Abstract
Background:
Blood transfusion is a life-saving measure in various medical and surgical emergencies. Transfusion medicine, apart from being important for the medical treatment of each patient, also has great public health importance.
Objectives:
The present study was conducted to estimate the prevalence of transfusion transmitted infections in voluntary blood donors at a rural tertiary care teaching hospital in western Maharashtra, India.
Materials and Methods:
All voluntary donors reporting to the blood bank were screened for HBsAg, Hepatitis C Virus (HCV), HIV and Syphilis by using the appropriate enzyme-linked immunosorbent assay. HIV infection was confirmed using a standard immunoblotting technique. Hepatitis B Virus (HBV) was tested for surface antigen (HBsAg) and HCV by the immunechromatographic method. The Venereal Disease Reference Laboratory (VDRL) test was used for estimation of syphilis infection. The study was designed for a duration of two years between January 2009 to December 2010. Medical reports of the donors were accessed from the hospital records and analyzed.
Results:
A total of 5661 voluntary blood donors were screened, of which 5394 (95.28%) were males and 267 (4.72%) were females. The overall seroprevalence of HBV and HCV were 1.09% and 0.74% respectively; for HIV and syphilis the seroprevalence was estimated to be 0.07% for each.
Conclusion:
Blood is still one of the main sources of transmission of infections. HIV, hepatitis B, hepatitis C viruses and syphilis are prevalent among voluntary donors in rural India.
Keywords: Seroprevalence, transfusion transmissible infections, voluntary blood donors
Introduction
India is the second most populous nation in the world. The Indian subcontinent is classified as an intermediate Hepatitis B Virus (HBV) endemic (HBsAg carriage 2-7%) zone and has the second largest global pool of chronic HBV infections.[1] India has a population of more than 1.2 billion with 5.7 (reduced to 2.5) million Human Immunodeficiency Virus (HIV) positive, 43 million HBV positive and 15 million HCV positive persons. The risk of transfusion transmission of these viruses may be alarming due to high seroprevalence of HIV, anti-HCV, and HBsAg (0.5%, 0.4%, and 1.4%, respectively) among blood donors.[2] Safety assessment of the blood supply, the quality of screening procedures and the risk of transfusion transmitted infectious diseases (TTIs) in any country can be estimated by review and analysis of the records of blood donors for screening procedures and the prevalence of serological markers of infectious diseases. Blood transfusion has been used since 1930 for various indications.[3] Transfusion therapy is a well-established treatment in various medical and surgical procedures.[4] Transfusion medicine, apart from being important for the medical treatment of each patient, also has a great public health importance worldwide.[5] After the introduction of the blood banks and better storage techniques, it became more widely used.[6] Blood is one of the major sources of transmission of hepatitis B, hepatitis C, HIV, syphilis, and many other diseases.[5,7] Discovery of these hazards brought a dramatic change in attitude of physicians and patients about transfusion of blood.[8] It is mandatory to test each donor's blood for syphilis by a Venereal Disease Reference Laboratory (VDRL), and for HBsAg, anti-HCV, and anti-HIV. In July 1989, consequent to the reports of high seroprevalence in commercial blood donors, mandatory screening of blood and blood products for HIV antibodies was initiated by Indian National AIDS Control Origination (NACO).[9] The objective of this study is to estimate the seroprevalence of transfusion transmitted infections among voluntary blood donors at a rural tertiary healthcare teaching hospital in western Maharashtra. This knowledge might give us the idea of disease burden of the society and the basic epidemiology of these diseases in the rural community.
Materials and Methods
A retrospective hospital record-based study was conducted at the blood bank of a rural tertiary care teaching hospital in Maharashtra, India. Data were collected for a period of 2 years from January 2009 to December 2010. Sera of civilian residents from various localities and of different age groups, who donated blood voluntarily was screened for HIV, HBsAg, HCV, and syphilis. A total of 5661 blood units were collected and studied. The ethics committee of the institute approved the study. No professional or honorary donor was bled. Exclusion criteria for blood donation were current history of medication, recent history of having undergone a surgical procedure, serious illness, previous blood transfusions, weight <50 kg, age <18 and >60 years, pregnant and lactating women.
Data collection
The outcome variable was serological status of the selected individual, whether positive or negative for any TTIs, which was determined from a blood sample.
