Abstract
Mutants of Salmonella typhimurium defective in adenylate cyclase (cya gene) or in cAMP receptor protein (crp gene) are lysogenized at reduced frequency by phage P22. One class of the bacterial mutants with an altered RNA polymerase (rif gene) is also lysogenized at reduced frequency. In the three types of mutant bacteria, the phage's decision between lysogeny and lysis is shifted to lysis and the phage form clear plaques. We propose that in wild-type bacteria the cAMP-receptor protein, in combination with cAMP, activates bacterial RNA polymerase to transcribe certain phage genes that are required for efficient lysogenization. Under conditions of strong catabolite repression, when the supply of energy and biosynthetic components is abundant and the concentration of cAMP is low, the phage would multiply and lyse the cell. When the supply of energy is deficient and the concentration of cAMP is high, the phage would lysogenize the cell.
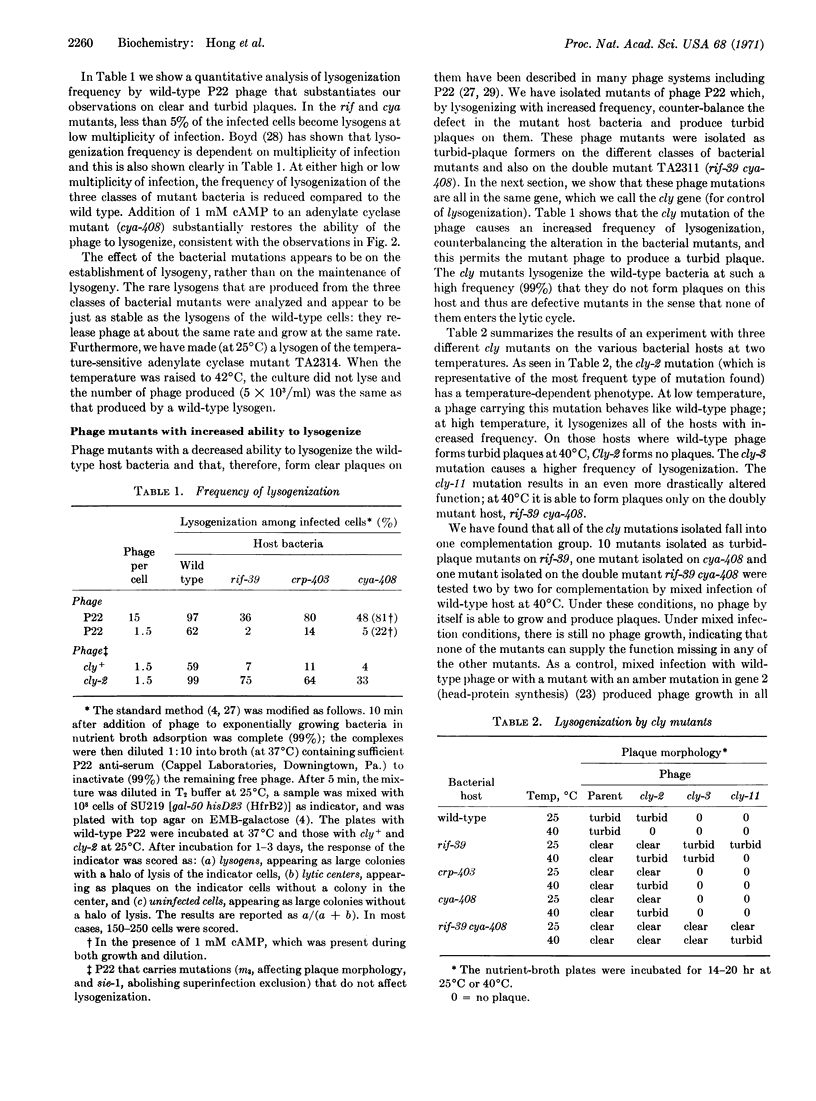
Phage mutants have been isolated that form turbid plaques on the three classes of bacterial mutants due to a higher frequency of lysogeny. These phage mutants have been shown by complementation to be defective in the same gene, which we have called the cly gene. These cly mutants lysogenize the wild-type bacteria with a 99% frequency and, thus, do not form plaques on them.
Other kinds of bacterial mutants are also lysogenized at reduced frequency by phage P22. They may be altered in other physiological control systems that influence the frequency of lysogenization.
