Abstract
MicroRNAs have emerged as important post-translational regulators of gene expression and are involved in several physiological and pathological states including the pathogenesis of human colon cancers. In regards to tumor development, microRNAs can act as oncogenes or tumor suppressors. Both hereditary predispositions (i.e. Lynch Syndrome and Familial Adenomatous Polyposis) contribute to the development of colon cancer. In addition, individuals who suffer from inflammatory bowel diseases such as Crohn’s disease or ulcerative colitis have a higher risk of developing colon cancer. Here, we discuss the occurrence of the deregulated expression of miRNAs in colon cancer that arise due to hereditary predisposition and inflammatory bowel disease.
Keywords: MicroRNA, HNPCC, FAP, Crohn’s Disease, Ulcerative Colitis
Introduction
Worldwide, colon cancer is the third most prevalent cancer in women, and second most common in men[1]. One million new cases, and 600,000 deaths worldwide each year occur due to colon cancer[2, 3]. Colon cancer is predominantly a disease of industrialized countries that practice a western diet and lifestyle; epidemiological studies indicate many risk factors including obesity, excessive alcohol consumption, excessive red meat as well as processed meat consumption[4], a diet high in fat, smoking, low socioeconomic status[5], and physical inactivity[6].
Almost two-thirds of colon cancer cases can be prevented by endoscopic screening or fecal occult-blood testing (FOBT); however, only 39% of patients are diagnosed at early stages when the disease is localized, and treatment is most effective[7]. The American Cancer Society recommends a colonoscopy every 5 years. Colonoscopies are invasive and expensive[8]; patients in a study by Bandi et al. found that only 4.4% of uninsured participants underwent endoscopy (including colonoscopy, sigmoidoscopy, or protoscopy)[9]. However, the percentage of persons having an endoscopic screening has increased almost 6% from 2008 to the present[10]. Fecal occult blood tests are less invasive because they do not require bowel preparation or sedation, but they can have low sensitivity (still in recommended range) ranging from 47%-73% in case control studies [8, 11]. In any case, epidemiologic studies in the United States indicate that only 45% of adults aged 50-65 are up to date with recommended colon cancer screening using either method[12].
The 5-year survival rate of patients with localized, early stage cancer is 90% compared to only 12% if the tumor has metastasized to distant organs [7, 13]. Prognosis is directly related to the stage-at-diagnosis. Detection of colon cancer at an early stage is difficult in the absence of screening because symptoms are often not apparent[8]. Therefore, there is a vital need for sensitive and non-invasive biomarkers for diagnosis.
Colon cancer etiology can be either hereditary or, more commonly, sporadic. In both cases, gene mutations lead to transformation of normal intestinal mucosal epithelia. New evidence also suggests an important role for microRNAs, which regulate gene transcription, in the pathogenesis of colon cancer. Current evidence supporting microRNA involvement in both hereditary- and inflammation-mediated colon cancers, a common risk factor for sporadic colon cancer, will therefore be the subject of this review, including consideration of the possible use of microRNAs as biomarkers for earlier, more accurate detection, and prevention of colon cancer.
MicroRNAs are single stranded RNA molecules that range from 20-25 nucleotides. MicroRNAs are derived as short non-coding RNAs with a hairpin shape, and regulate coding genes by base pairing with the mRNA transcripts typically at the 3′-untranslated region (3′-UTR) but also target binding sites for microRNAs have been find in the 5′ -UTR and in coding sequences [14] (Liu, C. Nucleic Acid Research 2013, 1-13). MicroRNAs are transcribed normally by RNA polymerase II (pol II), although some may be transcribed by polymerase III[15]. After transcription, primary-microRNAs (pri-microRNAs) are capped and polyadenylated for added stability. A microprocessor complex then crops the pri-microRNA into pre-microRNA, which has a length of 60-100 nucelotides. The modified hairpin structure is then exported from the nucleus to the cytoplasm by exportin-5. While in the cytoplasm, DICER protein cleaves pre-microRNA into a single stranded, ~22 nucleotide, mature microRNA[14]. Through a post-transcriptional binding mechanism, messenger RNA transcripts targeted by microRNAs are either degraded if the base-pair matching is identical, or silenced if there is a partial base-pair match. Repressed mRNAs are destabilized, and moved to cytosolic processing bodies (P-bodies) until they are eventually degraded[15]. Proper binding of microRNAs to their mRNA targets is essential for effective silencing. However, mutations in the microRNA processing machinery, target genes, or polymorphisms that occur in the microRNA or target gene sites can hinder successful binding, and can lead to unchecked or abnormal microRNA production. It is now understood that each microRNA can target hundreds of mRNA, and a particular mRNA transcript is usually the target of several different microRNAs (Ciafre A.S and Galardi, S. 2013, RNA Biol 10 (6) .
1.0 MicroRNAs and Colon Cancer
Close to 8000 journal articles have been published on the relationship between cancer and microRNAs, about 350 of those pertain to colon cancer specifically. There are two main categories of microRNAs that are involved in cancer progression: those that enhance cell growth, survival, and proliferation (onco-microRNAs [oncomiRs]), and those that suppress these activities (tumor-suppressor [TS]-microRNAs).
