This study assesses the risk of being female in addition to the well-known factors of age and apolipoprotein E ε4 status in the development and progression of Alzheimer disease based on longitudinal brain atrophy, cognitive decline, and CSF markers. APOE ε4 accelerated rates of decline, especially in women. The gender effect was at least as important as APOE ε4 status and showed weaker relationships to CSF markers.
Abstract
BACKGROUND AND PURPOSE:
Age and the apolipoprotein E ε4 allele are well-known risk factors for Alzheimer disease, but whether female sex is also a risk factor remains controversial. It is also unclear how these risk factors affect rates of structural brain and clinical decline across the spectrum of preclinical to clinical Alzheimer disease. Our objective is to estimate the effects of apolipoprotein E ε4 and sex on age-specific rates of morphometric and clinical decline in late-onset sporadic Alzheimer disease.
MATERIALS AND METHODS:
With the use of linear mixed-effects models, we examined the effect of age, apolipoprotein E ε4, and sex on longitudinal brain atrophy and clinical decline among cognitively normal older individuals and individuals with mild cognitive impairment and Alzheimer disease (total = 688). We also evaluated the relationship between these effects and CSF biomarkers of Alzheimer disease pathology.
RESULTS:
Apolipoprotein E ε4 significantly accelerated rates of decline, and women in all cohorts had higher rates of decline than men. The magnitude of the sex effect on rates of decline was as large as those of ε4, yet their relationship to measures of CSF biomarkers were weaker.
CONCLUSIONS:
These results indicate that in addition to apolipoprotein E ε4 status, diagnostic and therapeutic strategies should take into account the effect of female sex on the Alzheimer disease process.
The clinical presentation of Alzheimer disease (AD) is not uniform across individuals: in addition to atypical presentations1,2 of AD, recent results show that the disease also presents differently in older compared with younger patients.3,4 It is unclear, however, whether common genetic risk variants and sex also affect how the disease manifests and progresses.
In the United States, two-thirds of AD cases are women,5 but because women live longer than men and older age is a known risk factor for AD, there remains controversy over whether women are at greater risk of development of AD than men. Several large epidemiology studies have found evidence of higher age-specific rates of incidence6–10 and prevalence11 of AD in women compared with men, though other studies have found no difference.12,13 Elderly women, however, have higher amounts of AD pathology than elderly men,14 and women with AD perform more poorly than men on cognitive assessment.15 Assessing sex differences in age-specific cognitive and structural rates of decline may help elucidate this controversy.
The strongest known common genetic risk factor for sporadic AD is the apolipoprotein E (APOE) ε4 allele.16,17 APOE ε4 increases the age-specific risk of development of AD in a dose-dependent manner18,19 and lowers the age of onset.18,20 Recently, we showed3 that rates of both cognitive and structural decline decreased with age in individuals with mild cognitive impairment (MCI) and AD, but increased with age for the cognitively healthy elderly. Because ε4 lowers the age of onset, age differences in rates of decline may have arisen partially from differences in ε4 prevalence with age. Thus, to better understand AD biomarker trajectories, it is important to assess simultaneously the effects of ε4 and age, as well as those of sex, on rates of clinical and structural decline.
We analyzed baseline and longitudinal data from cognitively healthy elderly (HC), MCI, and mild AD cohorts, age 65–90 years. We investigated the effects of ε4 status and sex on cognitive and structural rates of change, and assessed whether such effects could be explained by baseline CSF concentrations of Aβ1–42 and the neurodegeneration-associated τ and phosphorylated τ181p (p-τ) proteins.
Materials and Methods
Participants
We examined participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI, www.adni-info.org). Participant enrollment criteria, MR image acquisition, and CSF collection and analysis methods are provided in the On-line Appendix.
We evaluated 688 participants, age ≥65 years at baseline, who had longitudinal cognitive evaluations: 211 HC, 333 patients with MCI, and 144 patients with AD. Of these, 188 HC, 273 patients with MCI, and 105 patients with AD also had longitudinal structural MR imaging data (Table 1). Longitudinal evaluations were performed at 6- or 12-month intervals for up to 24 (AD) or 36 (HC and MCI) months. The research protocol was approved by each local institutional review board, and written informed consent was obtained from each participant.
