Abstract
Objective and Background:
Polycystic ovary syndrome (PCOS) is a complex condition and has been described in women who have polycystic ovaries as the underlying cause of hirsutism and chronic anovulation. Studies on PCOS in the Saudi population are very few. The aim of this study was to investigate the reproductive hormones levels in patients with PCOS. Effect of age and body mass index (BMI) on the hormonal findings was eliminated through a multivariate analysis.
Materials and Methods:
A comparative study was conducted on Saudi subjects attending the outpatient clinic of National Guard Hospital in Riyadh. A total of 62 cases with PCOS and 40 healthy Saudi women were included in this study. Physical evaluation and laboratory investigations were carried out. Blood luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol (E2), dehydroepiandrosterone sulfate (DHEA-SO4), sex hormone-binding globulin (SHBG), total testosterone, prolactin, and progesterone were determined. To adjust for the potentially confounding effect of age and BMI, we carried out multivariate linear regression analyses for the association between each of the reproductive hormones and PCOS.
Results:
Serum levels of FSH, SHBG, and progesterone were significantly lower in PCOS compared to controls (respective P values 0.001, 0.001, and 0.002), while LH/FSH and testosterone levels were higher in PCOS cases than in controls (P = 0.008 and 0.003, respectively). When multivariate linear regression analyses were carried out, LH/FSH and total testosterone were positively correlated with the disease [95% confidence interval (CI) = 0.02–0.35 and 0.02–0.17, respectively], whereas FSH, SHBG, and progesterone were negatively correlated with the disease (95% CI = –0.06 to 0.001, –0.01 to 0.001, and –0.17 to –0.03, respectively), independent of age and BMI.
Conclusion:
Our study suggests that regardless of the age and weight factors, Saudi patients with PCOS have higher levels of LH/FSH and total testosterone; but have lower levels of FSH, SHBG, and progesterone compared to controls.
Keywords: Age, body mass index, polycystic ovary syndrome, reproductive hormones, Saudi Arabia
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of reproductive age, with a prevalence of up to 10%. It is a complex condition that was first described in women who had polycystic ovaries as the underlying cause of hirsutism and chronic anovulation.[1] With the new guidelines for diagnosis of PCOS,[2] the condition remains to be named so even if ovarian cysts are absent. However, polycystic ovary as a phenomenon is very common in women with idiopathic hirsutism and oligomenorrhea.[3] The principal findings in patients with PCOS include irregular menstruation, acne, and excessive amounts of androgenic hormones.[4,5] Obesity is a common finding of women with PCOS,[6] but it is not part of the diagnostic criteria. PCOS is not merely a disease of the reproductive system, since type 2 diabetes, metabolic syndrome, and sometimes cardiovascular disease have been associated with this condition.[6]
The pathogenesis of PCOS remains unclear.[7] Establishing the diagnosis of PCOS is quite complex; the Rotterdam PCOS consensus workshop concluded that two out of three criteria need to be present in order to establish PCOS diagnosis.[2] These are chronic anovulation, clinical and/or biochemical evidence of hyperandrogenism, and presence of polycystic ovaries by ultrasound or by laparoscopic findings.[2] Diagnosis is confirmed only after exclusion of other known disorders with similar clinical presentations, such as thyroid dysfunction and hyperprolactinemia.[2] The etiology of PCOS remains unknown; however, familial predisposition seems to play an important role.[8]
In the literature, several groups have investigated the reproductive hormones of women with PCOS in comparison to healthy controls. Follicle stimulating hormone (FSH) and sex hormone-binding globulin (SHBG) were found to be lower in several studies.[9,10,11,12] In addition, mean concentration of testosterone was higher in patients with PCOS compared to controls.[13] Furthermore, it has been shown that luteinizing hormone (LH)[14,15] and LH/FSH ratio were elevated in PCOS patients compared to normal controls.[16] The results, however, depended on the day of the cycle on which the hormones are measured, wherein LH was significantly elevated in PCOS patients only late in the menstrual cycle but not earlier.[11] In addition, body mass index (BMI) seems to have a controversial influence on these results; an inverse relationship between LH and BMI was described in some studies[17,18,19,20] but not in others.[11,21,22,23] Diagnostic markers of PCOS are of great importance especially for general practitioners (GPs) and primary care clinicians who are the gatekeepers and the primary encounter of such patients.