The majority of microRNAs involved in tumor promotion are TS-microRNAs. Tumor suppressor microRNAs are downregulated in cancer cells, and would normally inhibit the translation of protooncogenes[16]. Suppression of certain TS-microRNAs can occur through epigenetic hypermethylation of their promoter region. Certain microRNAs are also epigenetically controlled based on cell type. For example, miR-124a-1, 124a-3 and miR-18b were found to be more often hypermethylated in colon cancer compared to endometrial cancer[17]. One early piece of evidence defining microRNA involvement in colon cancer progression came from Akao et al. who transfected SW480 colon cancer cells with increasing concentrations of miR-143 and miR-145 transcripts resulting in decreased cell viability. This study also identified miR-143 and miR-145 as downregulated in tumors of the colon; therefore these two microRNAs are considered to be tumor suppressors[18].
Upregulated microRNAs in cancer cells are termed oncomiRs. miR-155 was one of the first oncomiRs discovered. miR-155 over-expression was first identified in B-cell lymphoma, and has now also been implicated in breast[19], colon[20] and lung cancers[21].
Numerous microRNAs have been implicated in colon cancer (Table 1, column 2); the relative expression in colon cancer (vs. normal) cells or tissues reflects their functional characteristics with oncomiRs having increased expression and TS-microRNAs being decreased (Table 1). In several studies, transfection of TS-microRNAs in colon cancer cells reduced cell proliferation[22, 23], oncogene activation[18, 22-25], or invasion[26]. For example, cells transfected with hsa-let7a-1 and hsa-miR-126 decreased cell proliferation and viability. Let-7a-1 inhibited C-MYC and RAS activation, and miR-126 inhibited AKT[22, 23]. Conversely, cells or tissues said to have high levels of a particular oncomiR, were drug resistant[27], invasive[28-30]or lead to poor prognosis[29]. Colon cancer patients in one study witnessed a decrease in plasma levels of certain oncomiRs (miR-17-3p and miR-92) [31]. These data support the link between microRNAs and colon cancer progression.
Table 1.
MicroRNAs and expression levels in colon cancera
| MicroRNA | Expression in CC | Colitis/Inherited | Reference |
|---|---|---|---|
| hsa-miR-15 | Up | UC | [57] |
| hsa-miR-16 | Up | CD | [58] |
| hsa-miR-16-2 | Up | HNPCC | [39] |
| hsa-miR-18b | Hypermethylated | NS | [17] |
| hsa-let-7f | Up | UC | [57] |
| hsa-miR-21 | Up | CD, UC | [57] |
| hsa-miR-23a | Up | UC | [57] |
| hsa-miR-23b | Up | CD | [58] |
| hsa-miR-24 | Up | UC | [57] |
| hsa-miR-26b | Down | NS | [65] |
| hsa-miR-29a | Up | IBS | [57] |
| hsa-miR-30a | Up | HNPCC | [39] |
| hsa-miR-31 | Up | IBD | [62] |
| hsa-miR-106 | Up | CD | [58] |
| hsa-miR-124a-1 | Hypermethylated | NS | [17] |
| hsa-miR-124a-3 | Hypermethylated | NS | [17] |
| hsa-miR-126 | Up | Active UC | [57] |
| hsa-miR-143 | Down | NS | [18, 46, 47] |
| hsa-miR-145 | Down | UC | [18, 46, 47] |
| hsa-miR-155 | Up | CD, UC | [20, 55, 60] |
| hsa-miR-191 | Up | CD | [58] |
| hsa-miR-192 | Down | UC | [57] |
| hsa-miR-195 | Up | UC | [57] |
| hsa-miR-223 | Up | CD | [58] |
| hsa-miR-275 | Up | Active UC | [58] |
| hsa-miR-362-5p | Up | HNPCC | [39] |
| hsa-miR-375 | Down | UC | [57] |
| hsa-miR-422 | Down | UC | [57] |
| hsa-miR-542-3p | Down | NS | [67] |
| hsa-miR-594 | Up | CD | [58] |
| hsa-mR-622 | Down | HNPCC | [39] |
| hsa-miR-1238 | Down | HNPCC | [39] |
| hsa-let-7f | Up | UC | [57] |
Partial list of microRNAs and expression levels in either colitis-associated or hereditary colon cancer. Abbreviations: CD, Crohn’s Disease; HNPCC, hereditary nonpolyposis colon cancer; IBS, Irritable Bowel Syndrome; NS, Not Specified; UC, Ulcerative Colitis.
1.1 microRNAs and HNPCC
Although APC mutations are widely known to increase colon cancer risk, the majority of inherited cases are due to mutations in DNA mismatch repair (MMR) genes[36]. DNA mismatch repair promotes genomic stability by correcting base-base and small insertion/deletion mispairs that arise during DNA replication and recombination. Pathogenic mutations in MMR genes lead to an impairment in the mismatch repair response which in turn leads to genomic instability and contributes to cancer formation. [36]. Lynch syndrome is the most common colon cancer predisposition that is distinguished by mutations in human mutS homolog 2 (hMSH2), human mutL homolog 1 (hMLH1), hMSH6, or human postmeiotic segregation increased 2 (hPMS2). The most common mutations are hMSH2 and hMLH1[37]. DNA replication errors arising from MMR mutations can result in microsatellite instability in repetitive sequences of DNA, such as those found in certain tumor suppressor genes (eg. TGFβRII, IGF2R, and BAX), causing a loss of function therefore naming this the “mutator pathway”, which leads to tumorigenesis[38].