Sample collection and laboratory testing
Five milliliter blood each was collected from subjects into plain, sterile bottle following informed consent. Blood samples were centrifuged and the sera were separated and analyzed. Two kits were used based on WHO recommendation of two different testing strategies involving enzyme-linked immunosorbent assay (ELISA) and/or simple or rapid assays for surveillance. Samples were analyzed for antibodies to HIV 1 and 2, HBsAg and HCV by ELISA. Test for syphilis was done by VDRL. Any serum found reactive by the first assay was retested using a second assay based on different antigen preparations and/or different test principle using the anti-HIV test, HBsAg which is an immunochromatographic sandwich assay and HCV by the anti-HCV test. The validity of the test is assured as per the given criterion and the results were computed.
Statistical analysis
The data entry was carried out using Microsoft Office Excel worksheet and percentage and proportions for each variable was calculated. The chi-square test and Fisher's exact test were used as a test of significance.
Results
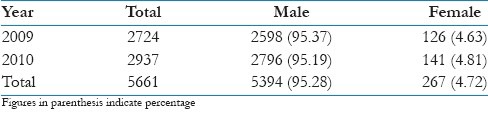
In the present study, out of total 5661 voluntary blood donors, 5394 (95.28%) were males and 267 (4.72%) were females which shows predominance of males as compared to females for the two studied years [Table 1].
Table 1.
Distribution of voluntary blood donors in the study population
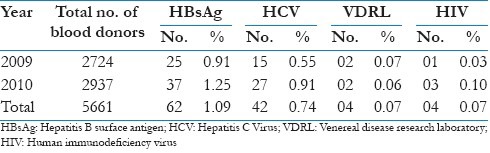
The prevalence of HBsAg, anti-HCV, VDRL, and anti-HIV among voluntary blood donors in the study population is showed in Table 2. The overall seroprevalence of HBV and HCV was 1.09% and 0.74% respectively, while the prevalence of VDRL and HIV was 0.07%. The highest prevalence was observed for HBV followed by HCV, syphilis and HIV in decreasing order.
Table 2.
Prevalence of HBsAg, anti-HCV, VDRL, and anti-HIV among voluntary blood donors in the study population
All infections summed together the highest prevalence (55.75%) was within the age group 21-30 years, followed by 36.28% within the age group 31-40 years, with the lowest prevalence was observed with in the age group of <20 years (1.76%) and ≥51 (0.88%) [Table 3].
Table 3.
Distribution of blood donors with transfusion transmitted infections according to the age
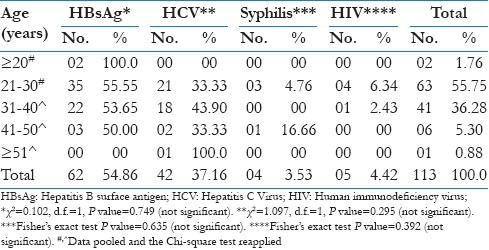
With respect to the individual TTIs, it is observed that the prevalence of HBV was highest within at the age groups <20 years (100%), HCV within the age group between 31 and 40 years (43.90%), syphilis within the age group between 41 and 50 years (16.66%) and HIV between 21 and 30 years (6.34%). The difference of the prevalence of transfusion transmitted diseases among different age groups was statistically not significant (P>0.05).
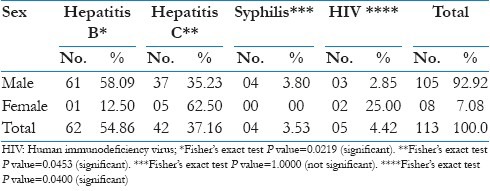
As mentioned in Table 4 that the prevalence of hepatitis B and syphilis was higher among males as compared to females (58.09% and 3.80%) respectively, while for hepatitis C, it was higher among females as compared to males (62.50%), the difference of prevalence by sex was statistically significant (P<0.05).
Table 4.
Distribution of blood donors with transfusion transmitted infections according to the sex
Discussion
Blood transfusion is an integral and life-saving procedure of modern medicine, but simultaneously it carries the risk of transmitting the life threatening transfusion transmissible infectious. HIV, hepatitis B, and hepatitis C are major public health problems in developing countries. They are transmitted parenterally, vertically, or through high-risk sexual behaviors and can cause fatal acute and chronic life-threatening disorders. Blood transfusion is a potential route of transmission of these TTIs.[10,11] Screening of blood is now mandatory for many diseases and is undertaken routinely in blood banks. Transmission of TTIs during the serologically window period still poses a threat to blood safety in environments where there is high rate of TTIs. HBV and HCV are the two established causes of post transfusion hepatitis. The prevalence of TTIs among the Indian blood donors is reported to be ranging as follows; HBV – 0.66% to 12%, HCV – 0.5% to 1.5%, HIV– 0.084% to 3.87%, and syphilis – 0.85% to 3% respectively.[12]
The present study revealed seroprevalence of HBV at 1.09% among the donors which is similar to findings by Chattoraj et al.,[12] Kaur et al.,[13] and Singh B et al.,[14]. Variable results of 0.66%,[15] 2.45%,[7] 3.44%,[16] 5.86%,[17] 25%[18] have also been reported in various other studies. Seroprevalence of HBV among blood donors differs. The major route of HBV transmission is parenteral and it is most infective among blood-borne viruses and chronic carrier state is associated with chronic liver disease, cirrhosis and hepatocellular carcinoma.