In the present study, we carried out a comparative study of the reproductive hormones on a group of patients with PCOS. To eliminate the effect of BMI on hormonal findings, we performed a multivariate analysis.
Materials and Methods
This study was conducted on women who attended the Obstetrics and Gynecology Clinic at King Abdulaziz Medical City in Riyadh, Saudi Arabia, between June 2005 and June 2007. Women aged between 18 and 45 years were screened for the presence of PCOS.
Study subjects
A total of 62 Saudi patients diagnosed with PCOS based on the Revised 2003 Rotterdam Criteria during this study period were included in the study. According to the Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group, two out of three of the following must be present to establish diagnosis: Oligo- and/or anovulation, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovaries. Exclusion of other etiologies (congenital adrenal hyperplasias, androgen-secreting tumors, Cushing's syndrome) was conducted.[2] For the purpose of this study, all diagnoses were made by a consultant gynecologist in association with a senior ultrasonographer.
A total of 40 healthy women, age and ethnically matched, represented the control group. Controls were free from any fertility, endocrinological, or dermatological problems.
All subjects included in this study were aged between 18 and 45 years. They had not been taking oral contraceptives for at least 3 months; they were pre-menopausal (FSH < 12 IU/L) and free from any severe disease. All participants signed an informed consent form. The study was approved by the Institutional Review Board.
Hormonal investigations
Blood samples were withdrawn between days 1 and 5 of the period, with comparable numbers in cases and controls. On average, samples were withdrawn on day 2.5 and 2.6 of the cycle in controls and cases, respectively.
LH, FSH, estradiol (E2), dehydroepiandrosterone sulfate (DHEA-SO4), SHBG, total testosterone, prolactin, and progesterone were determined by immunoassay (Immulite, Siemens, New York, USA).
Serum levels of thyroid stimulating hormone (TSH) and 24-h urinary cortisol were not measured unless thyroid dysfunction or hypercortisolemia was suspected.
Other information
Information about menstrual cycle was obtained from all subjects. Regular menstruation was defined as 9–16 cycles of 21–35 days duration within a year, and no more than a 4-day difference in duration between cycles. The subjects were checked for the presence of acne and/or blackheads on the face, neck, upper arm, chest, and back. Using the modified Ferriman and Gallwey score,[24] the subjects were checked for hirsutism. A Ferriman and Gallwey score higher than 7 indicated hirsutism.
Using a mercury sphygmomanometer, systolic and diastolic blood pressure were measured twice. Hypertension was defined if systolic pressure was ≥140 mmHg or diastolic blood pressure was ≥90 mm Hg.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) program, version 17, was used for the management and analyses of the data. Descriptive statistics was performed by providing the number and percent for categorical variables and the mean and standard deviation for continuous variables. The association between the outcome (PCOS) and other categorical variables was found using the Chi-square test or Fisher's exact test, as appropriate, and the t-test and the Wilcoxon–Mann–Whitney test, as appropriate. To adjust for the potentially confounding effect of age and BMI, we carried out multivariate linear regression analyses for the association between each of the reproductive hormones and PCOS. The results were represented by providing the regression coefficient and 95% confidence interval (CI). P value was calculated and was considered significant if <0.05.