There is evidence that patients with Lynch syndrome also have a unique microRNA profile when compared to normal individuals (Table 1, column 3). For example, miR-30a, miR-16-2, and miR-362-5p were upregulated significantly, and miR-1238 and miR-622 were repressed in one study with Lynch syndrome patients[39]. Microarray analysis of microRNAs was used as a powerful tool to discriminate normal and tumor tissues, as well as within tumor type. In this same study, the researchers were able to match microRNA profiles between suspected and proven Lynch syndrome cases. Balaguer and colleagues were able to distinguish the type of microsatellite instability (MSI) involved in malignancy based on microarray data of microRNA status[39]. Mismatch repair proteins hMSH2, hMLH1, hMSH6, can be targeted and repressed by microRNAs; miR-155 significantly decreased the mRNA expression of hMSH2, hMLH1, and hMSH6,. These data support the evidence that microRNAs modulate protein expression post-translationally, and can specifically target MMR proteins involved in colon cancer progression[40]. MicroRNAs could, therefore; contribute to triggering the molecular and cellular changes that occur in Lynch syndrome that lead specifically to colon cancer.
1.2 microRNAs and FAP
The second most common inherited colon cancer predisposition after Lynch syndrome is FAP. This disease occurs with a germline mutation in the APC gene, and confers 100% colon cancer lifetime risk[41, 42]. This germline mutation, that occurs in the APC gene located between 5q21 and 5q22, causes a truncated APC protein to be formed[43]. A dysfunctional APC molecule allows for the constitutive activation of β-catenin-mediated transcription of C-MYC and cyclin D2[36], and therefore contributes to uncontrolled proliferation. Loss of the APC gene can be bi-allelic or a single allele mutation that can lead to additional alterations in KRAS or TP53 genes as well[44]. Abnormal chromosomal segregation can lead to loss of heterozygocity usually in tumor suppressor genes APC or TP53 [2, 38]. Mutated KRAS has been shown to be a mediator in the early stages of colon carcinogenesis that involve unchecked cell proliferation and growth[45]. Chen et al. found that miR-143 targets KRAS in colon cancer, acting as a tumor suppressor. Cancer cell KRAS levels were inversely correlated to miR-143 expression, additionally, expression of miR-143 was decreased dramatically in all colon cancer samples tested[46, 47].
Staphylococcal nuclease tudor domain containing 1 (SND1) is a novel component of the RNA-induced silencing complex (RISC) involved in RNA-interference (RNAi) mechanisms mediated by siRNAs and microRNAs, and has been shown to be over expressed in human colon cancers and also in models of colon cancer [48]. Over expression of SND1 may induce activation of the Wnt-B-catenin signaling pathway through down regulation of the APC protein. [48]. However more studies are required to further understand how SND1 contributes to colon cancer.
2.0 MicroRNA and Inflammatory Bowel Diseases (IBD) Associated with Colon Cancer
As surmised from the previous discussion of associations between microRNAs and genetic colon cancer risk, chronic intestinal inflammation has long been associated with increased colon cancer risk, and our current understanding of microRNAs contributes to a stronger link between inflammation and cancer. More specifically, colitis-associated colon cancer is associated with ulcerative colitis (UC) and Crohn’s disease, two inflammatory bowel disorders (IBD) of uncertain etiology that are associated with 20-fold increased risk of colon cancer with high (50%) mortality [2]. Both disorders have a strong genetic predisposition, including associations with defects in genes related to innate immune system receptors that interact with microbial products at the epithelial interface of the gut[49, 50]. Additional interactions between the gut microbiota and gastric epithelium are also thought be to causative, including disregulation of microbiota secondary to antibiotics or viral illnesses [51]. A lack of concordance in identical twin studies for both diseases and geographic patterns of occurrence, which trump ethnicity, are also suggestive of causative environmental factors, including possible effects of western diets[50]. UC and Crohn’s disease differ pathologically in that UC results in mucosal lesions that are limited to the colon, while Crohn’s disease involves transmural lesions that can occur at any level of the digestive tract[51]. Crohn’s disease and ulcerative colitis each have distinct but overlapping microRNA profiles. An additive level of complexity exists in that there is also a difference in microRNA expression of inflamed versus quiescent UC and Crohn’s disease[52].
2.1 microRNAs and oncogenic mechanisms in chronic inflammation
Compromised tight junctions of intestinal cells are characteristic of both UC and Crohn’s disease. Ye et al. found that the inflammatory cytokine, TNF-α, triggers miR-122a expression, which targets occludin, a protein involved in enterocyte permeability that is decreased in UC, Crohn’s, and other irritable bowel disorders[53]. Furthermore, with powerful microarray analysis, several microRNAs were found to be differently expressed in patients with ulcerative colitis including miR-21, whose expression increased over 3-fold compared to normal tissues. Evidence from a mouse model of dextran sodium sulfate induced colitis confirms that genetic abnormality in combination with colits is sufficient to cause colonic dysplasia and cancer[54]. Deregulated microRNAs that increase chronic inflammatory pathways may increase colon cancer risk in patients who have germline or somatic mutations in DNA mismatch repair genes. A clear microRNA-mediated link between inflammatory and hereditary risks associated with colon cancer can be seen for miR-155. The “mutator” pathway associated with HNPCC is promoted by miR-155. Mutation rates increase in SW620 colon cancer cells with increasing expression of miR-155[55]. In addition, endotoxin, a component of gram negative bacteria and the inflammatory cytokine TNF, triggered upregulation of miR-155 in SW620 colon cancer cells.