Table 1:
Demographic data for participants with longitudinal structural and clinical measures
| Diagnostic Group | ε4− |
ε4+ |
||||||
|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||
| N | Age, y (SD) | N | Age, y (SD) | N | Age, y (SD) | N | Age, y (SD) | |
| HC | 70 | 75.87 (4.63) | 67 | 76.84 (4.94) | 26 | 76.63 (5.42) | 25 | 75.67 (3.13) |
| MCIa | 74 | 78.52 (5.96) | 42 | 78.70 (4.08) | 102 | 76.07 (5.42) | 55 | 73.64 (5.34) |
| MCIcb | 23 | 78.53 (5.50) | 12 | 78.95 (3.81) | 45 | 75.51 (5.28) | 30 | 72.76 (4.97) |
| ADc | 13 | 75.51 (5.70) | 13 | 78.52 (4.21) | 42 | 75.52 (5.90) | 37 | 75.09 (5.07) |
Note:—N indicates number of participants. Values are mean (standard deviation, SD). MCIc = MCI converters to AD.
MCI: ε4+ women are significantly younger than all other groups (all P < .01); ε4+ men are significantly younger than ε4− men and ε4− women (P < .01).
MCIc: ε4+ women are significantly younger than all other groups (all P < .05); ε4+ men are significantly younger than ε4− men and ε4− women (P < .05).
AD: ε4+ women are significantly younger than ε4− women (P < .05).
MR Image Processing
We quantified anatomical regional change in serial MR imaging with the use of Quarc.21,22 We analyzed data from all available time points that passed local quality control (total = 2244). Images that had degradation caused by motion, technical problems, significant clinical abnormalities (eg, hemispheric infarction), or changes in scanner vendor during the time series were excluded.23 We examined rates of change in medial and lateral temporal lobe structures affected in early AD24–26 and in whole-brain volume.
Genetic, CSF, and Clinical Measures
We grouped participants with respect to sex and APOE ε4 status (none, ε4–, versus at least 1 ε4 allele, ε4+) (Table 1 and On-line Table 7). Baseline CSF data were available on approximately half of the ADNI participants. All participants were scored for Clinical Dementia Rating Scale, sum of boxes (CDR-SB),27,28 cognitive subscale of the Alzheimer Disease Assessment Scale (ADAS-Cog),29,30 and Mini-Mental State Examination (MMSE)31 at each visit.
Mixed-Effects Modeling
Longitudinal cognitive and structural MR imaging atrophy outcomes (Yij) represent change with respect to baseline. This is expressed as the difference in test scores for cognitive measures and as a percentage of baseline size for cortical thickness change and region of interest volume change.
With the use of all available time points per participant, we investigated the dependence of atrophy rate and rate of clinical decline on ε4 status and sex by use of a linear mixed-effects model,32 controlling for baseline age, education, and, in the case of atrophy, baseline clinical severity. For each diagnostic group, the longitudinal outcome measurement Yij at time tij for participant i at follow-up time point j is
Here, b0, bCog, bEdu, bAge, bAPOE, and bSex are group regression parameters to be determined; Ci, Di, Ai, Ei, and Si are covariates for participant i, respectively, mean-centered baseline clinical severity as measured by ADAS-Cog (for atrophic measures only: Ci 0 when Yij is a cognitive measure), mean-centered educational level (years of education), mean-centered baseline age, ε4 status (Ei = 0 for ε4–, Ei = 1 for ε4+), and sex status (Si = 0 for male, Si = 1 for female); and εij is the within-participant error, assumed to be independent and identically normally distributed with zero mean and variance σε2. The first term on the right side of Eq. (1) incorporates mixed effects, allowing for different participant-specific rates of change: b0 is the group fixed effect slope and β0i is the corresponding between-participant random effect slope, with zero mean, assumed to be normally distributed with variance σ02. Subsequent covariate terms involve fixed effects only. We estimated the model parameters (including σ0 and σε) by use of the Matlab (R2009b) function nlmefit (MathWorks, Natick, Massachusetts). A follow-up set of analyses incorporated additional terms in Equation 1 for baseline CSF Aβ and p-τ concentrations to assess whether ε4 or sex effects could be explained by CSF biomarker values.