HCV infection is an evolving public health problem globally. For hepatitis C, the estimated prevalence in this study was 0.74%, similar to that reported by the other studies 0.79%,[12] 0.88%[19] and 0.78%[13]; whereas a few studies reported much lower level of prevalence such as 1.09%,[15] 1.57%,[20] 2.8%,[21] and 6.21%[17] and a yet another set of studies reported it to be at higher levels of 0.28%[16] and 0.50%.[14] Transmission of HCV is primarily through blood exposure and majority of the infected persons progress to chronic infection and chance of cirrhosis and hepatocellular carcinoma is more as compared to HBV. Blood is one of the main sources of transmission of Hepatitis C; hence, donor selection is of paramount importance.
In the present study, the prevalence of HIV was found to be 0.07%. Similar findings by Gupta et al.,[15] and Tiwari et al.,[22] reported 0.084% and 0.054% prevalence of HIV among blood donors, whereas lower seroprevalence of 0.0%[17] and higher seroprevalence of 0.13%,[12] 0.19%,[23] 0.26%,[13] 0.47%,[16] 3.8%[24] and 11.7%[18] have been reported.
For syphilis, the seroprevalence was found to be 0.07% in the present study, which was much lower than reported by other studies 0.85%[15] and 1.2%.[17]
Regarding sex, the study found that blood transfusion transmitted diseases are more prevalent among males than females, the difference of prevalence by sex was statistically significant (P<0.05), which was comparable to other studies.[7,17] A sex-wise difference in seroprevalence might be due to differences in the risk behavior.
Availability of safe blood for transfusion is a must for the recipients and the community as well. This can be achieved by vigorous screening of donors and donated bloods. Effective control strategies including a sensitive and stringent screening of all blood donors, public awareness programs, and institution of adequate public health measures are urgently needed. It may be possible through proper donor selection and education, uniform implementation of laboratory screening tests, and adequate supply of blood through voluntary blood donations along with restriction of donation by professional donors.
Limitations
Most of the previous studies were from urban areas; this report is first of its kind from rural area of western Maharashtra, India. The major limitation of the study is that there is no previous data available from this rural area for comparison and analysis of trends. Hence, we hereby recommend for future studies to look into trends for TTIs from this area.
Conclusion
Blood is still one of the main sources of transmission of hepatitis B, hepatitis C, HIV, and syphilis. The majority of donors in our country are voluntary, relatives or friends, who are apparently healthy, but this study found that these diseases are prevalent among donors. Hence, strict selection of blood donors with the emphasis on getting voluntary donors and comprehensive screening of donors for TTIs using standard methods are highly recommended to ensure the safety of blood for recipient.
Acknowledgement
We express our deep sense of gratitude to the Management, Pravara Medical Trust and Principal, Rural Medical College, Loni. We also acknowledge the help and support of Dr. S.D. Dongre, Professor and Head, Department of Pathology and medical interns- Sakshi Jain and Nighat Parveen.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures: A review. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Nancy Singh. NAT: Safe Blood, Safe India. [Last accessed on 2011 Dec 09]. Available from: http://www.expresshealthcare.in/200810/knowledge02.shtml .