Wu et al. found that miR-192, miR-375, and miR-422 were downregulated, yet miR-15, miR-21, miR-23a, miR-24, miR-29a, miR-126, miR-195, and let-7f were upregulated in UC[57]. Differences in expression of microRNAs during active vs inactive UC could provide a better understanding of which microRNAs are acute-acting, chronic-acting, oncomiRs, or tumor-suppressive. For example, miR-275 and miR-422b expression was increased in inactive UC tissue samples, while miR-192 expression did not change in inactive UC, but was decreased in active UC patients compared to healthy controls. Not only are there differences in inflammation state, but also different microRNAs are expressed in different locations within the colon, at least in patients with Crohn’s. In the sigmoid colon of active Crohn’s patients, miR-23b, miR-106, and miR-191 were all increased. However, in the terminal ileum, miR-16, miR-21, miR-223, and miR-594 were increased[58].
MicroRNAs miR-375 and miR-23a were also highly expressed in infectious colitis (IC) and irritable bowl syndrome (IBS)[58]. Again, miR-21, and miR-126 were increased in active UC, but not in normal, inactive, or IBS sigmoid colonic tissues[59]. Interestingly, high levels of miR-126 were inversely correlated with mRNA levels for IκBα, an inhibitor of NF-κB [59], a key transcription factor responsible for inflammatory cytokine production whose disregulation is a hallmark of IBD. Similarly, HT29 colon cancer cells transfected with miR-126 had decreased IκBα protein expression compared to normal cells[59]. Oncogenic microRNAs, miR-21 and miR-155, which can both target mismatch repair genes, as well as the let-7 family are highly over-expressed in active UC patients compared to healthy controls[60]. This evidence may provide a better understanding of colon cancer incidence as a direct result of UC or IBD. miR-145 also is downregulated in UC tissue samples[61]. In a unique study by Olaru, micoRNA-31 expression increased when analyzed between normal, IBD, to neoplasia, which implicates microRNAs in the progression of inflammatory diseases into cancer[62].
3.0 Altered COX-2 Regulation Mediated by MicroRNAs in Hereditary and Colitis-associated Colon Cancer
Over-expression of cyclooxygenase-2 (COX-2) could be a direct result of aberrant expression of microRNAs, which could be an important factor in connecting inflammation to cancer incidence. Several mechanisms other than the APC mutation discussed previously can cause uncontrolled activation of β-catenin; these include EP2 receptor activation and prostaglandin E2 ligand interaction[2]. Only a few studies have reported on the effects of microRNAs on COX-2 and downstream signaling. Strillacci and others have shown that miR-101 and miR-101a are inversely correlated with COX-2 expression in SW620, Caco2, HT29, and LoVo colon cancer cell lines[63]. This inverse relationship remains true for other cancer types as well; miR-101a was found to suppress COX-2 in HC11 mammary epithelial cells[64]. With the use of TargetScan, an in silico microRNA prediction tool, researchers were able to reveal that miR-26b has a unique binding site within the COX-2 3′UTR, and cells transfected with miR-26b after nine days formed fewer colonies compared to cells transfected with non-coding vector[65]. When the APC gene was expressed in HT29 cells, COX-2 mRNA expression decreased, but protein expression remained the same[66]. Proposed tumor suppressor, miR-101, responds to NSAIDs in a COX-2-dependent manner. In HCA-7 colon cancer cells treated with toxins that trigger inflammation, such as LPS, synthetic PGE2, or PMA, miR-101 mRNA expression was decreased, but then recovered with the treatment of NSAIDs. This microRNA could inhibit the genetic translation of COX-2 mRNA, and is normally expressed in low-grade, well-differentiated colon cancer tissues, but is not expressed in high-grade, poorly differentiated tissues[63].
In addition to COX-2, the EP4 prostanoid receptor has been found to be a direct target of miR-101. Furthermore, there is an inverse relationship between miR-101 level and EP4 receptor expression[67]. Thus miR-101 appears to be able to control key nodal points along the prostaglandin signaling pathway that can contribute to malignant progression.
4.0 MicroRNAs and Single Nucleotide Polymorphisms (SNPs)
Genetic variants can also occur in microRNA coding sequences, which influences biogenesis. SNPs in rs7372209 and rs1834306, variants in pri-miR-26a-1, and pri-miR-100 genes, respectively, correlated with longer time to disease progression, which can be translated to a better quality of life[68]. Alterations in microRNAs that affect its binding motif for target mRNAs may increase or decrease affinity. Landi et al. were the first to discover SNPs of CD86 and INSR genes that conferred increase colon cancer risk. The microRNAs that targeted CD86 were miR-337, miR-582, miR-200a*, miR-184 and miR-212. With a single point mutation, miR-337, miR-582 and miR-200a* bound weakly to the CD86 3′ UTR. However, miR-184 and miR-212 increased their binding strength[69].
