Mice
AM14 Tg BALB/c (IgHa) or AM14 Tg CB.17 (IgHb) mice were crossed to the Act1−/− BALB/c mice. AM14 Tg Act1+/− mice were used to generate AM14 Tg Act1−/− and AM14 Tg Act1+/+ control mice on IgHa or IgHb backgrounds. Animals were maintained under specific pathogen-free conditions at the Biological Resource Unit of the Cleveland Clinic. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
FACS analysis
FACS analysis was performed as described (32). Antibodies used for FACS analysis included: FITC-conjugated anti-IgM (121-15F9), PE-conjugated anti-CD93 (AA4.1), PE-conjugated anti-CD22 (Cy-34.1), APC-conjugated anti-CD44 (IM7), FITC-conjugated anti-CD69 (H1.2F3), Biotin-conjugated anti-GL7 (GL-7) and Streptavidin-PerCP-Cy5.5 (e-Biosciences, San Diego, CA); FITC-conjugated and PE-conjugated anti-CD21/35 (7G6), FITC- and PerCP conjugated anti-B220 (RA3–6B2) (BD Biosciences, Franklin Lakes, NJ); APC-conjugated anti-CD23 (Caltag Laboratories, Carlsbad, CA). Anti-Id (4–44) Ab, which recognizes the AM14 H chain only in combination with L chain of Vκ8–19 or Vκ8–28, joined to Jκ4 or Jκ5, combinations that encode anti-IgG2a specificity, was described previously (22). All samples were analyzed on a BD FACS Calibur or LSRII (BD Biosciences). All data were analyzed using FlowJo 7.5 software (TreeStar, Ashland, OR).
ELISA
Serum AM14 levels were determined by ELISA as previously described (25). Briefly, ELISA plates were pre-coated with 10 µg/ml goat anti-mouse IgM (Chemicon International, Inc., Temecula, CA) overnight in PBS, washed and blocked. Serum samples were incubated in duplicates for 2 hours and plates were washed and incubated for 1 hour with biotinylated anti-idiotype Ab 4–44 (0.25 µg/ml in 1% BSA/ PBS). After washing, plates were incubated with streptavidin-alkaline phosphatase (R&D Systems, Inc., Minneapolis, MN), and, finally, development with p-nitrophenylphosphate. The OD at 405 nm was read on a Perkin Elmer plate reader (Wellesley, MA).
ELISpot analysis
96-well MultiScreen™-IP plates (Millipore, Billerica, MA) were pre-coated with 10 µg/ml goat anti-mouse IgM (Chemicon International) and incubated overnight at 4°C. Cells were suspended in RPMI medium with 2.5% FBS at concentrations 1.5×107 cells /ml. Cells were plated in duplicate and then serially diluted by a factor of 3. ELISpot plates were centrifuged and incubated at 37°C, 5% CO2 for 12–14 hours. After washing, plates were incubated with biotin-conjugated anti-AM14 (4–44) Ab (1:500 in 1% BSA). Immune reaction was detected using an ELISpot Blue Color Module, (RnD Systems, Minneapolis, MN). Immune spots were analyzed using an ImmunoSpot®-S1 Analyzer and the ImmunoSpot®-4.0 Software (Cellular Technologies Ltd., Cleveland, OH). Final values were calculated as number spots per 1×106 cells.
Histology
Tissue samples were snap-frozen in OCT medium and stored at −80°C. Sections were prepared and stained with Abs as described previously (35). Abs used include 4–44-biotin, anti-CD4 Alexa 647 (GK1.5), anti-CD19 Alexa 647 (1D3.2), anti-CD90.2 Alexa 647 (30H12), PNA-FITC (Vector Laboratories, Inc., Burlingame, CA), anti-CD45R Alexa 488 (6B2), anti-F4/80 Alexa 647 (BM8), streptavidin Alexa 555 (Molecular Probes), streptavidin Brilliant Violet 421 (Biolegend), anti-Ki67 rabbit IgG supernatant (SP6), and anti-rabbit IgG Alexa 568 (Molecular Probes). A 5 × 5 array of tiled images at 100x power was captured for each sample at a resolution of 1600 × 1200 pixels per image. Images were captured with a Nikon Eclipse Ti-U microscope and processed using Nikon Software and composited and cropped using Adobe Photoshop.
CD40L Blocking
AM14 Tg Act1−/− mice with comparable levels of serum AM14 (OD reading between 0.4 and 0.55) were selected for this experiment. Mice were injected i.p on days 1 and 3 with 250 µg sterile anti-CD40L mAb (MR1) or control hamster IgG (BD Biosciences), diluted in sterile PBS. Mice were sacrificed on day 7 days after the first injection and spleens and cLNs were analyzed for the presence of CD22hi 4–44+, CD22lo 4–44+, and GL7+4–44+ B220+ B cells by FACS.
Statistical analysis
Differences between various groups were determined using unpaired two-tailed student t test. All analyses were performed using GraphPad Prism 5.0 Software (La Jolla, CA). Values are reported as mean ± SD; P values < 0.05 were considered significant.
RESULTS
Development of AM14 Tg B cells on Act1−/− background
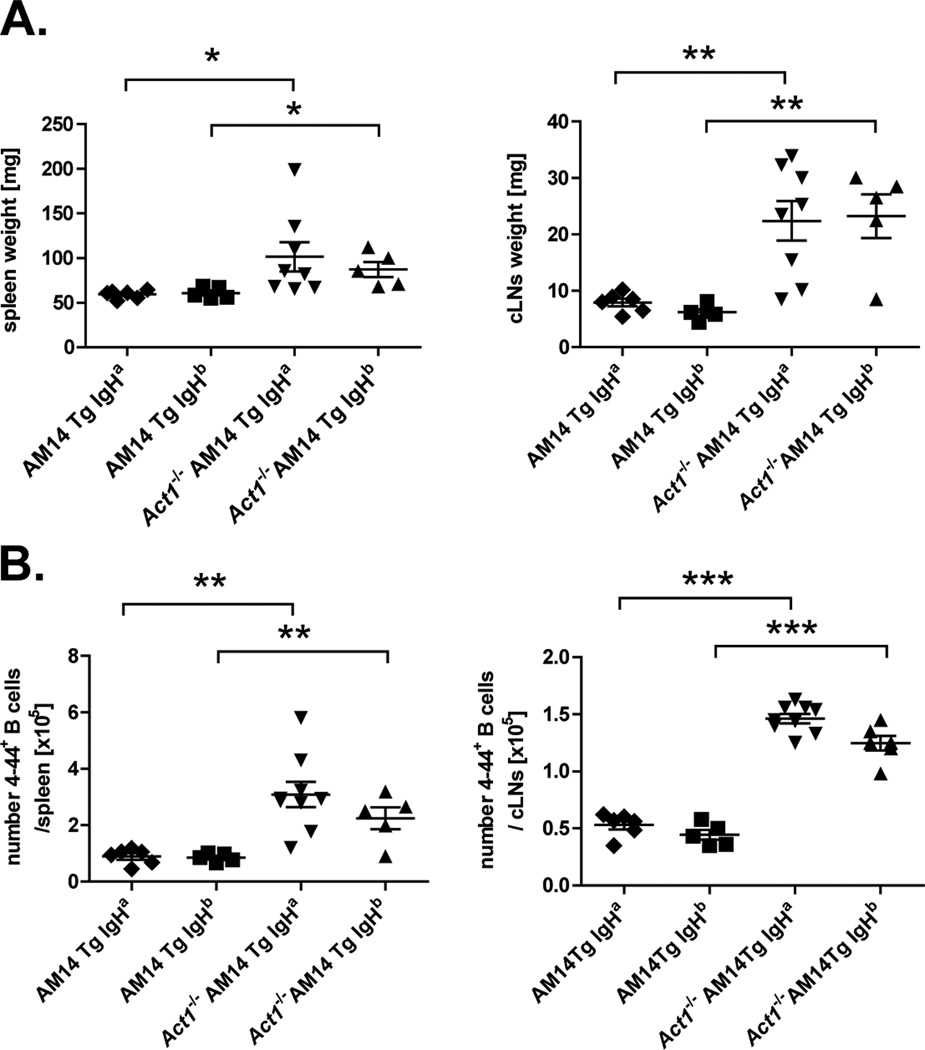
To study the effect of Act1 deficiency on the development of RF-specific (AM14 Tg) B cells in the presence or absence of endogenous Ag, we generated AM14 Tg Act1−/− mice on two different backgrounds: AM14 Tg Act1−/− BALB/c (IgHa) and AM14 Tg Act1−/− CB.17 (IgHb). At 8–10 weeks of age, AM14 Tg Act1−/− mice on both backgrounds had visibly enlarged spleens and cervical LNs when compared to their AM14 Tg Act1+/+ littermates (Fig. 1A). FACS analysis confirmed that lymphoid organ enlargement was associated with an increase in the number of IgM+B220+ B cells (data not shown). To evaluate if the B cell expansion also involved AM14 Tg B cells, we performed flow cytometry of spleen and cLN cells using anti-Id (4–44) Ab, which recognizes the 2–3% of B cells in AM14 H Tg mice that have an L chain that confers BCR specificity for IgG2a. Results from these analyses revealed a 2-fold (spleen) and 2.3–fold (combined cLNs) increase in the number of 4–44+ cells in AM14 Tg Act1−/− IgHa compared to AM14 Tg Act1+/+ IgHa. Similar increases were observed in spleens and cLNs of AM14 Tg Act1−/− IgHb mice as compared to AM14 Tg Act1+/+ IgHb mice (Fig. 1B).
