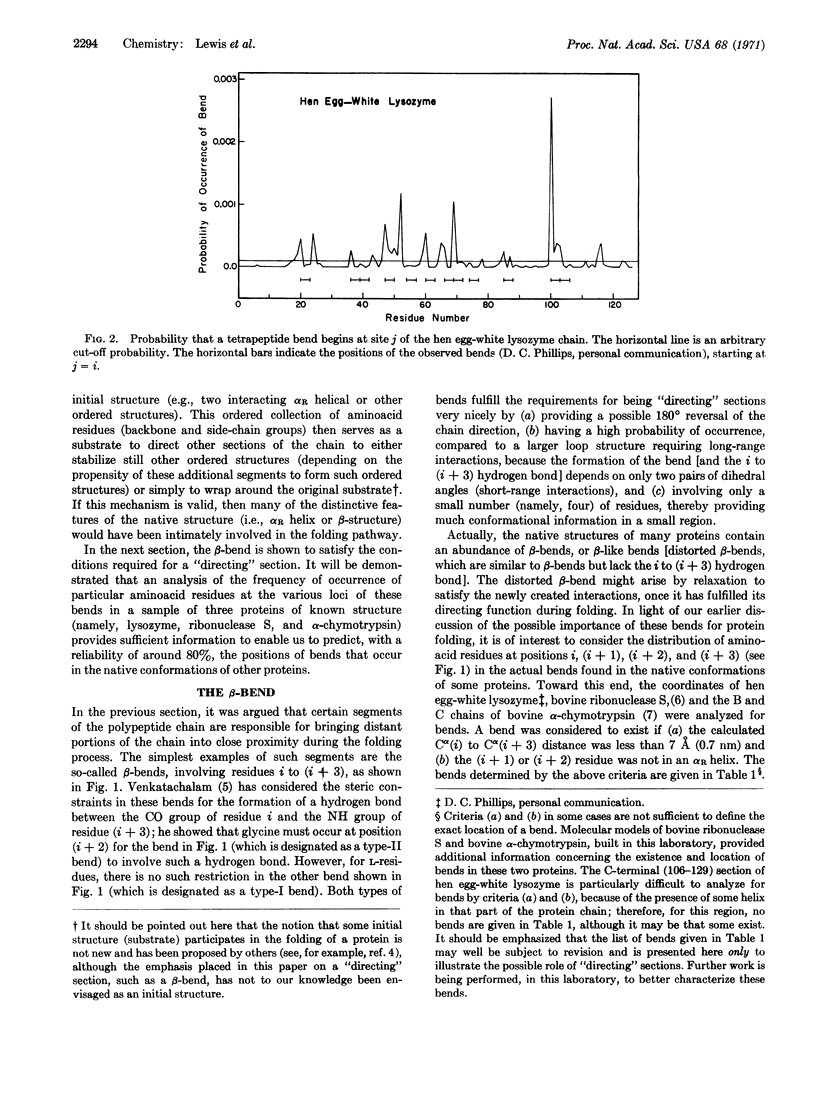
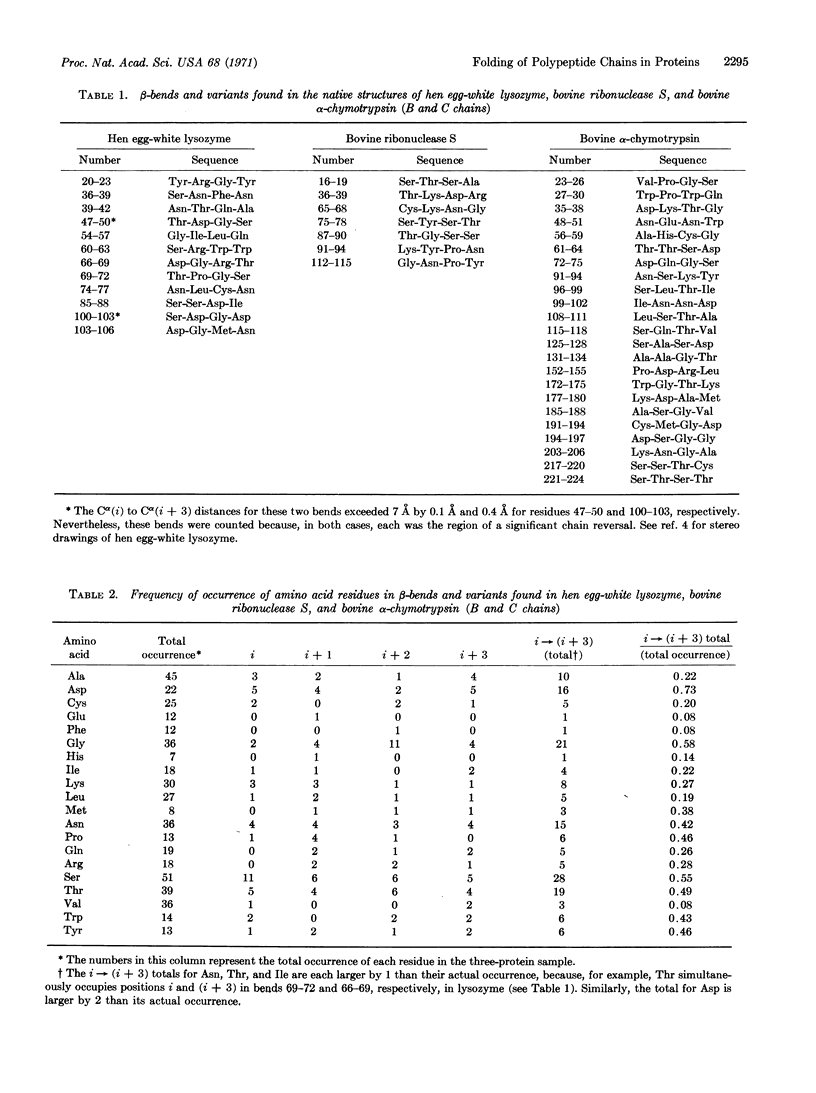
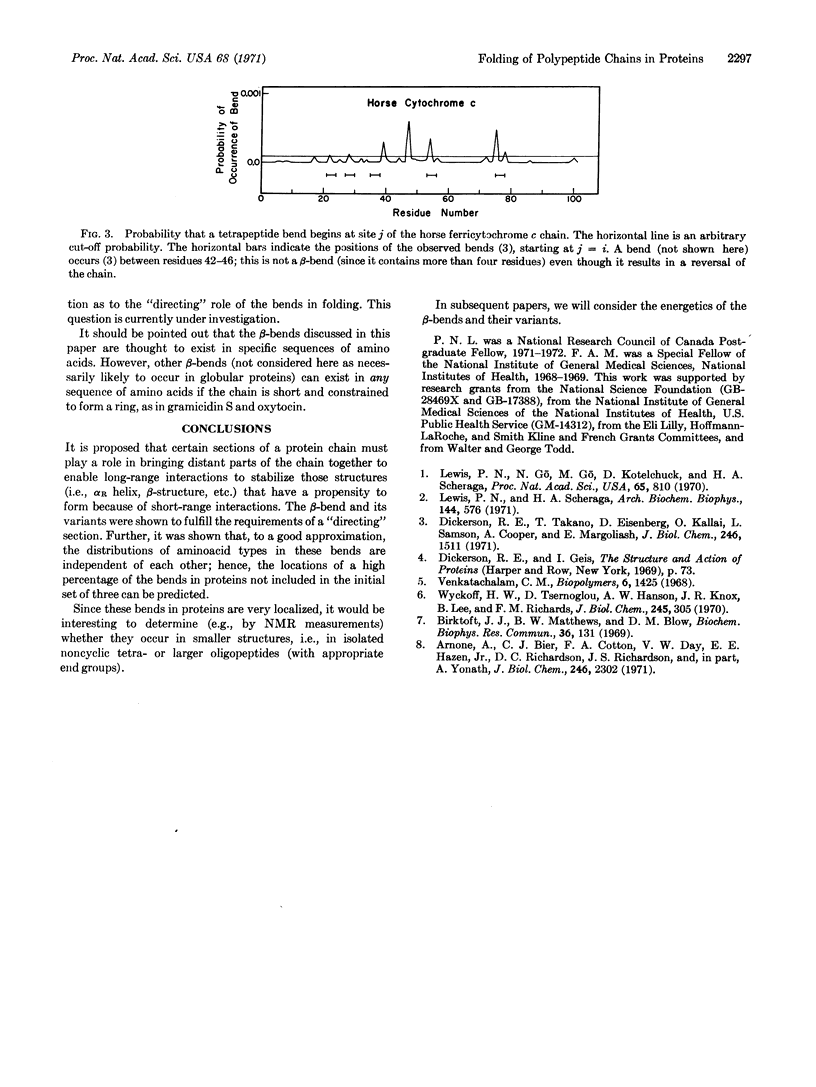
Abstract
A mechanism is proposed for the folding of protein chains. On the basis of short-range interactions, certain aminoacid sequences have a high propensity to be, say, α-helical. However, these short helical (or other ordered) regions can be stabilized only by long-range interactions arising from the proximity of two such ordered regions. These regions are brought near each other by the directing influence of certain other aminoacid sequences that have a high probability of forming β-bends or variants thereof, also on the basis of short-range interactions. An analysis is made of the tendency of various amino acids to occur in β-bends, and it is possible to predict the regions of a chain in which a β-bend will occur with a high degree of reliability.