A frame-shift mutation in the exportin 5 gene, EXPO5, that helps to export microRNAs from the nucleus to the cytoplasm, was found in HCT-15 and DLD-1 colon cancer cells. This type of mutation is not uncommon in tumors that exhibit microsatellite instability, or HNCPP tumors. About 26% of tissue samples with colon cancer deriving from germline mutations (HNPCC) had mutated EXPO5. Sporadic colon cancer cell lines and tissue samples with microsatellite instability expressed a higher mutation rate of EXPO5 compared to microsatellite stable cells and tissues[70]. Another example of a SNP was found in the IL-23 receptor, which is suspected as a risk factor for UC and Crohn’s disease. This variant, rs10889677, lead to both an increase in mRNA as well as protein, which suggests a loss in microRNA regulation at the 3′ UTR site. Let-7e and Let-7f were found to decrease mRNA and protein expression of IL-23R[71]. Differential expression of cyclooxygenases-2 (COX-2) may influence the susceptibility of patients to overt colon cancer. Results from a study by Moore, et al. show that the T8473 SNP in the COX-2 gene was found to produce COX-2 mRNA that could escape miR-542-3p inhibition. Colon cancer cells transfected with miR-542-3p rapidly degraded COX-2 mRNA and downregulated protein expression as well as inhibited downstream effects of COX-2 such as PGE2 production. Moreover, colorectal patient samples homozygous for the T8473 variant had significantly high COX-2 mRNA expression, and heterozygous and homozygous tissue samples had high COX-2 protein expression[72]. These data support several phenomena: microRNAs can directly influence the expression and functionality of inflammatory molecules involved in colon cancer progression, and common polymorphic variation in inflammatory molecules such as COX-2 at a particular binding site for microRNAs can allow escape from genetic silencing. It is not uncommon to discuss SNPs in the context of population variability. In a Taiwanese population, an APC SNP, g.4479G>A, was highly expressed in homozygous carriers with colon cancer. This polymorphism identified increased colon cancer risk[73]. Similarly, a SNP mutation in the IC53 gene, whose upregulation has been documented in SW480 colon cancer cells and is associated with colon cancer progression, has been demonstrated to create a target for miR-379. Homozygous carriers of this mutation (rs2737 allele) appear to be protected from colon cancer, which has a much later age of onset in these individuals.
MicroRNA-146a is a TS-microRNA that indirectly suppresses NF-κB. Colon cancer risk associated with a particular SNP in miR-146a has been studied in a Chinese population, as well as in other populations for bladder, breast, and gastric cancer. Individuals homozygous and heterozygous for the miR-146a rs 2910164 variant had reduced colon cancer risk[74].
A marked decrease in colitis-associated invasive colon cancer occurs in mice with a genotype named Hiccs, which is a 1.7-megabase area that has eight known genes and five microRNAs (that also carry SNPs). With this Hiccs interval protection from H. hepaticus infection, innate immune responses of certain proinflammatory cytokines such as TNF, IL-1β, and INF-γ, were dampened[75]. This finding further confirms that some microRNAs are genetically destined to control inflammatory mediators.
5.0 Clinical Utility of Detecting MicroRNA Changes
Since colonoscopies are expensive and invasive procedures, and FOBT can have low specificity, novel biomarkers may be useful for early diagnosis of colon cancer. When it comes to treatment over the past decade, more financially well off, educated, Caucasian, and insured individuals have turned to expensive colonoscopy screening. Elderly individuals with a fixed income, poor, publically insured or uninsured persons continue to use FOBT[9]. There is, therefore, a socioeconomic divide between screening options that could be closed with the use of novel biomarkers screened for in blood or other body fluids. Plasma microRNAs are fairly stable[76], and possibly only several key microRNAs would need to be identified and analyzed during a clinical examination compared to a large cohort of mRNAs known to be involved in cancer promotion.
In several studies, an increase in survival was observed in colon cancer patients with high let-7a[77] and miR-143[47] expression detected in serum; furthermore, progression free survival was positively associated with both high let-7a, and miR-143 expression, which denotes not only a longer life, but also a better quality of life. Deciding on proper treatment is a critical factor that helps formulate patient survival rates. Patients who would eventually discover that they are unresponsive to EGFR therapy might benefit from early prognostic tests that reveal microRNA status. For example, in bladder cancer cells, miR-200 family microRNAs were able to reverse insensitivity to EGFR therapy and EMT development[78]. Use of microRNAs as biomarkers to distinguish IBD from colon cancer has also been performed. miR-92 was used as a plasma biomarker to establish differences in IBD and colorectal cancer. Since it is not upregulated in IBD or gastric cancer, but is in colorectal cancer, miR-92 is specific for malignant transformation in the GI tract as well as its regional location. [31].
Conclusion
In this review we have described how microRNA expression influences pathogenesis of both hereditary colon cancer and inflammatory disease-associated colon cancers. Better classification, clinical diagnosis, prognosis, and tailored therapy can be accomplished by understanding the molecular etiology as well as the biological and pathological nature of colon cancer. Aberrant microRNA expression can be influenced by polymorphisms in genes that code for microRNA machinery proteins or proteins that are known to be linked to carcinogenesis, and are targets of microRNAs.
We have found that, in particular, microRNAs involved in the onset of familial adenomatous polyposis and progression to colon cancer have yet to be discovered. This particular knowledge gap needs to be addressed to provide a more complete picture of microRNA role in the two most common hereditary predisposition syndromes that put patients at a higher risk for colon cancer. While changes in microRNA expression have yet been associated with FAP, it remains possible that altered APC expression in FAP could be further exacerbated by abnormal formation of microRNA machinery which could lead to the production of oncomiRs, tumor suppressive microRNAs that deregulate APC’s downstream targets. Therefore, possibly with a microarray analysis, these interactions could reveal those microRNAs that contribute to FAP patients’ high risk for colon cancer. Albeit it was quite informative, only one study far has shown that specific microRNAs are deregulated in HNPCC as well. Deregulation of gene expression occurs in each cause of colon cancer, but a genetic screening and microRNA profile generation may become an important clinical tool for diagnosing, predicting prognosis, and providing more personalized treatment options.