Figure 1.
Expansion of AM14 Tg (4–44+) B cells in spleen and cLNs of AM14 Tg Act1−/− mice associated with splenomegaly and lymphadenopathy. A. Aged-matched AM14 Tg or AM14 Tg Act1−/− (IgHa and IgHb backgrounds) were analyzed for the development of splenomegaly and lymphadenopathy. Data are presented as mg tissue (spleen or combined superficial cervical LNs), for each individual mouse. B. Summary of FACS data showing the number of 4–44+ cells per spleen or combined cLN. The percentage of 4–44+ of total cells was determined by FACS and the cell numbers were calculated based on the total cell count for each individual mouse. ** p < 0.01, *** p < 0.001.
Previous analyses of peripheral B cell subsets in Act1−/− mice revealed defective selection of immature B cells as they passed the T1-to-T2 transitional checkpoint, as well as an increase in the number of T2, FO and MZ B cells within the spleen (32). To address whether the lack of Act1 similarly affected the development of 4–44+ B cells, we examined their representation within different B cell subsets in the spleen, including within T1 (B220+AA4.1+CD23−IgM+), T2 (B220+AA4.1+CD23+IgMhi), Fo (B220+AA4.1−CD23+IgM+) and MZ (B220+AA4.1−CD23−IgMhi) B cells. We found no significant differences in the percentage of 4–44+ B cells between AM14 Tg IgHa and AM14 Tg Act1−/− IgHa mice within any of these B cell subsets. As previously reported (22, 35), we observed that the majority of 4–44+ B cells in the spleens of both AM14 Tg Act1+/+ and AM14 Tg Act1−/− mice (age 3–4.5 months) displayed a phenotype of either Fo (72–80% of 4–44+ cells in the spleen) or T2 B cells (4–16% of 4–44+ cells in the spleen) (Supplementary Fig. 1). Thus, the 2-fold increase in the number of AM14 Tg B cells in AM14 Tg Act1−/− mice was proportional to the overall increase in the numbers of Fo and T2 B cells in these mice.
In summary, we observed a significant increase in the number of AM14 Tg B cells in the spleen and cLN of AM14 Tg Act1−/− mice, with no obvious skew in their development. In agreement with previous studies in AM14 Tg MRL.Faslpr mice, we did not find evidence of toleralization or elimination of 4–44+ B cells in the periphery.
Spontaneous, Ag-driven activation of AM14 Tg B cells in AM14 Tg Act1−/− mice
Previous studies have demonstrated that 4–44+ B cells on the autoimmune-prone MRL.Faslpr background become spontaneously activated and differentiate into Ab-secreting plasmablasts (26). Such activation requires the presence of endogenous Ag and occurs only in IgHa expressing mice, but not in IgHb expressing mice.
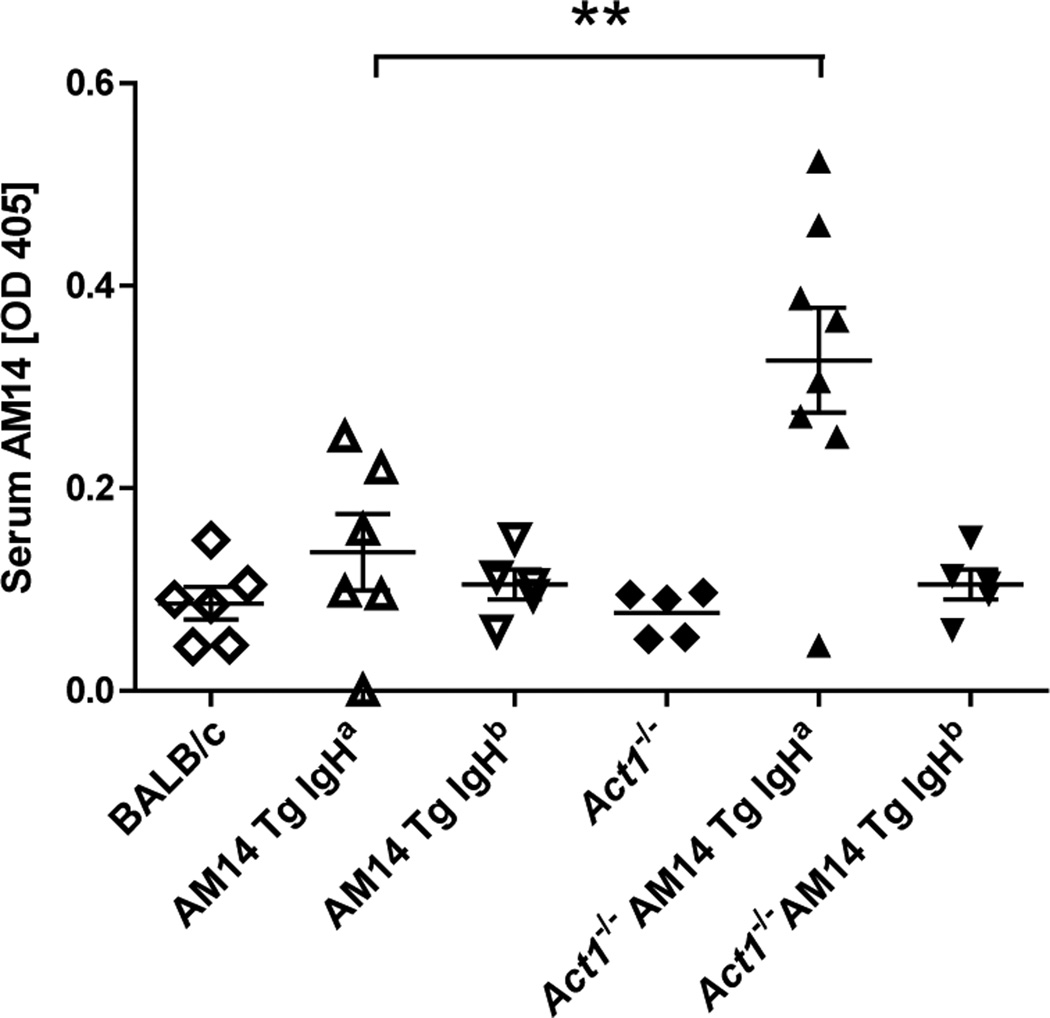
To test whether Act1 deficiency was sufficient to mediate spontaneous activation of RF-producing B cells, we measured the concentration of AM14 Abs (RF) in the serum of BALB/c (wild type), AM14 Tg Act1+/+ IgHa, AM14 Tg Act1+/+ IgHb, AM14 Tg Act1−/− IgHa and AM14 Tg Act1−/− IgHb mice. ELISA results showed a significant increase of AM14 Ab titers in the serum of AM14 Tg Act1−/− IgHa mice, while no significant levels of AM14 Ab were detected in AM14 Tg Act1+/+ IgHa, AM14 Tg Act1+/+ IgHb and AM14 Tg Act1−/− IgHb mice (Fig. 2).