0 when Yij is a cognitive measure), mean-centered educational level (years of education), mean-centered baseline age, ε4 status (Ei = 0 for ε4–, Ei = 1 for ε4+), and sex status (Si = 0 for male, Si = 1 for female); and εij is the within-participant error, assumed to be independent and identically normally distributed with zero mean and variance σε2. The first term on the right side of Eq. (1) incorporates mixed effects, allowing for different participant-specific rates of change: b0 is the group fixed effect slope and β0i is the corresponding between-participant random effect slope, with zero mean, assumed to be normally distributed with variance σ02. Subsequent covariate terms involve fixed effects only. We estimated the model parameters (including σ0 and σε) by use of the Matlab (R2009b) function nlmefit (MathWorks, Natick, Massachusetts). A follow-up set of analyses incorporated additional terms in Equation 1 for baseline CSF Aβ and p-τ concentrations to assess whether ε4 or sex effects could be explained by CSF biomarker values.
Results
Rates of Decline in Healthy Controls
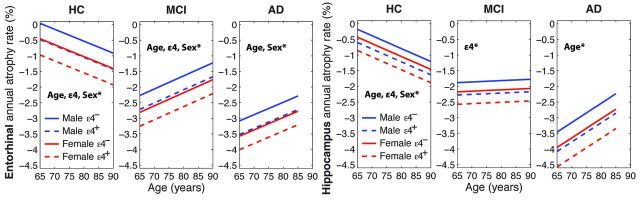
Table 2 shows the effects of age, ε4 status, and sex on rates of atrophy and clinical decline in HCs. For all brain regions, HC participants showed significant decline over time. The annual rate of change, expressed as a percentage of baseline size, ranged from −0.39%/year for the entorhinal cortex to −0.64%/year for the hippocampus (Table 2, b0 column). Older age at baseline was associated with a higher rate of change in medial temporal lobe structures, with an additional 0.04%/year loss in the hippocampus, entorhinal cortex, and amygdala for each additional year of age above the group mean (Table 2, bAge column). The presence of an ε4 allele showed a large effect on annual rate of change in the same medial temporal regions, contributing an additional −0.42%/year loss in the hippocampus, −0.52%/year loss in the entorhinal cortex, and −0.63%/year loss in the amygdala (Table 2, bAPOE column).
Table 2:
Effects of age, APOE ε4, and sex on rates of change in HC
| HC Measure | b0 | bCog | bEdu | bAge (SE; P) | bAPOE (SE; P) | bSex (SE; P) |
|---|---|---|---|---|---|---|
| Hippocampus | −0.64a | −0.06a | −0.04a | −0.04a (.01; .002) | −0.42a (.13; .002) | −0.25a (.13; .044) |
| Amygdala | −0.41a | −0.03 | −0.05 | −0.04a (.02; .028) | −0.63a (.16; 1 × 10−4) | −0.53a (.15; 5 × 10−4) |
| Entorhinal | −0.39a | −0.04 | −0.05 | −0.04a (.02; .025) | −0.52a (.17; .003) | −0.49a (.16; .002) |
| Inferior parietal | −0.50a | 0.00 | −0.01 | −0.01 (.01; .5) | −0.14 (.10; .2) | 0.03 (.10; .8) |
| Middle temporal | −0.61a | −0.02 | −0.02 | 0.00 (.01; .7) | −0.19 (.12; .1) | −0.02 (.11; .9) |
| Med-orbito-frontal | −0.48a | −0.03a | −0.01 | −0.01 (.01; .5) | −0.18a (.09; .050) | −0.03 (.09; .8) |
| Whole brain | −0.41a | 0.00 | −0.01 | 0.00 (.01; .8) | −0.08 (.06; .2) | −0.03 (.06; .7) |
| CDR-SB | 0.10a | – | −0.01 | 0.00 (.00; .4) | 0.01 (.05; .9) | −0.02 (.04; .6) |
| ADAS-Cog | −0.29a | – | −0.04 | 0.05a (.02; .008) | 0.27 (.20; .2) | −0.21 (.18; .2) |
| MMSE | 0.02 | – | −0.02 | −0.02a (.01; .009) | −0.14 (.08; .1) | −0.06 (.08; .4) |
Note:—b-Values are coefficients in Equation 1; for structural measures, units are annual thickness or volume change as a percentage of baseline size (%/year), and for cognitive measures they are annual score change, per ADAS-Cog unit in the case of bCog, and per year in the case of bEdu and bAge. ROIs: N = 188; mean age = 76.30 years; mean ADAS-Cog = 6.17; mean years education = 16.02. Clinical: N = 211; mean age = 76.35 years; mean years education = 16.03. SE indicates standard error; Med-orbito-frontal, medial orbito-frontal cortex.