Results
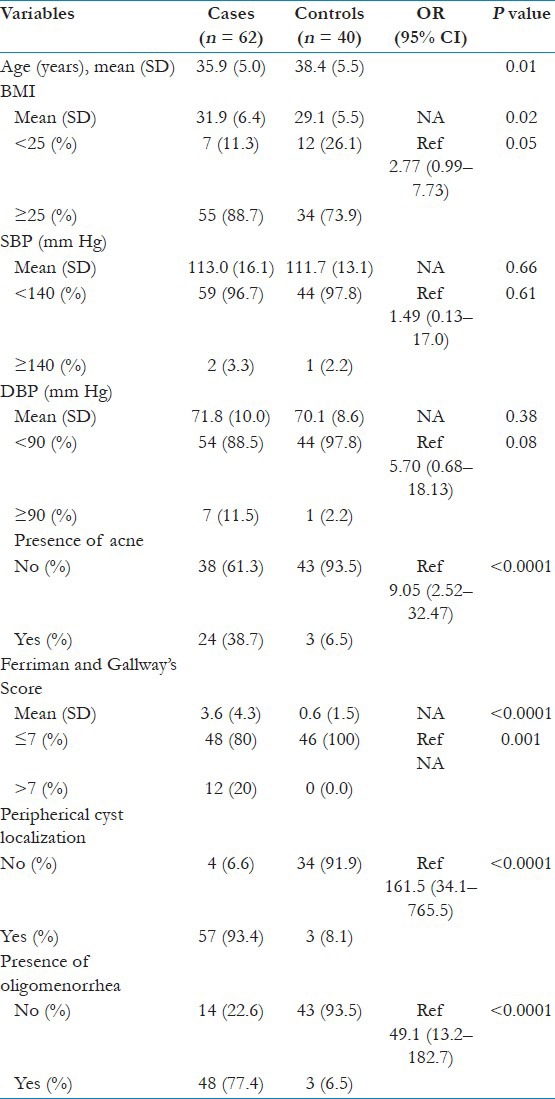
The main demographics of cases and controls are presented in Table 1. The mean age of the cases was 35.9 ± 5.5 years, whereas the mean age for the controls was 38.4 ± 5.0 years, which was found to be statistically significant (P = 0.01). Cases had a significantly higher BMI compared to controls (31.9 ± 6.4 vs. 29.1 ± 5.5, respectively; P = 0.02), and Odds Ratio (OR) was 2.77. No significant difference in blood pressure measurements (systolic “OR 1.49” and diastolic “OR 5.7”) was seen between cases and controls [Table 1]. In line with the PCOS characteristics, it was found that cases had significantly higher prevalence of acne (OR 9.05), hirsutism, ovarian cysts (OR 161.5), and oligomenorrhea (OR 49.1) [Table 1].
Table 1.
Comparison of the characteristics of cases and controls
Unadjusted comparison of the levels of various reproductive hormones between cases and controls is presented in Table 2. The mean FSH for cases was 6.4 IU/L ± 2.3, whereas that for the controls was 8.3 IU/L ± 3.7 (P = 0.001). The mean LH/FSH for cases was found to be 0.9 (±0.7) compared to 0.6 (±0.4) in controls (P = 0.008). In addition, the mean SHBG for cases was significantly lower (32.7 nmol/L ± 18.4) than that for controls (45.4 nmol/L ± 21.1) (P = 0.001). The mean total testosterone (nmol/L) for cases was measured as 2.2 ± 1.3, whereas that for controls was 1.5 ± 1.0 (P = 0.003). Progesterone levels (nmol/L) were 3.1 ± 1.0 and 3.8 ± 1.4 for cases and controls, respectively (P = 0.002). In summary, LH/FSH and total testosterone were significantly increased in PCOS cases, whereas FSH, SHBG, and progesterone were significantly decreased in cases compared to controls. The remaining measured hormones, i.e., LH, E2, DHEA-SO4, prolactin, and cortisol, were not significantly different between cases and controls. Although LH did not show significant difference between cases and controls, cases had higher levels of LH (IU/L) than controls (5.8 ± 4.1 vs. 4.7 ± 2.9).
Table 2.
Unadjusted comparison between cases and controls in terms of reproductive hormones
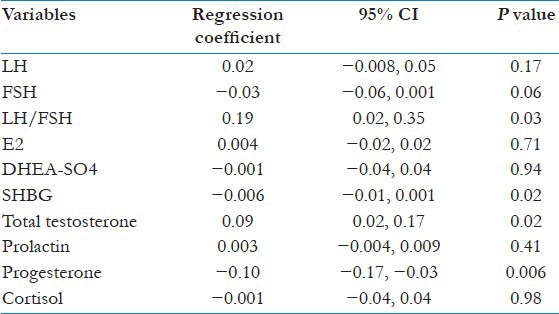
To eliminate the potentially confounding bias of age and BMI, we carried out multivariate analyses [Table 3]. This enabled us to confirm the findings of the unadjusted comparison in Table 2. Actually, both adjusted and unadjusted analyses led to the same conclusion. Indeed, LH/FSH and total testosterone were positively correlated with the disease (regression coefficient = 0.19 and 0.09, respectively, with P = 0.03 and 0.02, respectively). However, FSH, SHBG, and progesterone were negatively correlated with the disease (regression coefficient = −0.03, −0.006, and −0.10, respectively, with P = 0.06, 0.02, and 0.006, respectively).