Figure 2.
Increase of AM14 protein levels in AM14 Tg Act1−/− IgHa mice. Serum levels of AM14 protein were measured by ELISA as described in Materials and Methods. Samples were collected from 3 to 4.5-months-old mice. Each symbol represents one individual mouse. ** p < 0.01.
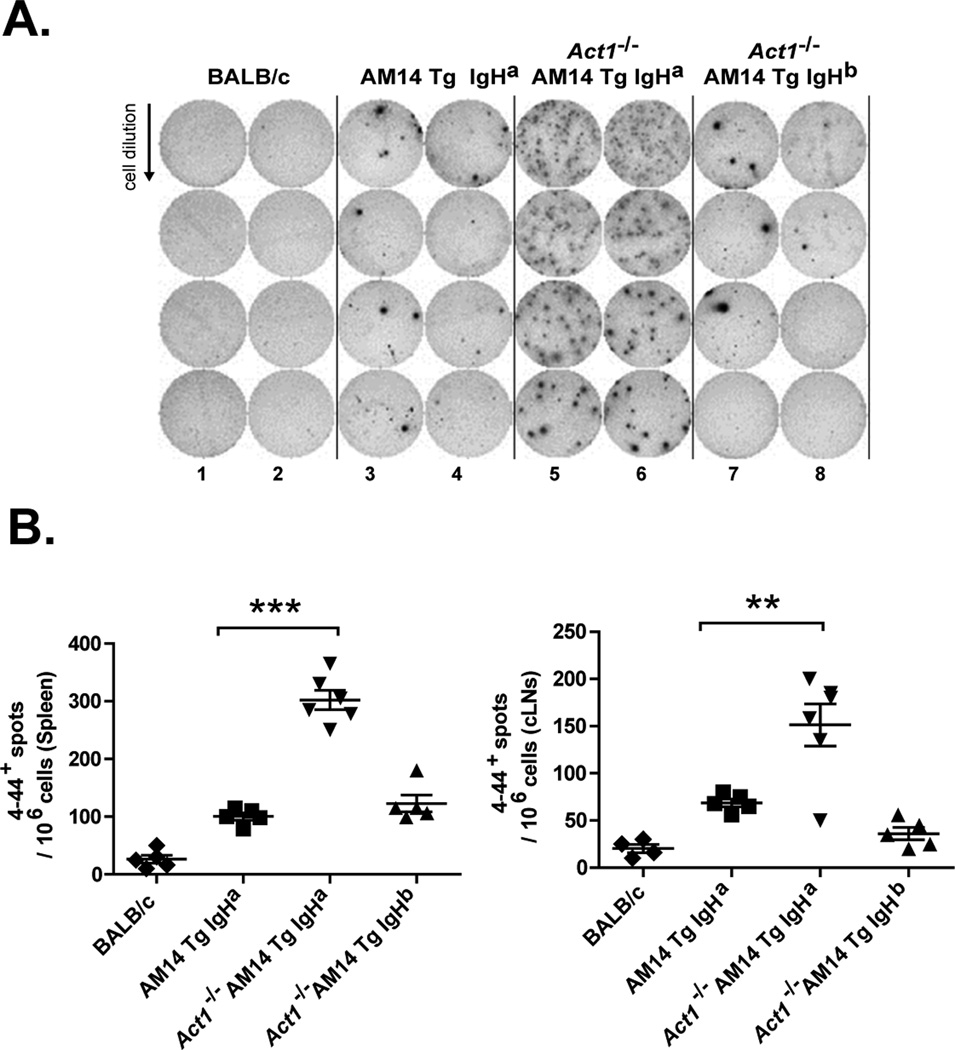
The presence of AFCs in AM14 Tg Act1−/− IgHa mice was further confirmed in ELISpot assays (Fig. 3). Splenocytes from BALB/c AM14 Tg Act1+/+ IgHa, AM14 Tg Act1−/− IgHa and AM14 Tg Act1−/− IgHb mice (6 mice per group) had a 5-to-6-fold increase in the number of AM14 (4–44+) AFCs in the AM14 Tg Act1−/− IgHa mice as compared to AM14 Tg Act1+/+ IgHa mice (Fig. 3A). Only low frequencies of AFCs were observed in AM14 Tg Act1−/− IgHb mice, supporting a requirement for endogenous Ag for the activation of AM14 Tg Act1−/− B cells. Interestingly, high numbers of AM14-specific AFC cells were also detected in cervical LNs isolated from AM14 Tg Act1−/− IgHa mice (Fig. 3B), suggesting that these LNs serve as an additional location for the accumulation and, possibly, for the activation of RF-specific cells in AM14 Tg Act1−/− mice.
Figure 3.
Accumulation of AM14 Tg AFCs in spleens and cLNs of AM14 Tg Act1−/− mice. A. Representative ELISpot analysis used to quantify splenic 4–44+ AFCs in AM14 Tg Act1+/+ or AM14 Tg Act1−/− mice (on IgHa and IgHb backgrounds). B. Number of 4–44+ AFCs detected by ELISpot in spleens or cLNs from individual AM14 Tg or AM14 Tg Act1−/− mice. Data is presented as number of spots per 106 cells. ** p < 0.01, *** p < 0.001.
Studies using AM14 Tg MLR.Faslpr mice identified two populations of B cells involved in the RF response: 4–44+CD22hiCD44hi cells, which do not secrete autoantibodies but instead appear to be immediate precursors of a second population, and 4–44+CD22loCD44brigt cells, which display features of differentiated plasmablasts capable of producing AM14 Ab.