Full text
PDF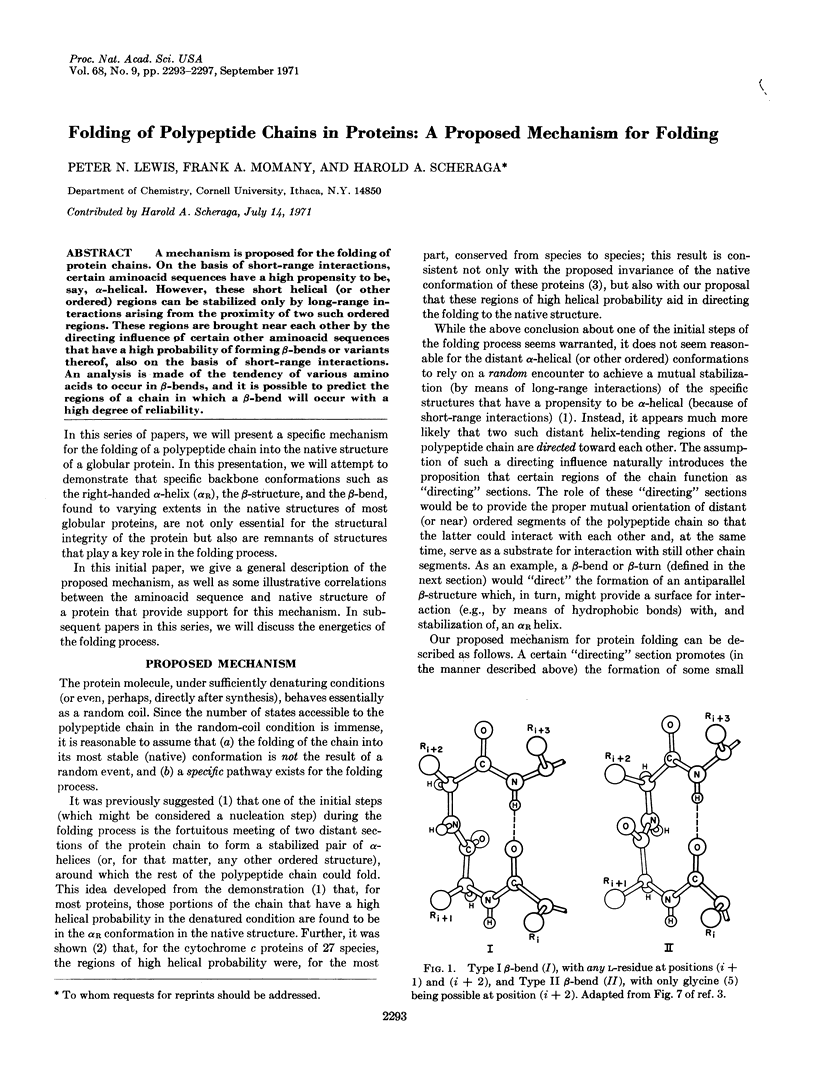

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnone A., Bier C. J., Cotton F. A., Day V. W., Hazen E. E., Jr, Richardson D. C., Yonath A., Richardson J. S. A high resolution structure of an inhibitor complex of the extracellular nuclease of Staphylococcus aureus. I. Experimental procedures and chain tracing. J Biol Chem. 1971 Apr 10;246(7):2302–2316. [PubMed] [Google Scholar]
- Birktoft J. J., Matthews B. W., Blow D. M. Atomic co-ordinates for tosyl-alpha-chymotrypsin. Biochem Biophys Res Commun. 1969 Jul 7;36(1):131–137. doi: 10.1016/0006-291x(69)90659-7. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Lewis P. N., Go N., Go M., Kotelchuck D., Scheraga H. A. Helix probability profiles of denatured proteins and their correlation with native structures. Proc Natl Acad Sci U S A. 1970 Apr;65(4):810–815. doi: 10.1073/pnas.65.4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. N., Scheraga H. A. Predictions of structural homologies in cytochrome c proteins. Arch Biochem Biophys. 1971 Jun;144(2):576–583. doi: 10.1016/0003-9861(71)90363-8. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Wyckoff H. W., Tsernoglou D., Hanson A. W., Knox J. R., Lee B., Richards F. M. The three-dimensional structure of ribonuclease-S. Interpretation of an electron density map at a nominal resolution of 2 A. J Biol Chem. 1970 Jan 25;245(2):305–328. [PubMed] [Google Scholar]