Table 3.
Age and BMI adjusted comparison of reproductive hormones
Discussion
In this study, we have measured the level of reproductive hormones of Saudi women diagnosed with PCOS according to the Rotterdam criteria. Recent research has proven that ethnicity seems to affect the clinical presentation of PCOS.[25] The importance of our study lies in the fact that studies describing PCOS in the Saudi population are very scarce.
In our studied population, cases were younger than controls, and this age difference is sometimes unavoidable in observational studies. In addition, both cases and controls had higher than normal mean BMI (<25 kg/m2), which reflects the fact that obesity is a common finding in PCOS and also in the general Saudi population. Moreover, cases had significantly higher BMI compared to controls, with a mean BMI greater than 30 kg/ m2. PCOS had higher risk of overweight compared to control (OR 2.77).
In the literature, BMI has been suggested to influence the levels of reproductive hormones with some contradictory results. Indeed, some studies found that higher BMI was associated with lower LH,[19,20,26,27] but others described that BMI had no influence on LH.[22,23] On the other hand, recent research has indicated that age can also influence both the clinical presentation and metabolic manifestations of PCOS.[28,29] Owing to the age and BMI difference between the two studied groups, we have adjusted for both these factors by carrying out multivariate regression analyses.
In the unadjusted comparison, we found a significantly lower level of FSH, SBHG, and progesterone, and significantly higher LH/FSH ratio and testosterone. However, the following hormones did not show significant difference between cases and controls: LH, E2, DHEA-SO4, prolactin, and cortisol. In the adjusted comparison between cases and controls, we were able to confirm those results. In fact, the results of the multivariate regression analyses confirmed that regardless of the age and weight factor, cases had higher levels of LH/FSH and total testosterone, and that cases had lower level of FSH, SHBG, and progesterone. Even in the adjusted comparison, we could not find a significant increase in LH levels.
Unlike the previous published studies,[14,15] we have failed to find a significant increase in LH in patients with PCOS. A major factor that tends to affect the results is the variation of hormonal level with the menstrual cycle. According to a published study,[11] elevated LH levels are not very reproducible in the early menstrual cycle, which was the time at which we measured LH in this study. Furthermore, it was previously demonstrated that LH is more elevated in lean PCOS patients compared to obese PCOS patients.[16] Having most of our patients with BMI >25 (88%) could have been a major influence of the results. However, this was shown not to be the case, since both BMI adjusted and unadjusted comparisons have yielded the same results. Several studies in the literature have reported that not all PCOS patients have elevated LH,[13,14,15] and this could be very well the reason why the average increase in LH observed in our study was not statistically significant.
In fact, the reasons mentioned above were given for not including LH in the diagnostic criteria set by the Rotterdam consensus group.[2] The group did state, however, that further research is required to elucidate the role of LH in PCOS.[2] Our results should be of importance especially for primary care clinics which is the first encounter for such patients. Hormonal study of FSH, LH, and testosterone should be on the list of tests for diagnostic purposes of patients with PCOS.
The results of this study should be interpreted bearing in mind its limitations. A major limitation was the sample size (62 cases and 40 controls). Future studies with a larger number are required to further clarify the hormonal variations in PCOS. Another limitation is the unavailability of a measurement for insulin level for the study subjects. Most of the subjects, whether cases or controls, were overweight or obese, which is a predisposing factor for diabetes or the metabolic syndrome. On the other hand, insulin resistance is significantly related to serum LH. Therefore, the absence of data on insulin levels of both groups has impacted some constraints on the interpretation of the hormonal findings.
In conclusion, our study suggests that regardless of the age and weight factor, PCOS patients have higher levels of LH/FSH and total testosterone, but lower levels of FSH, SHBG, and progesterone.