Values significant at P ≤ .05.
Values in the b0 column show the expected rate of change for an APOE ε4−negative male subject of mean age, mean education, and with a mean level of cognitive function. The remaining columns show the additional rate of change caused by the other factors of interest, and the amount of change experienced by a given individual can be calculated on the basis of the sum of the relevant coefficients. For example, for hippocampal atrophy, each point above the mean baseline ADAS-Cog score contributes an additional 0.06% to the annual atrophy rate; each year of education below the mean contributes an additional 0.04% to annual atrophy rate, as does each year of age above the mean at baseline; presence of an APOE ε4 allele contributes an additional 0.42% to rate of decline, and female sex contributes an additional 0.25%. Thus, an APOE ε4+ female subject, of mean age, education, and cognitive function at baseline, would show a hippocampal atrophy rate of 1.31% (0.64 + 0.42 + 0.25).
Sex significantly affected rate of change (Table 2, bSex column), with women showing higher rates of change than men for the hippocampus (an additional −0.25%/year), the entorhinal cortex (−0.49%/year), and the amygdala (−0.53%/year).
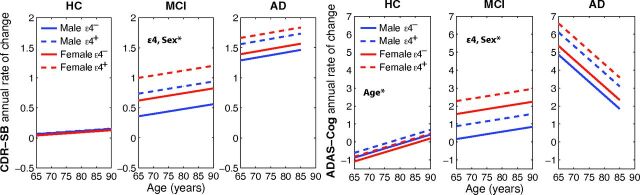
In contrast to the strong effects of ε4 and sex on medial temporal atrophy rates, we did not find a significant association between these factors and rate of decline on any of the clinical measures in HCs.
The effects of age, ε4, and sex on rates of decline in the entorhinal cortex and hippocampus are shown in Fig 1 for the HC, MCI, and AD cohorts, at the group average ages, educational levels, and ADAS-Cog scores. Fig 2 shows the effects of age, ε4, and sex on rates of decline in CDR-SB and ADAS-Cog for the 3 cohorts, at the group average ages and educational levels.
Fig 1.
Entorhinal and hippocampal annual atrophy rates with respect to age for HC, MCI, and AD participants at their group mean educational level and cognitive performance. Where significant, effects of age (slope), ε4 and sex (shifts along y-axis) are noted by *. Also see Tables 2–4.
Fig 2.
Annual rates of cognitive decline, measured with CDR-SB and ADAS-Cog, with respect to age for HC, MCI, and AD participants at their group mean educational level. Where significant, effects of age (slope), ε4 and sex (shifts along y-axis) are noted by *. Also see Tables 2–4.
Rates of Decline in MCI
Table 3 shows the effects of age, ε4 status, and sex on atrophy rates and rates of clinical decline in the MCI cohort. With the exception of the hippocampus and amygdala, increased age was associated with a slower rate of decline (bAge coefficients are positive) for all brain regions examined. Significant effects of ε4 status were observed for all medial temporal lobe structures and for the inferior parietal cortex, with the additive effect of ε4 on annual atrophy rate ranging from −0.28%/year to −0.94%/year. Independent of ε4, sex significantly affected rate of change in all brain regions examined, except for the hippocampus: Women atrophied faster than did men, with the magnitude of the additive effect exceeding that of the ε4 effect.