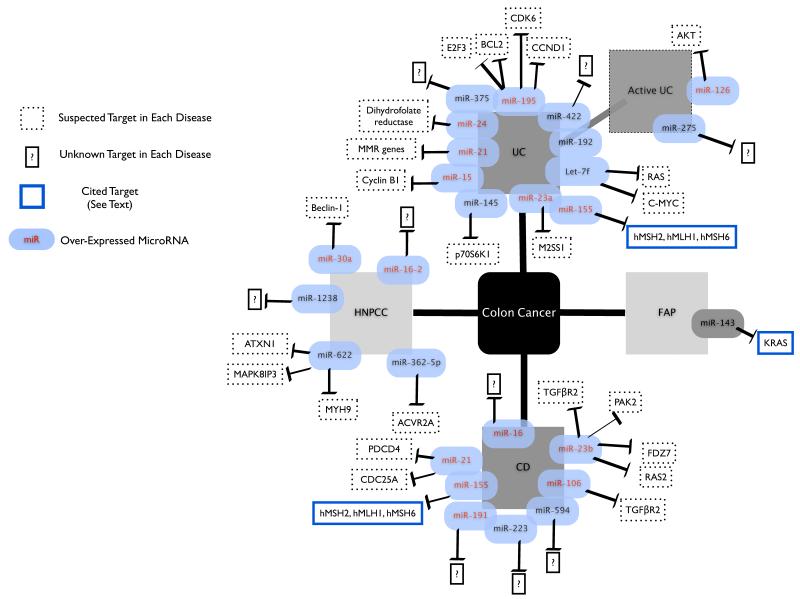
Figure1.
Several MicroRNAs have been implicated in ulcerative colitis (UC), Crohn’s disease (CD), familial adenopolyposis (FAP), and hereditary non-polyposis colon cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heavey PM, McKenna D, Rowland IR. Colorectal cancer and the relationship between genes and the environment. Nutrition and Cancer-an International Journal. 2004;48(2):124–141. doi: 10.1207/s15327914nc4802_2. [DOI] [PubMed] [Google Scholar]
- 2.Terzic J, et al. Inflammation and Colon Cancer. Gastroenterology. 2010;138(6):2101–U119. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 3.Schetter AJ, Harris CC. Alterations of MicroRNAs Contribute to Colon Carcinogenesis. Seminars in Oncology. 2011;38(6):734–742. doi: 10.1053/j.seminoncol.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan DSM, et al. Red and Processed Meat and Colorectal Cancer Incidence: Meta-Analysis of Prospective Studies. Plos One. 2011;6(6) doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doubeni CA, et al. Socioeconomic status and the risk of colorectal cancer An Analysis of More Than a Half Million Adults in the National Institutes of Health-AARP Diet and Health Study. Cancer. 2012;118(14):3636–3644. doi: 10.1002/cncr.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonja H, Damjan G. MicroRNAs as novel biomarkers in colorectal cancer. Frontiers in Genetics. 2012;3(180):1–9. doi: 10.3389/fgene.2012.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Society AC. In: Colorectal Cancer Facts and Figures 2011-2013. Rick Alteri M, et al., editors. American Cancer Society; Atlanta GA: 2011. [Google Scholar]
- 8.Kanaan Z, et al. Plasma MiR-21 A Potential Diagnostic Marker of Colorectal Cancer. Annals of Surgery. 2012;256(3):544–551. doi: 10.1097/SLA.0b013e318265bd6f. [DOI] [PubMed] [Google Scholar]
- 9.Bandi P, et al. Trends in Colorectal Cancer Screening With Home-Based Fecal Occult Blood Tests in Adults Ages 50 to 64 Years, 2000-2008. Cancer. 2012;118(20):5092–5099. doi: 10.1002/cncr.27529. [DOI] [PubMed] [Google Scholar]
- 10.Smith RA, Cokkinides V, Brawley OW. Cancer Screening in the United States, 2012 A Review of Current American Cancer Society Guidelines and Current Issues in Cancer Screening. Ca-a Cancer Journal for Clinicians. 2012;62(2):129–142. doi: 10.3322/caac.20143. [DOI] [PubMed] [Google Scholar]
- 11.Burch JA, et al. Diagnostic accuracy of faecal occult blood tests used in screening for colorectal cancer: a systematic review. Journal of Medical Screening. 2007;14(3):132–137. doi: 10.1258/096914107782066220. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, et al. Trends in Colorectal Cancer Test Use among Vulnerable Populations in the United States. Cancer Epidemiology Biomarkers & Prevention. 2011;20(8):1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Society AC. Cancer Facts and Figures 2012, 2012. American Cancer Society; Atlanta GA: [Google Scholar]
- 14.Kim VN. MicroRNA biogenesis: Coordinated cropping and dicing. Nature Reviews Molecular Cell Biology. 2005;6(5):376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 15.Cowland JB, Hother C, Gronwaek K. MicroRNAs and cancer. Apmis. 2007;115(10):1090–1106. doi: 10.1111/j.1600-0463.2007.apm_775.xml.x. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 17.Pavicic W, et al. Altered Methylation at MicroRNA-Associated CpG Islands in Hereditary and Sporadic Carcinomas: A Methylation-Specific Multiplex Ligation-Dependent Probe Amplification (MS-MLPA)-Based Approach. Molecular Medicine. 2011;17(7-8):726–735. doi: 10.2119/molmed.2010.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akao Y, Nakagawa Y, Naoe T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncology Reports. 2006;16(4):845–850. [PubMed] [Google Scholar]
- 19.Mattiske S, et al. The Oncogenic Role of miR-155 in Breast Cancer. Cancer Epidemiology Biomarkers & Prevention. 2012;21(8):1236–1243. doi: 10.1158/1055-9965.EPI-12-0173. [DOI] [PubMed] [Google Scholar]
- 20.Pu J, et al. Adrenaline promotes cell proliferation and increases chemoresistance in colon cancer HT29 cells through induction of MiR-155. Biochemical and Biophysical Research Communications. 2012;428(2):210–215. doi: 10.1016/j.bbrc.2012.09.126. [DOI] [PubMed] [Google Scholar]
- 21.Miaomiao Y, et al. High expression of miR-21 and miR-155 predicts recurrence and unfavourable survival in non-small cell lung cancer. European Journal of Cancer. 2012;49(3):604–615. doi: 10.1016/j.ejca.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 22.Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biological & Pharmaceutical Bulletin. 2006;29(5):903–906. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 23.Guo C, et al. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes & Cancer. 2008;47(11):939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito Y, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9(6):435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Nagel R, et al. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Research. 2008;68(14):5795–5802. doi: 10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- 26.Hu M, et al. MicroRNA-141 Regulates Smad Interacting Protein 1 (SIP1) and Inhibits Migration and Invasion of Colorectal Cancer Cells. Digestive Diseases and Sciences. 2010;55(8):2365–2372. doi: 10.1007/s10620-009-1008-9. [DOI] [PubMed] [Google Scholar]