Conclusion
Elevated levels of LH/FSH and testosterone and reduced FSH, SHBG, and progesterone were predictors of PCOS. This was independent of BMI or age. Future studies with larger sample size and data on insulin levels are needed for greater understanding of the manifestation of PCOS in the Saudi population.
Acknowledgment
The authors would like to express their gratitude to Dr. Hani Tamim for his assistance in the statistical analysis and interpretations of the results.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–91. [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-SponsoredPCOSConsensusWorkshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 3.Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 1986;293:355–9. doi: 10.1136/bmj.293.6543.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alemzadeh R, Kichler J, Calhoun M. Spectrum of metabolic dysfunction in relationship with hyperandrogenemia in obese adolescent girls with polycystic ovary syndrome. Eur J Endocrinol. 2010;162:1093–9. doi: 10.1530/EJE-10-0205. [DOI] [PubMed] [Google Scholar]
- 5.Hassa H, Tanir HM, Yildiz Z. Comparison of clinical and laboratory characteristics of cases with polycystic ovarian syndrome based on Rotterdam's criteria and women whose only clinical signs are oligo/anovulation or hirsutism. Arch Gynecol Obstet. 2006;274:227–32. doi: 10.1007/s00404-006-0173-8. [DOI] [PubMed] [Google Scholar]
- 6.Setji TL, Brown AJ. Polycystic ovary syndrome: Diagnosis and treatment. Am J Med. 2007;120:128–32. doi: 10.1016/j.amjmed.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 7.Matalliotakis I, Kourtis A, Koukoura O, Panidis D. Polycystic ovary syndrome: Etiology and pathogenesis. Arch Gynecol Obstet. 2006;274:187–97. doi: 10.1007/s00404-006-0171-x. [DOI] [PubMed] [Google Scholar]
- 8.Franks S. Polycystic ovary syndrome: A changing perspective. Clin Endocrinol (Oxf) 1989;31:87–120. doi: 10.1111/j.1365-2265.1989.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 9.Franks S, Kiddy D, Sharp P, Singh A, Reed M, Seppälä M, et al. Obesity and polycystic ovary syndrome. Ann N Y Acad Sci. 1991;626:201–6. doi: 10.1111/j.1749-6632.1991.tb37915.x. [DOI] [PubMed] [Google Scholar]
- 10.Penttilä TA, Anttila L, Törmä A, Koskinen P, Erkkola R, Irjala K. Serum free testosterone in polycystic ovary syndrome measured with a new reference method. Fertil Steril. 1996;65:55–60. doi: 10.1016/s0015-0282(16)58027-3. [DOI] [PubMed] [Google Scholar]
- 11.Iwasa T, Matsuzaki T, Murakami M, Shimizu F, Kuwahara A, Yasui T, et al. Reproducibility of luteinizing hormone hypersecretion in different phases of the menstrual cycle in polycystic ovary syndrome. J Obstet Gynaecol Res. 2009;35:514–9. doi: 10.1111/j.1447-0756.2008.00998.x. [DOI] [PubMed] [Google Scholar]
- 12.Iwasa T, Matsuzaki T, Minakuchi M, Tanaka N, Shimizu F, Hirata Y, et al. Diagnostic performance of serum total testosterone for Japanese patients with polycystic ovary syndrome. Endocr J. 2007;54:233–8. doi: 10.1507/endocrj.k06-159. [DOI] [PubMed] [Google Scholar]
- 13.Robinson S, Rodin DA, Deacon A, Wheeler MJ, Clayton RN. Which hormone tests for the diagnosis of polycystic ovary syndrome? Br J Obstet Gynaecol. 1992;99:232–8. doi: 10.1111/j.1471-0528.1992.tb14505.x. [DOI] [PubMed] [Google Scholar]
- 14.van Santbrink EJ, Hop WC, Fauser BC. Classification of normogonadotropic infertility: Polycystic ovaries diagnosed by ultrasound versus endocrine characteristics of polycystic ovary syndrome. Fertil Steril. 1997;67:452–8. doi: 10.1016/s0015-0282(97)80068-4. [DOI] [PubMed] [Google Scholar]