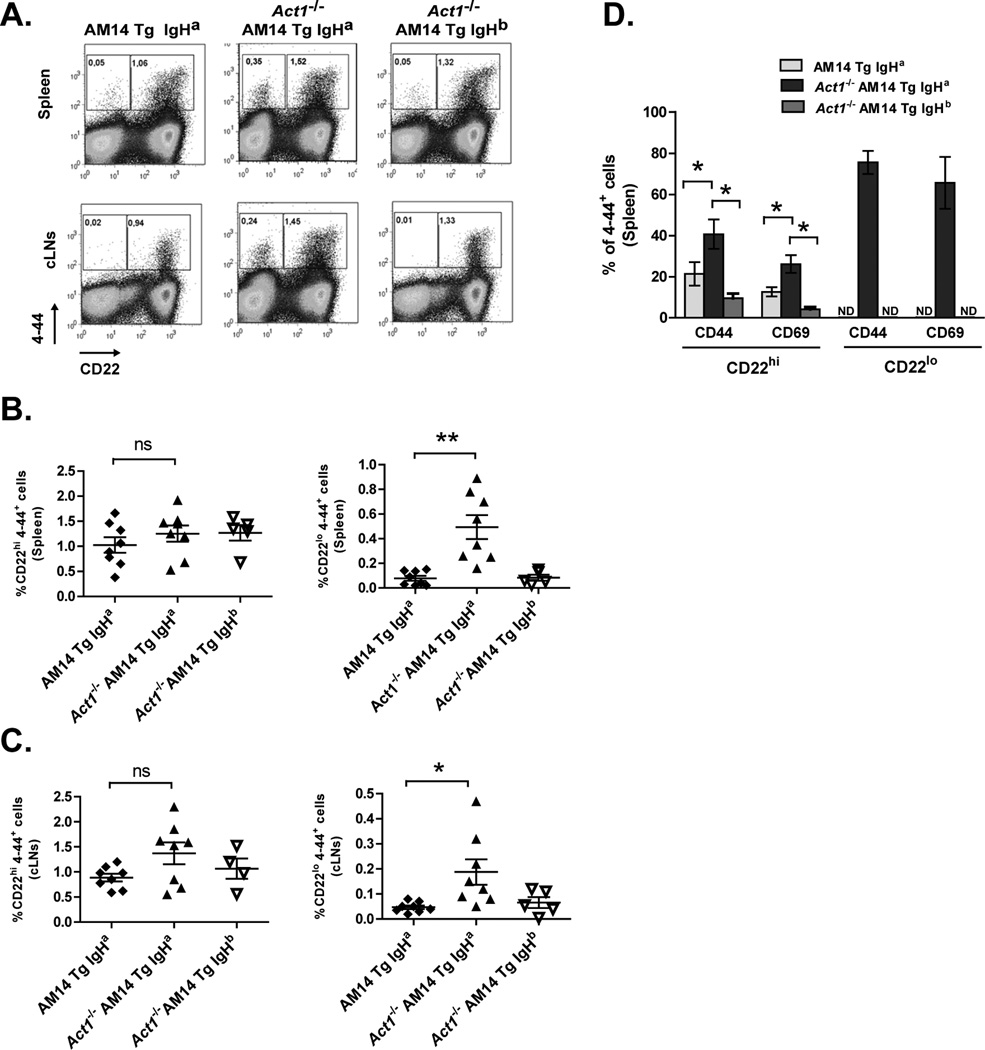
We used FACS analyses to identify these two cell populations and compared them between Act1−/− and Act1+/+ AM14 Tg mice (on IgHa or IgHb backgrounds). Consistent with the elevated levels of serum AM14 Ab in the AM14 Tg Act1−/− IgHa mice, we found a significant increase in the frequency of 4–44+CD22lo B cells in the spleens of AM14 Tg Act1−/− IgHa mice (0.48 ± 0.11 % versus 0.08 ± 0.01 and 0.08 ± 0.04 % in AM14 Tg Act1+/+ and AM14 Tg Act1−/− IgHb, respectively (Fig. 4A, upper panel and Fig. 4B). 4–44+CD22lo Tg B cells were also clearly detectable in the cervical LNs of AM14 Tg Act1−/− IgHa mice (Fig. 4A, lower panel and Fig. 4C).
Figure 4.
Detection of AM14 Tg plasmablast in AM14 Tg Act1−/− mice. A. Representative FACS plots of cells from AM14 Tg Act1+/+, AM14 Tg Act1−/− IgHb and AM14 Tg Act1−/− IgHa mice. Gates identify 4–44+ CD22hi AM14 Tg B cells and 4–44+ CD22lo AM14 Tg cells. B and C. Summary of FACS data, showing the percentage of 4–44+ CD22hi and 4–44+ CD22lo B cells of total live cells in spleens and cLNs from individual AM14 Tg IgHa, AM14 Tg Act1−/− IgHa and AM14 Tg Act1−/− IgHb mice. D. Summary of FACS data of the percentage of 4–44+ CD22hi and 4–44+ CD22lo, expressing CD44 and CD69 activation markers. No results available for 4–44+ CD22lo in AM14 Tg mice due to low cell count. AM14 Tg IgHa mice (n=5), AM14 Tg Act1−/− IgHa mice (n=6) and AM14 Tg Act1−/− IgHb mice (n=3) * p < 0.05, ** p < 0.01, *** p < 0.001.
The frequency of 4–44+CD22lo Tg B cells in the periphery was variable between individual AM14 Tg Act1−/− IgHa mice. Still, their frequency in the spleen positively correlated with the increased serum levels of AM14 Ab (R2=0.47, p=0.015, data not shown). Consistent with data from AM14 Tg MRL.Faslpr mice (26), the frequency of 4–44+CD22lo Tg B cells increased in AM14 Tg Act1−/− IgHa mice as they aged (data not shown).
Further analyses of 4–44+CD22hi B cells in the spleen of AM14 Tg Act1−/− IgHa mice and control mice showed a significant increase in the percentage of cells expressing CD44 and CD69 markers on their surface (Fig. 4D), suggesting that these cells were also undergoing Ag-driven activation. As expected, the majority of 4–44+CD22lo cells in AM14 Tg Act1−/− IgHa mice expressed high levels of CD44 and CD69. In contrast, we did not observe significant accumulation of CD44+ or CD69+ 4–44+CD22lo cells in AM14 Tg Act1−/− IgHb mice (Fig. 4D).
Due to the low frequency of 4–44+CD22lo cells in AM14 Tg Act1+/+ IgHa and AM14 Tg Act1−/− IgHb mice, we were unable to determine the levels of CD44 and CD69 expression in these cells (Fig. 4D). Based on these observations, we concluded that Act1 deficiency in 4–44+ B cells (IgHa background) leads to the spontaneous activation and differentiation of these cells from ignorant follicular B cells into functional AFCs.
Development of 4–44+ germinal center B cells in spleens and cLNs of AM14 Tg Act1−/− mice
Previously, we found an extensive and presumably spontaneous formation of germinal centers (GC) in the cLNs and spleens of Act1−/− mice (34). The exact mechanisms triggering this GC formation are still under investigation, although we suspect a role for T cell-dependent CD40 ligation, as both T-cell deficient Act1−/− mice (36) and Act1−/−CD40−/−-double deficient mice show a complete abrogation of GC formation (Y. Qian, N. Giltiay, unpublished data). Activated 4–44+ B cells in AM14 Tg MLR.Faslpr autoimmune mice displayed a phenotype of short-lived plasmablasts and were found to actively proliferate and to undergo somatic hypermutations outside of the GC-follicles (30, 37).