Table 3:
Effects of age, APOE ε4, and sex on rates of change in MCI
| MCI Measure | b0 | bCog | bEdu | bAge (SE; P) | bAPOE (SE; P) | bSex (SE; P) |
|---|---|---|---|---|---|---|
| Hippocampus | −1.83a | −0.13a | 0.03 | 0.00 (.02; .8) | −0.40a (.20; .045) | −0.29 (.20; .1) |
| Amygdala | −1.57a | −0.15a | 0.01 | 0.03 (.02; .1) | −0.94a (0.21; 7 × 10−6) | −0.98a (.21; 2 × 10−6) |
| Entorhinal | −1.78a | −0.12a | 0.00 | 0.04a (.02; .006) | −0.44a (.17; .011) | −0.54a (.17; .002) |
| Inferior parietal | −0.91a | −0.08a | 0.02 | 0.06a (.01; 2 × 10−6) | −0.28a (.14; .040) | −0.40a (.14; .004) |
| Middle temporal | −1.40a | −0.11a | 0.00 | 0.07a (.02; 9 × 10−6) | −0.28 (.18; .1) | −0.52a (.17; .003) |
| Med-orbito-frontal | −0.78a | −0.04a | 0.04a | 0.02a (.01; .023) | 0.03 (.11; .8) | −0.24a (.11; .026) |
| Whole brain | −0.74a | −0.04a | 0.01 | 0.02a (.01; 4 × 10−4) | −0.09 (.08; .2) | −0.17a (.08; .22) |
| CDR-SB | 0.46a | – | 0.01 | 0.01 (.01; .4) | 0.38a (.11; 6 × 10−4) | 0.26a (.11; .021) |
| ADAS-Cog | 0.49a | – | 0.00 | 0.03 (.03; .3) | 0.72a (.31; .022) | 1.40a (.32; 2 × 10−5) |
| MMSE | −0.35a | – | 0.02 | 0.02 (.02; .4) | −0.81a (.20; 4 × 10−5) | −0.34 (.20; .1) |
Note:—See Table 2 for units and key.
Values significant at P ≤ .05.
ROIs: N = 273; mean age = 76.65 years; mean ADAS-Cog = 11.68; mean years education = 15.61. Cognitive: N = 211; mean age = 76.84 years; mean years education = 15.63.
Significant ε4 additive contributions to rates of cognitive decline were found for CDR-SB (0.38 points/year), ADAS-Cog (0.72 points/year), and MMSE (−0.81 points/year), whereas effects of female sex were significant for CDR-SB (0.26 points/year) and ADAS-Cog (1.40 points/year).
Rates of Decline in AD
Table 4 shows the effects of age, ε4 status, and sex on rates of atrophy and clinical decline in AD participants. The effect of age on rates of change was significant for all brain regions examined, with increased age associated with lower rates of decline. The additive contribution to rate of decline for ε4 was significant only for the amygdala (−0.91%/year) but showed a trend toward significance for the hippocampus and entorhinal cortex. Significant sex effects were found for all regions except for the hippocampus and amygdala, with women having higher rates of decline. There were no significant effects of ε4 status or sex on rate of decline on any of the cognitive measures.