Keywords: Salmonella typhimurium, P22 phage, adenylate cyclase, RNA polymerase
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Lysogeny. Adv Virus Res. 1958;5:151–193. doi: 10.1016/s0065-3527(08)60673-9. [DOI] [PubMed] [Google Scholar]
- BERTANI G., NICE S. J. Studies on lysogenesis. II. The effect of temperature on the lysogenization of Shigella dysenteriae with phage P1. J Bacteriol. 1954 Feb;67(2):202–209. doi: 10.1128/jb.67.2.202-209.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI L. E. The effect of the inhibition of protein synthesis on the establishment of lysogeny. Virology. 1957 Aug;4(1):53–71. doi: 10.1016/0042-6822(57)90043-0. [DOI] [PubMed] [Google Scholar]
- BOYD J. S. K. Observations on the relationship of symbiotic and lytic bacteriophage. J Pathol Bacteriol. 1951 Jul;63(3):445–457. doi: 10.1002/path.1700630311. [DOI] [PubMed] [Google Scholar]
- Berkowitz D. D-Mannitol utilization in Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):232–240. doi: 10.1128/jb.105.1.232-240.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdek M., Amati P. Properties of P22 and A related Salmonella typhimurium phage. I. General features and host specificity. Virology. 1967 Feb;31(2):272–278. doi: 10.1016/0042-6822(67)90171-7. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN J. R. Effect of chloramphenicol on lysogenization by temperate phage P1. Virology. 1957 Aug;4(1):184–185. doi: 10.1016/0042-6822(57)90054-5. [DOI] [PubMed] [Google Scholar]
- Calendar R. The regulation of phage development. Annu Rev Microbiol. 1970;24:241–296. doi: 10.1146/annurev.mi.24.100170.001325. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel D. H., Hutchins J. E. Mutations affecting RNA polymerase associated with rifampicin resistance in Escherichia coli. Nature. 1968 Oct 19;220(5164):276–277. doi: 10.1038/220276a0. [DOI] [PubMed] [Google Scholar]
- Goldenbaum P. E., Dobrogosz W. J. The effect of cyclic 3',5'-AMP on catabolite repression of beta-galactosidase synthesis in Escherichia coli. Biochem Biophys Res Commun. 1968 Dec 9;33(5):828–833. doi: 10.1016/0006-291x(68)90235-0. [DOI] [PubMed] [Google Scholar]
- Gough M. Second locus of bacteriophage P22 necessary for the maintenance of lysogeny. J Virol. 1968 Oct;2(10):992–998. doi: 10.1128/jvi.2.10.992-998.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D., CHASE M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952 May;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- LEVINE M. Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology. 1957 Feb;3(1):22–41. doi: 10.1016/0042-6822(57)90021-1. [DOI] [PubMed] [Google Scholar]
- LEVINE M., SMITH H. O. SEQUENTIAL GENE ACTION IN THE ESTABLISHMENT OF LYSOGENY. Science. 1964 Dec 18;146(3651):1581–1582. doi: 10.1126/science.146.3651.1581. [DOI] [PubMed] [Google Scholar]
- LIEB M. The establishment of lysogenicity in Escherichia coli. J Bacteriol. 1953 Jun;65(6):642–651. doi: 10.1128/jb.65.6.642-651.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A., KAPLAN A. S., RITZ E. Recherches sur la lysogénisation de Salmonella typhimurium. Ann Inst Pasteur (Paris) 1954 Feb;86(2):127–148. [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew K. K., Roth J. R. Isolation of UGA and UAG nonsense mutants of bacteriophage P22. Virology. 1970 Apr;40(4):1059–1062. doi: 10.1016/0042-6822(70)90153-4. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. The role of the lac promotor locus in the regulation of beta-galactosidase synthesis by cyclic 3',5'-adenosine monophosphate. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1336–1342. doi: 10.1073/pnas.61.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Perlman R., Pastan I. Cyclic 3'5-AMP: stimulation of beta-galactosidase and tryptophanase induction in E. coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):656–664. doi: 10.1016/0006-291x(68)90563-9. [DOI] [PubMed] [Google Scholar]
- Rabussay D., Zillig W. A rifampicin resistent rna-polymerase from E. coli altered in the beta-subunit. FEBS Lett. 1969 Oct 21;5(2):104–106. doi: 10.1016/0014-5793(69)80305-4. [DOI] [PubMed] [Google Scholar]
- Reichardt L., Kaiser A. D. Control of lambda repressor synthesis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Reiness G., Zubay G. Purification and DNA-binding properties of the catabolite gene activator protein. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1222–1225. doi: 10.1073/pnas.68.6.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. O., LEVINE M. TWO SEQUENTIAL REPRESSIONS OF DNA SYNTHESIS IN THE ESTABLISHMENT OF LYSOGENY BY PHAGE P22 AND ITS MUTANTS. Proc Natl Acad Sci U S A. 1964 Aug;52:356–363. doi: 10.1073/pnas.52.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Revised linkage map of Salmonella typhimurium. Bacteriol Rev. 1967 Dec;31(4):354–372. doi: 10.1128/br.31.4.354-372.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A. E., Magasanik B., Reznikoff W. S., Miller J. H., Beckwith J. R. Catabolite sensitive site of the lac operon. Nature. 1969 Mar 15;221(5185):1012–1014. doi: 10.1038/2211012b0. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Marino P., Colvill A. J. Mutant of E. coli containing an altered DNA-dependent RNA polymerase. Nature. 1968 Oct 19;220(5164):275–276. doi: 10.1038/220275a0. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Monod J. Cyclic AMP as an antagonist of catabolite repression in Escherichia coli. FEBS Lett. 1968 Nov;2(1):57–60. doi: 10.1016/0014-5793(68)80100-0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Staehelin M. Action of rifamycin on RNA-polymerase from sensitive and resistant bacteria. Biochem Biophys Res Commun. 1968 Jul 26;32(2):284–288. doi: 10.1016/0006-291x(68)90382-3. [DOI] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T., Igarashi K. RNA polymerase mutants of Escherichia coli. I. Mutants resistant to streptovaricin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1313–1319. doi: 10.1073/pnas.61.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]