- 15.Laven JS, Imani B, Eijkemans MJ, Fauser BC. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet Gynecol Surv. 2002;57:755–67. doi: 10.1097/00006254-200211000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2248–56. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 17.Su HW, Chen CM, Chou SY, Liang SJ, Hsu CS, Hsu MI. Polycystic ovary syndrome or hyperprolactinaemia: A study of mild hyperprolactinaemia. Gynecol Endocrinol. 2011;27:55–62. doi: 10.3109/09513590.2010.487606. [DOI] [PubMed] [Google Scholar]
- 18.Panidis D, Farmakiotis D, Rousso D, Koliakos G, Kaltsas T, Krassas G. Decrease in adiponectin levels in women with polycystic ovary syndrome after an oral glucose tolerance test. Fertil Steril. 2005;83:232–4. doi: 10.1016/j.fertnstert.2004.05.105. [DOI] [PubMed] [Google Scholar]
- 19.Katsikis I, Karkanaki A, Misichronis G, Delkos D, Kandaraki EA, Panidis D. Phenotypic expression, body mass index and insulin resistance in relation to LH levels in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;156:181–5. doi: 10.1016/j.ejogrb.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Dale PO, Tanbo T, Vaaler S, Abyholm T. Body weight, hyperinsulinemia, and gonadotropin levels in the polycystic ovarian syndrome: Evidence of two distinct populations. Fertil Steril. 1992;58:487–91. doi: 10.1016/s0015-0282(16)55249-2. [DOI] [PubMed] [Google Scholar]
- 21.Moran C, Garcia-Hernandez E, Barahona E, Gonzalez S, Bermudez JA. Relationship between insulin resistance and gonadotropin dissociation in obese and nonobese women with polycystic ovary syndrome. Fertil Steril. 2003;80:1466–72. doi: 10.1016/j.fertnstert.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Toprak S, Yönem A, Cakir B, Güler S, Azal O, Ozata M, et al. Insulin resistance in nonobese patients with polycystic ovary syndrome. Horm Res. 2001;55:65–70. doi: 10.1159/000049972. [DOI] [PubMed] [Google Scholar]
- 23.Tropeano G, Vuolo IP, Lucisano A, Liberale L, Barini A, Carfagna P, et al. Gonadotropin levels in women with polycystic ovary syndrome: Their relationship to body weight and insulin levels. J Endocrinol Invest. 1996;19:139–45. doi: 10.1007/BF03349856. [DOI] [PubMed] [Google Scholar]
- 24.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 25.Guo M, Chen ZJ, Eijkemans MJ, Goverde AJ, Fauser BC, Macklon NS. Comparison of the phenotype of Chinese versus Dutch Caucasian women presenting with polycystic ovary syndrome and oligo/amenorrhoea. Hum Reprod. 2012;27:1481–8. doi: 10.1093/humrep/des018. [DOI] [PubMed] [Google Scholar]
- 26.Grulet H, Hecart AC, Delemer B, Gross A, Sulmont V, Leutenegger M, et al. Roles of LH and insulin resistance in lean and obese polycystic ovary syndrome. Clin Endocrinol (Oxf) 1993;38:621–6. doi: 10.1111/j.1365-2265.1993.tb02144.x. [DOI] [PubMed] [Google Scholar]
- 27.Banaszewska B, Spaczyński RZ, Pelesz M, Pawelczyk L. Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo-and hyperinsulinemia. Rocz Akad Med Bialymst. 2003;48:131–4. [PubMed] [Google Scholar]
- 28.Johnstone EB, Davis G, Zane LT, Cedars MI, Huddleston HG. Age-related differences in the reproductive and metabolic implications of polycystic ovarian syndrome: Findings in an obese, United States population. Gynecol Endocrinol. 2012;28:819–22. doi: 10.3109/09513590.2012.671389. [DOI] [PubMed] [Google Scholar]
- 29.Glintborg D, Mumm H, Ravn P, Andersen M. Age associated differences in prevalence of individual Rotterdam criteria and metabolic risk factors during reproductive age in 446 Caucasian women with polycystic ovary syndrome. Horm Metab Res. 2012;44:694–8. doi: 10.1055/s-0032-1304608. [DOI] [PubMed] [Google Scholar]