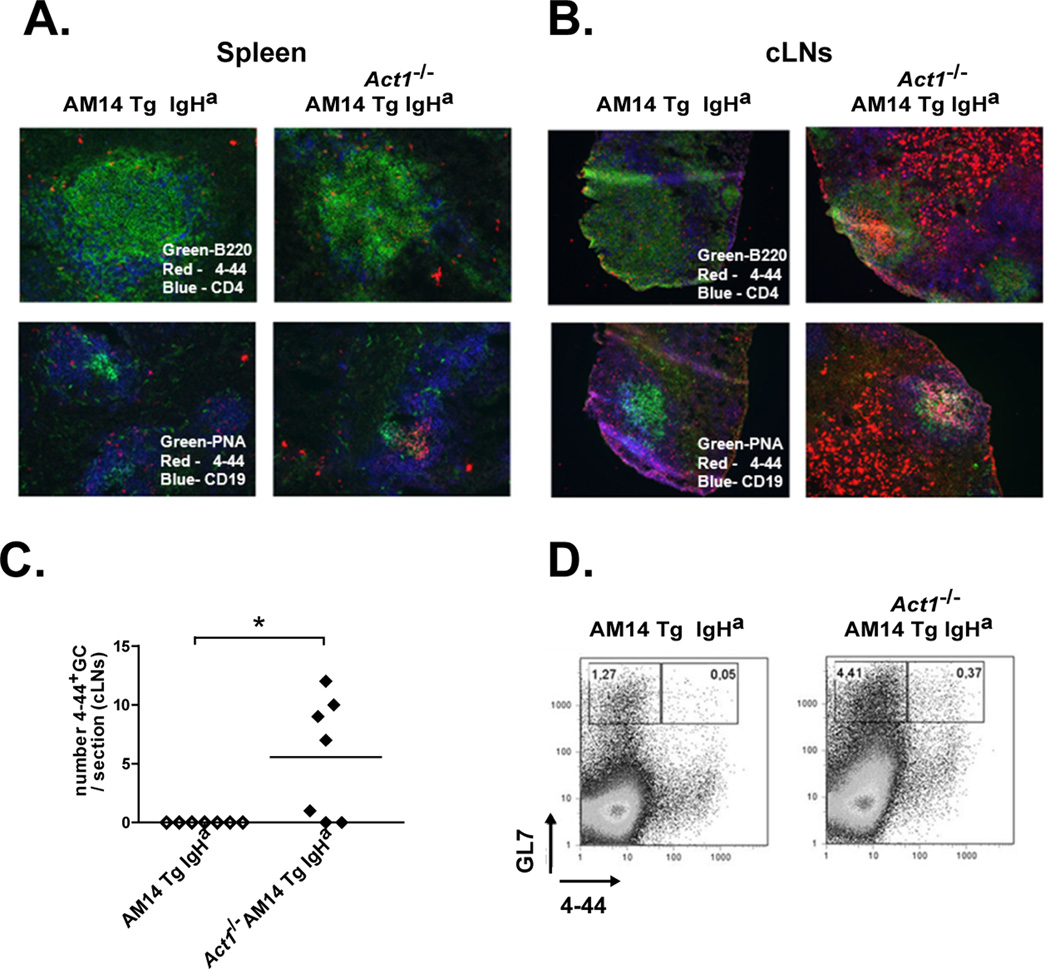
To determine the location of expanded 4–44+ B cells in AM14 Tg Act1−/− mice, we analyzed splenic and cLNs sections. In agreement with our FACS and ELISpot data, consistent with an extrafollicular response 4–44+ splenic cells from AM14 Tg Act1−/− mice were observed to cluster outside of the B cell follicles (Fig. 5A). The expansion of 4–44+ B cells was more apparent in the cLN of AM14 Tg Act1−/− mice, where 4–44+ B cells showed both follicular and extrafollicular localization (Fig. 5B). We found that a significant percentage of AM14 Tg Act1−/− mice (~60%) developed 4–44+ positive GCs in their cLNs (Fig. 5B, right panel). This result was supported by flow cytometry analysis that showed a significant increase in the percentage of GL7+ 4–44+ B cells in cLNs from AM14 Tg Act1−/− mice cells as compared to AM14 Tg Act1+/+ controls (Fig. 5C–D).
Figure 5.
AM14 Tg B cells in the AM14 Tg Act1−/− mice undergo activation at extra-follicular sites and form GCs. A and B. Micrographs of immunoflorescent staining of splenic (A) and cLN (B) sections from AM14 Tg Act1+/+ IgHa and AM14 Tg Act1−/− IgHa mice showing: B220+ B cells (green), CD4+ T cells (blue) and 4–44+ AM14 Tg cells (red) – upper panel; and PNA+ GC cell (green), CD19+ B cells (blue) and 4–44+ AM14 Tg cells (red) – lower panel. C. Graph showing the number of GC per section of cLN from AM14 Tg Act1+/+ and AM14 Tg Act1−/− mice. D. Representative FACS plots showing the percentage of CD44+ GL7+ in cLNs from AM14 Tg and AM14 Tg Act1−/− mice. * p < 0.05.
Immunohistological analyses of cLNs from Act1−/− AM14Tg IgHa using Ki67 revealed the presence of proliferating 4–44+ B cells both in the GC as well as at extrafollicular sites (Supplementary Figure 2).
Dependence of 4–44+ Act1−/− B cells on CD40-mediated signaling
In our previous studies we found that intact CD40/CD40L interaction is required for the formation of disease-associated autoantibodies in the Act1−/− mice (33). In ex vivo settings, we observed that Act1−/− mature B cells responded more vigorously to CD40-ligation (anti-CD40 Ab) as compared to WT B cells, and differentiated much faster into proliferating blast-like cells (Supplementary Figure 3). Previously we also showed that agonistic anti-CD40Ab treatment was associated with a more significant up-regulation of the BclXL protein in Act1−/− B cells as compared to WT B cells (34).
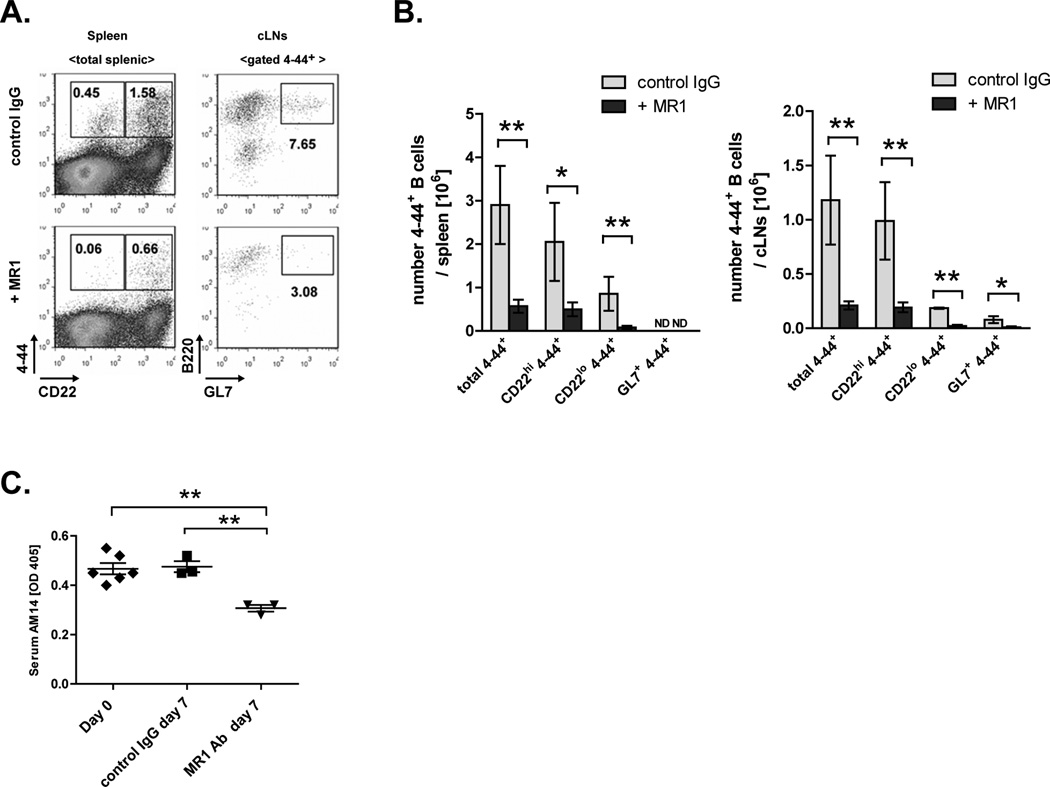
To test if blocking CD40 ligation in vivo had an effect on the activation of 4–44+ B cells and the formation of plasmablasts and GCs in AM14 Tg Act1−/− mice, we treated mice with anti-gp39 blocking Ab (clone MR-1). Spleens and cLNs of injected mice were harvested on day 7 after the initial injection and were analyzed by FACS. Results from this analysis showed that blocking of CD40 resulted in significantly decreased frequencies of 4–44+ CD22hi and 4–44+ CD22lo B cells in the spleen and cLN of AM14 Tg Act1−/− mice (Fig. 6 A and B). No significant decrease in total B cells was observed (not shown). MR-1 treatment also led to decrease in the frequency of 4–44+ GL7+ cells in the cLNs of AM14 Tg Act1−/− mice (Fig. 6 A and B).