- 3.Zafar N. A survey of blood transfusion practices. J Coll Physicians Surg Pak. 2000;10:90–2. [Google Scholar]
- 4.Isbister JP. Risk management in transfusion medicine. Transfus Med. 1996;10:183–202. doi: 10.1016/s0887-7963(96)80059-9. [DOI] [PubMed] [Google Scholar]
- 5.Grgicevic D, Balija M, Pirc-Tiljak D, Mihaljevic´ I, Gjenero-Margan I, Zupancic´-Salek S. Decreasing risk of viral transfusion-transmitted diseases in Croatia. Croat Med J. 2000;41:191–6. [PubMed] [Google Scholar]
- 6.Asif N, Kokher N, Ilahi F. Seroprevalence of HBV, HCV, and HIV infection among voluntary non remunerated and replacement donors in northern Pakistan. Pak J Med Sci. 2004;20:24–8. [Google Scholar]
- 7.Chaudhary IA, Samiullah, Khan SS, Masood R, Sardar MA, Mallhi AA. Seroprevalence of HBV and C among health donors at Fauji Foundation Hospital, Rawalpindi. Pak Med J. 2007;23:64–7. [Google Scholar]
- 8.Mujeeb SA, Kausar A, Khalid M. Seroprevalence of HBV, HCV, and HIV infection among college going voluntary donors. J Pak Med Assoc. 2000;50:269–70. [PubMed] [Google Scholar]
- 9.Kar HK. Global and National overview of HIV/AIDS epidemic. In: Sharma VK, editor. Sexually transmitted diseases and HIV/AIDS. 2nd ed. New Delhi: Viva Books Pvt. Ltd; 2009. pp. 99–109. [Google Scholar]
- 10.Irshad M, Peter S. Spectrum of viral hepatitis in thalassemic children receiving multiple blood transfusions. Indian J Gastroenterol. 2002;21:183–4. [PubMed] [Google Scholar]
- 11.Mollah AH, Nahar N, Siddique MA, Anwar KS, Hassan T, Azam MG. Common transfusion-transmitted infectious agents among thalassaemic children in Bangladesh. J Health Popul Nutr. 2003;21:67–71. [PubMed] [Google Scholar]
- 12.Chattoraj A, Bhel R, Kataria V. Infectious disease markers in blood donors. Med J Armed Forces India. 2008;64(1):33–5. doi: 10.1016/S0377-1237(08)80142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur H, Dhanon J, Pawar G. Hepatitis C infection amongst blood donors in Punjab – a six year study. Indian J Hematol Blood Transfus. 2001;19:21–2. [Google Scholar]
- 14.Singh B, Verma M, Verma K. Markers of transfusion associated hepatitis in North Indian blood donors: Prevalence and trends. Jpn J Infect Dis. 2004;57:49–51. [PubMed] [Google Scholar]
- 15.Gupta N, Vijay Kumar, Kaur A. Seroprevalence of HIV, HBV, HCV, and Syphilis in voluntary blood donors. Indian J Med Sci. 2004;58:255–7. [PubMed] [Google Scholar]
- 16.Garg S, Mathur DR, Garg DK. Comparison of seropositivity of HIV, HBsAg, HCV and syphilis in replacement and voluntary blood donors in Western India. Indian J Pathol Microbiol. 2001;44:409–12. [PubMed] [Google Scholar]
- 17.Mumtaz S, Rehman MU, Muzaffar M, Hassan MU, Iqbal W. Frequency of seropositive blood donors for hepatitis B, C and HIV viruses in railway hospital, Rawalpindi. Pak J Med Res. 2002;41(2):19–2. [Google Scholar]
- 18.Dessie A, Abera B, Fissehawale Seroprevalence of major blood borne infections among blood donors at Felege Hiwot referral hospital, Northwest Ethopia. Ethiop J Health Dev. 2007;21:68–9. [Google Scholar]
- 19.Bagga PK, Singh SP. Seroprevalence of hepatitis C antibodies in healthy blood donors-a prospective study. Indian J Pathol Microbiol. 2007;50:429–32. [PubMed] [Google Scholar]
- 20.Jain A, Rana SS, Chakravarty P, Gupta RK, Murthy NS, Nath MC, et al. The prevalence of hepatitis C virus antibodies among voluntary blood donors of New Delhi, India. Eur J Epidemiol. 2003;18:695–7. doi: 10.1023/a:1024887211146. [DOI] [PubMed] [Google Scholar]
- 21.Sood G, Chauhan A, Sehgal S, Agnihotri S, Dilawari JB. Antibodies to hepatitis C virus in blood donors. Indian J Gastroenterol. 1992;11:44. [PubMed] [Google Scholar]
- 22.Tiwari B, Ghimire P, Karkee S, Rajkarnikar M. Seroprevalence of human immunodeficiency Virus in Nepalese blood donors: A study from three regional blood transfusion services. Asian J Transf Sci. 2008;2:66–8. doi: 10.4103/0973-6247.42663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karkee S, Ghimire P, Tiwari B, Shrestha A. Seroprevalence of HIV and Hepatitis C coinfection among blood donors in Katmando valley, Nepal. Southeast Asian J Trop Med Public Health. 2009;40(1):66–70. [PubMed] [Google Scholar]
- 24.Matee M, Magesa PM, Lyamuya EF. Seroprevalence of human immunodeficiency virus, Hepatitis B and C viruses and Syphilis infections among blood donors at the Muhimbiili National Hospital in Dar es Salam, Tanzania. BMC Public Health. 2006;6:21. doi: 10.1186/1471-2458-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]