Table 4:
Effects of age, APOE ε4, and sex on rates of change in AD
| AD Measure | b0 | bCog | bEdu | bAge (SE; P) | bAPOE (SE; P) | bSex (SE; P) |
|---|---|---|---|---|---|---|
| Hippocampus | −2.80a | −0.06a | 0.03 | 0.06a (.03; .028) | −0.62 (.35; .08) | −0.49 (.30; .1) |
| Amygdala | −2.73a | −0.05 | 0.06 | 0.06a (.03; .043) | −0.91a (.36; .012) | −0.41 (.31; .2) |
| Entorhinal | −2.65a | −0.04 | −0.02 | 0.04a (.02; .045) | −0.43 (.25; .09) | −0.49a (.22; .025) |
| Inferior parietal | −1.68a | −0.06a | −0.03 | 0.15a (.02; <10−6) | −0.25 (.24; .3) | −0.69a (.21; .001) |
| Middle temporal | −2.48a | −0.10a | −0.05 | 0.17a (.02; <10−6) | −0.30 (.29; .3) | −0.88a (.25; .001) |
| Med-orbito-frontal | −0.96a | −0.02 | −0.02 | 0.05a (.02; .008) | 0.04 (.24; .9) | −0.64a (.21; .002) |
| Whole brain | −0.97a | −0.04a | −0.01 | 0.06a (.01; <10−6) | −0.19 (.14; .2) | −0.38a (.12; .002) |
| CDR-SB | 1.39a | – | 0.11a | 0.01 (.03; .8) | 0.27 (.33; .4) | 0.10 (.29; .7) |
| ADAS-Cog | 3.20a | – | 0.29 | −0.15 (.08; .069) | 1.25 (.98; .2) | 0.49 (.89; .6) |
| MMSE | −1.97a | – | −0.16 | 0.13a (.05; .007) | −0.20 (.57; .7) | 0.03 (.52; 1.0) |
Note:—See Table 2 for units and key.
Values significant at P ≤ .05.
ROIs: N = 105; mean age = 75.74 years; mean ADAS-Cog = 18.49; mean years education = 14.83. Cognitive: N = 144; mean age = 75.99 years; mean years education = 14.70.
Effects of APOE ε4 and Sex on Baseline CSF and Clinical Measures
Controlling for age and sex, ε4 carriers showed significantly lower CSF Aβ concentrations than noncarriers, with the magnitude of the effect decreasing from HC to patients with MCI to those with AD (Fig 3 and On-line Table 5A). Relative to noncarriers, ε4 carriers showed significantly higher CSF concentrations of τ and p-τ in the HC and MCI cohorts, but no significant differences were found for these biomarkers in the AD cohort.
Fig 3.
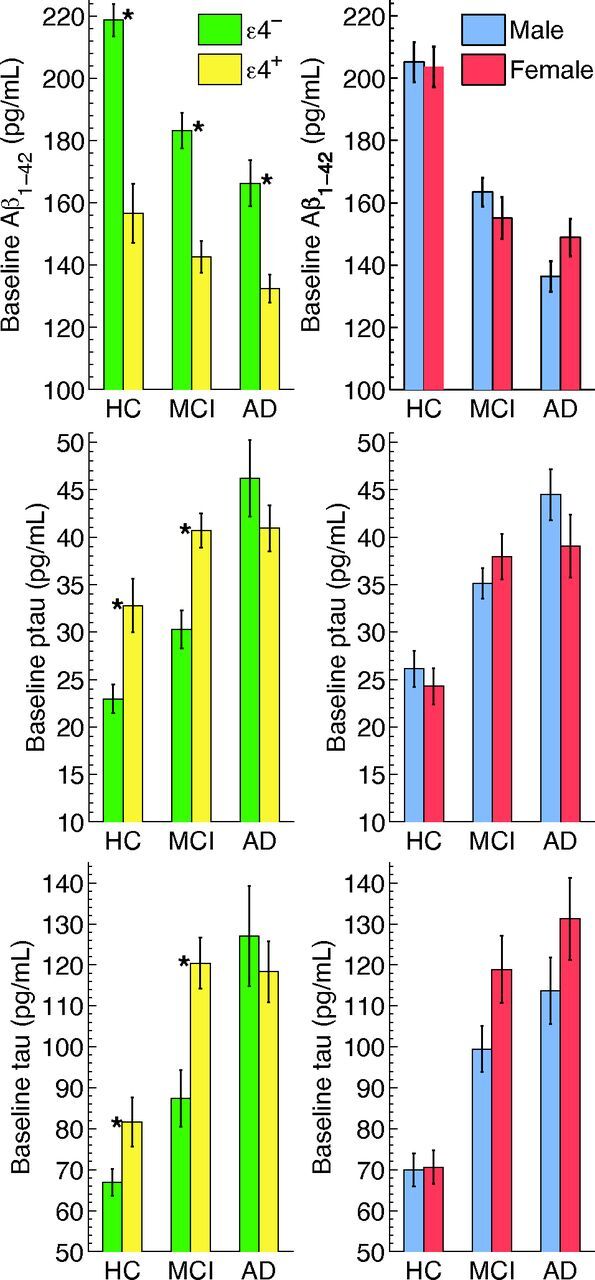
Baseline CSF values for Aβ, p-τ, and τ, by ε4 status (left) and sex (right) for the HC, MCI, and AD cohorts. Numeric values are in On-line Table 5A. *Significant differences.