Figure 6.
AM14 Tg Act1−/− B cells are susceptible to anti-CD40L treatment. A. Representative FACS plot showing the percentage CD44+CD22hi and CD44+CD22lo cells of splenic cells from AM14 Tg Act1−/− mice treated with control IgG (left top panel) or anti-CD40L Ab MR1 (left bottom panel). Right panels show the percentage of B220+GL7+ of gated CD44+ from cLN from mice, treated with control IgG (top panel) or MR1Ab (bottom panel). B. Numbers of total 4–44+, 4–44+CD22hi, 4–44+ CD22lo and 4–44+ GL7+ in spleen and cLNs of control and MR1 -injected mice analyzed at day 7. C. Levels of AM14 protein measured by ELISA in serums form AM14 Tg Act1−/− mice at day 0 and day 7 after the IgG or MR1 administration. * p < 0.05, ** p < 0.01.
Finally, we detected a reduction in serum AM14 Ab titers in anti-CD40L Ab-treated mice (Fig. 6 C), indicating that this serum Ab derives from short-lived AFCs, presumably plasmablasts, rather than from long-lived plasma cells, which would not be affected directly by MR-1 Ab treatment, nor substantially even indirectly via GC inhibition, over the space of a week (38).Thus, it appears that CD40 signaling is important for the optimal activation and differentiation of 4–44+ B cells in AM14 Tg Act1−/− mice in vivo. Based on these findings, we propose a model in which the lack of Act1 leads to dysregulation of CD40 signaling and results in spontaneous activation of 4–44+ B cells.
DISCUSSION
Loss of B cell tolerance plays a key role in the development of systemic autoimmunity. We previously reported that deficiency of Act1 in mice leads to dysregulation of B cell function in the periphery and results in the development of SjS- and SLE-like autoimmune diseases (33, 34).
In this study, we show that crossing of AM14 Tg mice to Act1−/− mice leads to the activation of AM14 Tg B cells. We found that AM14 Tg Act1−/− mice developed enlarged spleens and cLNs, and displayed expansions of RF-specific autoreactive B cells as compared to AM14 Tg Act1+/+ mice. Furthermore, our data indicated that in the presence of endogenous self-Ag (BALB/c background), AM14 Tg Act1−/− B cells became activated and differentiated into functional AFCs. Notably, we found that a significant number of activated AM14 Tg (4–44+) B cells accumulated in the cLN of AM14 Tg Act1−/− mice, where they differentiated into GC B cells as well as AFCs.
The exact mechanisms by which Act1 regulates B cell function in the periphery are not fully understood. In our previous studies, we found that Act1 interacts with TRAF family members and functions as an inhibitor of BAFF-R- and CD40-signaling pathways by regulating the activation of the NF-κB1/ NF-κB2 pathways and MAPK signaling (34). In addition to its regulatory function in BAFF-R and CD40 signaling in B cells, the Act1 molecule was identified as a key signaling component of IL-17R signaling in fibroblasts, endothelial and epithelial cells, where it plays an opposite function in promoting NF-κB1 activation (39–41).
Under normal conditions, several mechanisms function to prevent the activation of autoreactive B cells (2, 3). Alterations that enhance BCR signaling have been associated with a breakdown in B cell tolerance and the development of autoimmunity. For example, mutations in the Bruton’s tyrosine kinase (Btk) gene and tyrosine phosphatase nonreceptor type 22 (PTPN22) gene, two molecules that function downstream of the BCR, have been implicated in defective selection of autoreactive B cells at the central tolerance checkpoint in humans (42). Mice that lack the expression of Lyn, a Src family kinase that activates the inhibitory receptors CD22 and FcγRIIb, which are involved in the negative regulation of BCR signaling, develop autoimmunity associated with production of autoantibodies to nuclear Ags (43–47).
In addition to BCR-hyper-responsiveness, alterations in the strength of B cell pro-survival signals can disrupt the negative selection of autoreactive B cells. For example, overexpression of B cell survival factor BAFF in BAFF Tg mice has been shown to breach the peripheral tolerance checkpoint and to promote the expansion of T2-MZ precursors and MZ B cells, resulting in the development of SLE- and SjS-like diseases (15, 48, 49).
In previous studies, we have shown that Act1 deficiency resulted in dysregulation of BAFF signaling during transitional B cell maturation and promotes the maturation of HEL-specific autoreactive B cells (32). Here, we found that Act1 deficiency did not affect the development of AM14 Tg B cells in the periphery, as we did not find significant differences in the percentages of 4–44+ cells within T1, T2, Fo B cells subset in the AM14 Tg Act1−/− as compared to AM14 Tg Act1+/+ littermates. In addition, while previous analysis of Act1−/− mice showed an increase in MZ B cells (32, 34) we did not find a significant percentage of 4–44+ B cells expressing a MZ phenotype in the AM14 Tg Act1−/− mice. Thus, our data are in agreement with previously published studies in AM14 Tg mice, where 4–44+ B cells progress normally through immature stages and accumulate in the B cell follicles, but not in MZs (22, 35). The lack of impact on AM14 Tg B cell development by Act1 deficiency may reflect relatively weak self-reactivity, as well as the fact that AM14 Tg B cells are clonally ignorant.
The appearance of 4–44+ CD22lo cells in AM14 Tg Act1−/− mice suggests that Act1 deficiency leads to spontaneous activation of AM14 Tg B cells, and drives the differentiation of AM14 Tg B cells into AFCs. Similar to previous studies (26, 31) using the AM14 Tg MLR.Faslpr mouse model, we found that the spontaneous activation of 4–44+ B cells was associated with up-regulation of CD69 and CD44 markers, and occurred only in the presence of endogenous autoantigen (i.e. on the IgHa BALB/c background). The extent of AFC development was variable between individual AM14 Tg Act1−/− IgHa mice and increased with age, suggesting the stochastic nature of their activation.
Interestingly, we found a significant accumulation of AM14 Tg B cells in the cLNs, which were visibly enlarged. The exact mechanism driving the accumulation of activated autoreactive 4–44+ B cells in the cLN of AM14 Tg Act1−/− remains unclear, however it might be related to the fact that Act1−/− BALB/c mice develop symptoms of SjS, associated with infiltration of activated autoreactive B cells in the salivary and lacrimal glands (33).