- 27.Song B, et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28(46):4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asangani IA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 29.Schimanski CC, et al. High miR-196a levels promote the oncogenic phenotype of colorectal cancer cells. World Journal of Gastroenterology. 2009;15(17):2089–2096. doi: 10.3748/wjg.15.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burk U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. Embo Reports. 2008;9(6):582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng EKO, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58(10):1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 32.Lubbe SJ, et al. Clinical Implications of the Colorectal Cancer Risk Associated With MUTYH Mutation. Journal of Clinical Oncology. 2009;27(24):3975–3980. doi: 10.1200/JCO.2008.21.6853. [DOI] [PubMed] [Google Scholar]
- 33.Howe JR, et al. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. Journal of Medical Genetics. 2004;41(7):484–491. doi: 10.1136/jmg.2004.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehenni H, et al. Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity. American Journal of Human Genetics. 1998;63(6):1641–1650. doi: 10.1086/302159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan AOO, et al. Concordant CpG island methylation in hyperplastic polyposis. American Journal of Pathology. 2002;160(2):529–536. doi: 10.1016/S0002-9440(10)64872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nature Medicine. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 37.Jasperson KW, et al. Hereditary and Familial Colon Cancer. Gastroenterology. 2010;138(6):2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itzkowitz SH, Yio XY. Inflammation and cancer - IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2004;287(1):G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 39.Balaguer F, et al. Colorectal Cancers with Microsatellite Instability Display Unique miRNA Profiles. Clinical Cancer Research. 2011;17(19):6239–6249. doi: 10.1158/1078-0432.CCR-11-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valeri N, et al. Modulation of mismatch repair and genomic stability by miR-155. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(15):6982–6987. doi: 10.1073/pnas.1002472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishisho I, et al. MUTATIONS OF CHROMOSOME-5Q21 GENES IN FAP AND COLORECTAL-CANCER PATIENTS. Science. 1991;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura Y, et al. MUTATIONS OF THE APC (ADENOMATOUS-POLYPOSIS-COLI) GENE IN FAP (FAMILIAL-POLYPOSIS-COLI) PATIENTS AND IN SPORADIC COLORECTAL TUMORS. Tohoku Journal of Experimental Medicine. 1992;168(2):141–147. doi: 10.1620/tjem.168.141. [DOI] [PubMed] [Google Scholar]
- 43.Tighe A, Johnson VL, Taylor SS. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. Journal of Cell Science. 2004;117(26):6339–6353. doi: 10.1242/jcs.01556. [DOI] [PubMed] [Google Scholar]
- 44.Shi C, Washington K. Molecular Testing in Colorectal Cancer Diagnosis of Lynch Syndrome and Personalized Cancer Medicine. American Journal of Clinical Pathology. 2012;137(6):847–859. doi: 10.1309/AJCPI83DINULUJNI. [DOI] [PubMed] [Google Scholar]
- 45.Bos JL, et al. PREVALENCE OF RAS GENE-MUTATIONS IN HUMAN COLORECTAL CANCERS. Nature. 1987;327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28(10):1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- 47.Pichler M, et al. Down-regulation of KRAS-interacting miRNA-143 predicts poor prognosis but not response to EGFR-targeted agents in colorectal cancer. British Journal of Cancer. 2012;106(11):1826–1832. doi: 10.1038/bjc.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuchiya N, et al. SND1, a component of RNA-induced silencing complex, is up-regulated in human colon cancers and implicated in early stage colon carcinogenesis. Cancer Research. 2007;67(19):9568–9576. doi: 10.1158/0008-5472.CAN-06-2707. [DOI] [PubMed] [Google Scholar]
- 49.Dalal S, Kwon J. The Role of MicroRNA in Inflammatory Bowel Disease Gastroenterology & Hepatology. 2010;6(11):714–722. [PMC free article] [PubMed] [Google Scholar]
- 50.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nature Clinical Practice Gastroenterology & Hepatology. 2006;3(7):390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 51.Ordas I, et al. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 52.Fasseu M, et al. Identification of Restricted Subsets of Mature microRNA Abnormally Expressed in Inactive Colonic Mucosa of Patients with Inflammatory Bowel Disease. Plos One. 2010;5(10):12. doi: 10.1371/journal.pone.0013160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye D, et al. MicroRNA Regulation of Intestinal Epithelial Tight Junction Permeability. Gastroenterology. 2011;141(4):1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohonen-Corish MRJ, et al. Susceptibility of Msh2-deficient mice to inflammation-associated colorectal tumors. Cancer Research. 2002;62(7):2092–2097. [PubMed] [Google Scholar]