Controlling for age and ε4 status, there were no significant effects of sex on CSF Aβ or p-τ concentrations in any of the cohorts (Fig 3 and On-line Table 5A). For τ, the effect of sex approached significance for the MCI cohort only (P = .060), with women showing higher τ concentrations than men.
Controlling for age and sex, performance on the clinical tests was significantly affected by ε4 status in MCI participants only, with carriers showing worse performance than noncarriers for CDR-SB and ADAS-Cog, and showing a trend for worse performance on MMSE (On-line Table 5A and On-line Fig 1). Controlling for age and ε4 status, no sex differences were found on the clinical tests in the patient cohorts, though MCI showed a trend toward significance for MMSE (P = .072), with women performing more poorly.
Effects of Baseline CSF Aβ and p-τ on Rates of Decline
With Aβ in the model, significant effects of ε4 and sex remained for MCI and AD participants, signifying that APOE ε4 exerts an effect on atrophy rate in AD independent of its relation to Aβ (On-line Tables 3A–C). For HCs, however, there were no significant effects of ε4 with CSF Aβ in the model. Adding an additional term for p-τ concentrations did not alter these results (On-line Tables 4A–C), but this term was found to be significant in MCI for the amygdala, entorhinal cortex, ADAS-Cog, and MMSE, and in AD for the entorhinal cortex, rendering the Aβ term insignificant for all measures.
Discussion
Our results show that changes in brain structure and function related to aging and AD do not progress uniformly across individuals but instead depend on age, sex, and APOE ε4 status. Age differences in progressive atrophy and clinical decline, whereby older patients with MCI and AD decline at a slower rate than younger patients but older healthy adults decline at a faster rate than younger healthy adults, have been previously reported.3,33 However, our finding that sex differences in atrophy rates are as large as differences associated with the well-known genetic risk factor, APOE ε4, is novel, and has important implications for clinical practice, therapeutics research, and for advancing mechanistic understanding of AD.
The results showed that in all stages, from healthy aging through AD dementia, women had higher rates of brain atrophy than men, and the magnitude of the sex differences was at least as large as the magnitude of the APOE ε4 effects. In HCs, sex differences were restricted to the medial temporal areas first affected in AD. In MCI and AD, the sex differences were more widespread, with weaker effects observed in medial temporal areas than in other brain regions. Additionally in MCI, in women compared with men, higher rates of atrophy were accompanied by higher rates of clinical decline.
These findings are consistent with prior large epidemiology studies5–7,11,34 that showed higher rates of prevalence and incidence of AD in women than in men, with the differences between men and women comparable in magnitude to those between ε4 carriers and noncarriers. They are also consistent with a recent meta-analysis that found lower cognitive performance for women than men diagnosed with AD.15 A neuropathologic study35 showed that women, especially if ε4 carriers, are at higher risk of both neurofibrillary tangle (NFT) and amyloid plaque neuropathology than men in the earliest stages of AD (NFT stages I–III26).
One possible explanation for the sex differences in HCs, in which women showed faster rates of atrophy in medial temporal areas, is that the HC women may be showing early signs of AD-related neurodegeneration. However, the lack of sex differences in baseline CSF biomarkers of AD pathology in HCs does not support this view. The finding that CSF biomarkers did not explain the faster rates of decline occurring in women in any of the diagnostic groups suggests that other factors must be contributing to the sex differences. It has been argued that estrogens stimulate α-secretase activity and thus enhance nonamyloidogenic processing of amyloid-β precursor protein36,37; the diminution in estrogen levels after menopause would then contribute to higher levels of AD pathology and poorer cognitive performance in women than in men. However, further research is needed to elucidate the basis of the observed sex differences.