The activation of AM14 Tg cells in previously studied AM14 Tg MLR.Faslpr mice was found to occur mainly outside of the GC in the spleen resulting in the generation of short-lived plasmablasts (50). However, AM14 B cells did form GCs in the LNs of both BALB/c and MRL.Faslpr mice, but only in the presence of self-Ag (51). The observation that 4–44+ B cells form GCs in AM14 Tg Act1−/− mice suggests a parallel mechanism of action of Act1 in the regulation of autoreactive B cells.
It is well accepted that T-cells express CD40L, along with other co-stimulatory molecules and cytokines, and thus provide help to B cells, resulting in clonal expansion, enhanced Ab production, class-switching, and somatic hypermutation (52). The requirements of T-cell help for the spontaneous activation of AM14 cells was extensively studied in AM14 Tg MLR.Faslpr mice (31), as well as in AM14 BALB/c mice challenged with chromatin-containing immune complexes (29). These studies demonstrated that exposure of AM14 Tg cells to IgG2a anti-chromatin complexes can sustain the activation of AM14 Tg B cells even in the absence of antigen-specific T cell help. Following up on this observation, a number of recent studies have suggested an important role for TLRs in driving T-cell independent activation of autoreactive B cells (42, 53). Still, for isotype-switched responses, T cell help had a substantial effect on the magnitude of the response (4–5-fold), indicating that T cells can and do participate in generating extrafollicular plasmablasts (31).
Our recent studies showed that lack of T-cells in B6.Act1−/−TCRβ−/−/TCRδ−/− mice resulted in an almost complete inhibition of anti-nuclear IgG Abs in the serum, suggesting that T-cell help is required for the development of self-reactive antibodies in the B6.Act1−/− mouse (36). Likewise, we have previously shown that CD40−/− Act1−/− BALB/c mice fail to develop serum auto-Abs and/or SjS- and SLE-like disease (33, 34). Based on these observations, we predicted that stimulation through CD40 plays a key role in the activation of 4–44+ B cells in AM14 Tg Act1−/− mice and in the selection of 4–44+ cells to the GCs. Indeed, blocking CD40L by MR-1 Ab treatment led to a reduction in the percentage of 4–44+ B cells, decreased AM14 Ab serum titers, and reduced percentages and numbers of 4–44+ GL7+ cells. Together, these findings strongly support a model in which CD40-mediated signaling plays a key role in the activation of 4–44+ B cells in AM14 Tg Act1−/− mice. The development of Ag-driven GCs in AM14 Tg Act1−/− mice suggests the generation of cognate T cell help at the target LNs. The mechanisms by which the lack of Act1 can promote breakdown of T cell tolerance remain unknown. One possibility is that activated AM14 Tg Act1−/− B cells present Ags to self-reactive T cells, which express CD40L along with other cytokines and costimulatory molecules. Activation of B cells via CD40L-CD40 can promote the activation and differentiation of CD4+ T cells to T follicular helper cells, for example by ICOSL-ICOS interactions (54), thus mediating the formation of GCs.
Recent studies have implicated a role for Th17–derived cytokines IL-17 in promoting Ab-production by B cells and the formation of GC (55). Despite the fact that Act1−/− mice show an increase in the production of IL-17 (56), it is unlikely that IL-17-mediated signals contribute directly to the expansion of AM14 Tg and GC formation in the AM14 Tg Act1−/− mice due to their inability to mount IL-17 responses (41). Consistent with its role in IL-17 mediated inflammation, we and others have shown that Act1 is essential for the development of EAE and CIA (41, 57). Recent studies revealed a novel role of Act1 in controlling the activation of Th17 cells and showed an increased production of IL-22 by Th17 cells in Act1−/− mice (56). However, since Th17 cells can make other cytokines, most notably IL-21, they might contribute to the activation of AM14 Tg B cells (58, 59). Interestingly, lack of Act1 was recently shown to protect FcγRIIb−/− mice from development of lethal glomerulonephritis by preventing the influx of inflammatory myeloid-derived cells into the kidneys, without affecting the production of anti-nuclear Abs (60). Thus, the role of Act1 molecule in SLE pathology can be multi-faceted. Despite the lack of IL-17R signaling, Act1−/− BALB/c mice spontaneously develop autoimmune pathology, associated with GC formation and production of autoantibodies (33, 34), underscoring the important, cell-intrinsic role of Act1 in B cell regulation. Previous studies using Act1−/− mice have supported a role for Act1 in the selection of autoreactive B cells at the transitional checkpoint (32, 36). Results presented in this study further suggest that Act1 plays an important function in the suppression of AFC and GC formation by autoreactive B cells. This function of Act1 is most likely mediated via the regulation of CD40 responsiveness of B cells. Our findings support the requirement for a strict regulation CD40-mediated signaling in the toleralization of low affinity autoreactive B cells and suggest how we might interfere with pathogenic B cell responses in autoimmune diseases such as SLE and SjS.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Pavlina Baevova, Rebecca Sweet and Michelle Harris for providing reagents and protocols related to this study. We would like to thank Dr. Edward Clark for critical reading of the manuscript.
This study was supported by NIH grants AI 065470 (X.L.), and Research Grant from The Sjögren’s Syndrome Foundation (N.G.).
Abbreviations
- AFC
Antibody Forming cells
- BAFF
B cell-activating factor belonging to the TNF family
- BCR
B cell antigen receptor
- BM
bone marrow
- cLN
cervical lymph node
- FM
follicular mature
- MZ
marginal zone
- RF
rheumatoid factor
- SjS
Sjögren’s Syndrome
- SLE
Systemic Lupus Erythematosus
Footnotes
DISCLOSURES
The authors declare no financial or commercial conflicts of interests.