- 55.Tili E, et al. Mutator activity induced by microRNA-155 (miR-155) links inflammation and cancer. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):4908–4913. doi: 10.1073/pnas.1101795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Connell RM, et al. MicroRNA-155 is induced during the macrophage inflammatory response. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(5):1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu F, et al. Identification of MicroRNAs Associated with Ileal and Colonic Crohn’s Disease. Inflammatory Bowel Diseases. 2010;16(10):1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu F, et al. MicroRNAs Are Differentially Expressed in Ulcerative Colitis and Alter Expression of Macrophage Inflammatory Peptide-2 alpha. Gastroenterology. 2008;135(5):1624–1635. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 59.Feng X, et al. Up-Regulation of microRNA-126 May Contribute to Pathogenesis of Ulcerative Colitis via Regulating NF-kappaB Inhibitor IκBα. PLoS ONE. 2012;7(12):e52782. doi: 10.1371/journal.pone.0052782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takagi T, et al. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. Journal of Gastroenterology and Hepatology. 2010;25:S129–S133. doi: 10.1111/j.1440-1746.2009.06216.x. [DOI] [PubMed] [Google Scholar]
- 61.Pekow JR, et al. miR-143 and miR-145 are downregulated in ulcerative colitis: Putative regulators of inflammation and protooncogenes. Inflammatory Bowel Diseases. 2012;18(1):94–100. doi: 10.1002/ibd.21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olaru AV, et al. Dynamic Changes in the Expression of MicroRNA-31 During Inflammatory Bowel Disease-associated Neoplastic Transformation. Inflammatory Bowel Diseases. 2011;17(1):221–231. doi: 10.1002/ibd.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strillacci A, et al. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Experimental Cell Research. 2009;315(8):1439–1447. doi: 10.1016/j.yexcr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka T, et al. A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation. 2009;77(2):181–187. doi: 10.1016/j.diff.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Ji Y, et al. MiRNA-26b regulates the expression of cyclooxygenase-2 in desferrioxamine-treated CNE cells. Febs Letters. 2010;584(5):961–967. doi: 10.1016/j.febslet.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 66.Hsi LC, Angerman-Stewart J, Eling TE. Introduction of full-length APC modulates cyclooxygenase-2 expression in HT-29 human colorectal carcinoma cells at the translational level. Carcinogenesis. 1999;20(11):2045–2049. doi: 10.1093/carcin/20.11.2045. [DOI] [PubMed] [Google Scholar]
- 67.Chandramouli A, et al. MicroRNA-101 (miR-101) post-transcriptionally regulates the expression of EP4 receptor in colon cancers. Cancer Biology & Therapy. 2012;13(3):175–183. doi: 10.4161/cbt.13.3.18874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boni V, et al. Role of primary miRNA polymorphic variants in metastatic colon cancer patients treated with 5-fluorouracil and irinotecan. Pharmacogenomics Journal. 2011;11(6):429–436. doi: 10.1038/tpj.2010.58. [DOI] [PubMed] [Google Scholar]
- 69.Landi D, et al. Polymorphisms within micro-RNA-binding sites and risk of sporadic colorectal cancer. Carcinogenesis. 2008;29(3):579–584. doi: 10.1093/carcin/bgm304. [DOI] [PubMed] [Google Scholar]
- 70.Melo SA, et al. A Genetic Defect in Exportin-5 Traps Precursor MicroRNAs in the Nucleus of Cancer Cells. Cancer Cell. 2010;18(4):303–315. doi: 10.1016/j.ccr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Zwiers A, et al. Cutting Edge: A Variant of the IL-23R Gene Associated with Inflammatory Bowel Disease Induces Loss of MicroRNA Regulation and Enhanced Protein Production. Journal of Immunology. 2012;188(4):1573–1577. doi: 10.4049/jimmunol.1101494. [DOI] [PubMed] [Google Scholar]
- 72.Moore AE, Young LE, Dixon DA. A common single-nucleotide polymorphism in cyclooxygenase-2 disrupts microRNA-mediated regulation. Oncogene. 2012;31(12):1592–1598. doi: 10.1038/onc.2011.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen SP, et al. Single nucleotide polymorphisms of the APC gene and colorectal cancer risk: a case-control study in Taiwan. Bmc Cancer. 2006;6 doi: 10.1186/1471-2407-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma L, et al. A genetic variant in miR-146a modifies colorectal cancer susceptibility in a Chinese population Archives of Toxicology. 2013 doi: 10.1007/s00204-012-1004-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 75.Boulard O, et al. Identification of a genetic locus controlling bacteria-driven colitis and associated cancer through effects on innate inflammation. Journal of Experimental Medicine. 2012;209(7):1309–1324. doi: 10.1084/jem.20120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell PS, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruzzo A, et al. High Let-7a MicroRNA Levels in KRAS-Mutated Colorectal Carcinomas May Rescue Anti-EGFR Therapy Effects in Patients with Chemotherapy-Refractory Metastatic Disease. Oncologist. 2012;17(6):823–829. doi: 10.1634/theoncologist.2012-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adam L, et al. miR-200 Expression Regulates Epithelial-to-Mesenchymal Transition in Bladder Cancer Cells and Reverses Resistance to Epidermal Growth Factor Receptor Therapy. Clinical Cancer Research. 2009;15(16):5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng H, et al. Circulating Plasma MiR-141 Is a Novel Biomarker for Metastatic Colon Cancer and Predicts Poor Prognosis. Plos One. 2011;6(3) doi: 10.1371/journal.pone.0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]