REFERENCES
- 1.Basten A, Brink R, Peake P, Adams E, Crosbie J, Hartley S, Goodnow CC. Self tolerance in the B-cell repertoire. Immunological reviews. 1991;122:5–19. doi: 10.1111/j.1600-065x.1991.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 2.Shlomchik JM. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Current opinion in immunology. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 5.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 6.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. The Journal of experimental medicine. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radic MZ, Erikson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. The Journal of experimental medicine. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nature immunology. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 9.Carsetti R, Kohler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. The Journal of experimental medicine. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. Journal of immunology. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 11.Shlomchik MJ. Activating systemic autoimmunity: B's, T's, and tolls. Current opinion in immunology. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 13.Rifkin IR, Leadbetter EA, Beaudette BC, Kiani C, Monestier M, Shlomchik MJ, Marshak-Rothstein A. Immune complexes present in the sera of autoimmune mice activate rheumatoid factor B cells. Journal of immunology. 2000;165:1626–1633. doi: 10.4049/jimmunol.165.3.1626. [DOI] [PubMed] [Google Scholar]
- 14.Brink R. Regulation of B cell self-tolerance by BAFF. Seminars in immunology. 2006;18:276–283. doi: 10.1016/j.smim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Biancone L, Cantaluppi V, Camussi G. CD40-CD154 interaction in experimental and human disease (review) International journal of molecular medicine. 1999;3:343–353. doi: 10.3892/ijmm.3.4.343. [DOI] [PubMed] [Google Scholar]
- 17.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 18.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 19.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 20.Mandik-Nayak L, Seo SJ, Sokol C, Potts KM, Bui A, Erikson J. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. The Journal of experimental medicine. 1999;189:1799–1814. doi: 10.1084/jem.189.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C, Li H, Tian Q, Beardall M, Xu Y, Casanova N, Weigert M. Selection of anti-double-stranded DNA B cells in autoimmune MRL-lpr/lpr mice. Journal of immunology. 2006;176:5183–5190. doi: 10.4049/jimmunol.176.9.5183. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Shlomchik MJ. Autoantigen-specific B cell activation in Fas-deficient rheumatoid factor immunoglobulin transgenic mice. The Journal of experimental medicine. 1999;190:639–649. doi: 10.1084/jem.190.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 24.Santulli-Marotto S, Qian Y, Ferguson S, Clarke SH. Anti-Sm B cell differentiation in Ig transgenic MRL/Mp-lpr/lpr mice: altered differentiation and an accelerated response. Journal of immunology. 2001;166:5292–5299. doi: 10.4049/jimmunol.166.8.5292. [DOI] [PubMed] [Google Scholar]
- 25.Shlomchik MJ, Zharhary D, Saunders T, Camper SA, Weigert MG. A rheumatoid factor transgenic mouse model of autoantibody regulation. International immunology. 1993;5:1329–1341. doi: 10.1093/intimm/5.10.1329. [DOI] [PubMed] [Google Scholar]
- 26.Hannum LG, Ni D, Haberman AM, Weigert MG, Shlomchik MJ. A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. The Journal of experimental medicine. 1996;184:1269–1278. doi: 10.1084/jem.184.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martel C, Gondran G, Launay D, Lalloue F, Palat S, Lambert M, Ly K, Loustaud-Ratti V, Bezanahary H, Hachulla E, Jauberteau MO, Vidal E, Hatron PY, Fauchais AL. Active immunological profile is associated with systemic Sjogren's syndrome. Journal of clinical immunology. 2011;31:840–847. doi: 10.1007/s10875-011-9553-3. [DOI] [PubMed] [Google Scholar]
- 28.Ippolito A, Wallace DJ, Gladman D, Fortin PR, Urowitz M, Werth V, Costner M, Gordon C, Alarcon GS, Ramsey-Goldman R, Maddison P, Clarke A, Bernatsky S, Manzi S, Bae SC, Merrill JT, Ginzler E, Hanly JG, Nived O, Sturfelt G, Sanchez-Guerrero J, Bruce I, Aranow C, Isenberg D, Zoma A, Magder LS, Buyon J, Kalunian K, Dooley MA, Steinsson K, van Vollenhoven RF, Stoll T, Weisman M, Petri M. Autoantibodies in systemic lupus erythematosus: comparison of historical and current assessment of seropositivity. Lupus. 2011;20:250–255. doi: 10.1177/0961203310385738. [DOI] [PubMed] [Google Scholar]
- 29.Sweet RA, Ols ML, Cullen JL, Milam AV, Yagita H, Shlomchik MJ. Facultative role for T cells in extrafollicular Toll-like receptor-dependent autoreactive B-cell responses in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7932–7937. doi: 10.1073/pnas.1018571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shlomchik MJ, Euler CW, Christensen SC, William J. Activation of rheumatoid factor (RF) B cells and somatic hypermutation outside of germinal centers in autoimmune-prone MRL/lpr mice. Annals of the New York Academy of Sciences. 2003;987:38–50. doi: 10.1111/j.1749-6632.2003.tb06031.x. [DOI] [PubMed] [Google Scholar]
- 31.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giltiay NV, Lu Y, Allman D, Jorgensen TN, Li X. The adaptor molecule Act1 regulates BAFF responsiveness and self-reactive B cell selection during transitional B cell maturation. Journal of immunology. 2010;185:99–109. doi: 10.4049/jimmunol.0903312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian Y, Giltiay N, Xiao J, Wang Y, Tian J, Han S, Scott M, Carter R, Jorgensen TN, Li X. Deficiency of Act1, a critical modulator of B cell function, leads to development of Sjogren's syndrome. European journal of immunology. 2008;38:2219–2228. doi: 10.1002/eji.200738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, Fairchild RL, Omori SA, Rickert RC, Scott M, Kotzin BL, Li X. Act1, a negative regulator in CD40- and BAFF-mediated B cell survival. Immunity. 2004;21:575–587. doi: 10.1016/j.immuni.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Sweet RA, Christensen SR, Harris ML, Shupe J, Sutherland JL, Shlomchik MJ. A new site-directed transgenic rheumatoid factor mouse model demonstrates extrafollicular class switch and plasmablast formation. Autoimmunity. 2010;43:607–618. doi: 10.3109/08916930903567500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson AC, Davison LM, Giltiay NV, Vareechon C, Li X, Jorgensen TN. Lack of T cells in Act1-deficient mice results in elevated IgM-specific autoantibodies but reduced lupus-like disease. European journal of immunology. 2012;42:1695–1705. doi: 10.1002/eji.201142238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herlands AR, William J, Hershberg U, Shlomchik MJ. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. European journal of immunology. 2007;37:3339–3351. doi: 10.1002/eji.200737752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. The Journal of experimental medicine. 1995;182:1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X. Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling. Cytokine. 2008;41:105–113. doi: 10.1016/j.cyto.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, Li X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Science signaling. 2009;2 doi: 10.1126/scisignal.2000382. ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nature immunology. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 42.Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Annals of the New York Academy of Sciences. 2011;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 44.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 45.DeFranco AL, Chan VW, Lowell CA. Positive and negative roles of the tyrosine kinase Lyn in B cell function. Seminars in immunology. 1998;10:299–307. doi: 10.1006/smim.1998.0122. [DOI] [PubMed] [Google Scholar]
- 46.Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T. A double-edged kinase Lyn: a positive and negative regulator for antigen receptor-mediated signals. The Journal of experimental medicine. 1998;187:1343–1348. doi: 10.1084/jem.187.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith KG, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B cell by CD22: a requirement for Lyn. The Journal of experimental medicine. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. The Journal of experimental medicine. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 50.William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. Journal of immunology. 2005;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- 51.William J, Euler C, Primarolo N, Shlomchik MJ. B cell tolerance checkpoints that restrict pathways of antigen-driven differentiation. Journal of immunology. 2006;176:2142–2151. doi: 10.4049/jimmunol.176.4.2142. [DOI] [PubMed] [Google Scholar]
- 52.Klaus SJ, Berberich I, Shu G, Clark EA. CD40 and its ligand in the regulation of humoral immunity. Seminars in immunology. 1994;6:279–286. doi: 10.1006/smim.1994.1036. [DOI] [PubMed] [Google Scholar]
- 53.Mao C, Jiang L, Melo-Jorge M, Puthenveetil M, Zhang X, Carroll MC, Imanishi-Kari T. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 54.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual review of immunology. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 55.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C, Wu L, Bulek K, Martin BN, Zepp JA, Kang Z, Liu C, Herjan T, Misra S, Carman JA, Gao J, Dongre A, Han S, Bunting KD, Ko JS, Xiao H, Kuchroo VK, Ouyang W, Li X. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nature immunology. 2013;14:72–81. doi: 10.1038/ni.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pisitkun P, Claudio E, Ren N, Wang H, Siebenlist U. The adaptor protein CIKS/ACT1 is necessary for collagen-induced arthritis, and it contributes to the production of collagen-specific antibody. Arthritis and rheumatism. 2010;62:3334–3344. doi: 10.1002/art.27653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 59.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of experimental medicine. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pisitkun P, Ha HL, Wang H, Claudio E, Tivy CC, Zhou H, Mayadas TN, Illei GG, Siebenlist U. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity. 2012;37:1104–1115. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.