The APOE ε4 effects observed in the present study on longitudinal rates of change across cohorts are consistent with the elevated burdens of amyloid and τ pathology observed for ε4 carriers compared with noncarriers at baseline. These baseline differences in CSF biomarkers between carriers and noncarriers agree with earlier reports38,39 and with neuropathologic findings that ε4 was associated with greater senile plaque and neurofibrillary tangle pathology in the elderly.14 APOE ε4 has further been associated with a higher plaque stage for a given age and allocortical NFT stage (Braak stages I–III, which correspond roughly with HC and early MCI) for ε4 carriers compared with noncarriers, whereas at the later isocortical NFT stages (corresponding to late MCI and dementia), ε4 gene dose was not an important predictor of pathology burden,35,40 suggesting that ε4 might exert its strongest effects in the prodromal stages of AD. Recently, Koffie et al41 have shown that the ε4 gene increases the amount of the synaptotoxic oligomeric Aβ in neuropil and its colocalization at synapses, even in nondemented control subjects, leading to synaptic injury and loss, a strong correlate of cognitive decline.42 Our results showing elevated atrophy in ε4 carriers generally, and our finding of marginally significant higher atrophy rates in predementia stages of AD for the medial orbito-frontal cortex43 and inferior parietal lobule, sites of early amyloid deposition,26 are consistent with these neuropathologic findings.
How ε4 affects rates of cognitive decline across the preclinical, prodromal, and dementia stages of AD has been unclear,20,44,45 but some studies have suggested that the effect of ε4 is stronger in the earlier phases of the disorder.39,46,47 Our results suggest that the accelerating effect of ε4 on rates of decline diminishes with advancing disease stage, which comports with an earlier finding that ε4 gene dose does not have a significant effect on the duration of AD,20 and supports the hypothesis that as neurodegeneration advances, it becomes increasingly independent of initiating events.48
This study has several limitations: The ADNI sample is not representative of the general population, and there was sex bias in MCI enrollment, with men outnumbering women. The HC and AD cohorts, however, showed more balanced sex representation. Because similar sex effects were observed across groups, they are unlikely to have arisen from enrollment bias. There is insufficient information within ADNI to address issues of whether history of hormone replacement therapy or number of years since menopause may have influenced the observed sex differences. Finally, statistical power was limited with respect to analyses of CSF biomarker data. Larger population-based studies that can systematically address hormonal issues, and other medical issues that may differ between the sexes, are needed to elucidate the basis of the observed sex differences in rate of atrophy and cognitive decline.
Conclusions
Our results show that women and APOE ε4 carriers in ADNI have higher rates of decline in normal aging, MCI, and AD, and that these effects are not fully explained by baseline CSF concentrations of AD-related proteins. Because two-thirds of AD cases in the United States are women, and because the higher rates of decline in women compared with men were at least as large as those related to the major genetic risk factor, APOE ε4, it is of particular importance that sex differences in rates of decline in aging and AD be taken into account in the clinical setting and in therapeutics research. Greater understanding of the mechanistic basis of these differences likely will facilitate further understanding of AD etiology.
Supplementary Material
ABBREVIATIONS:
- AD
Alzheimer disease
- ADAS-Cog
cognitive subscale of the Alzheimer Disease Assessment Scale
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- APOE
apolipoprotein E
- CDR-SB
Clinical Dementia Rating Scale, sum of boxes
- HC
cognitively healthy elderly
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- NFT
neurofibrillary tangle
- p-τ
phosphorylated τ
Footnotes
Disclosures: Dominic Holland—RELATED: Grant: NIH NIA,* Comments: This research was supported by NIH grants R01AG031224, R01AG22381, U54NS056883, P50NS22343, and P50MH081755 (A.M.D.); NIA K01AG029218 (LKM); NIH-NIBIB T32 EB005970 (RSD); UNRELATED: Grants/Grants Pending: NIH*; Patents (planned, pending or issued): UCSD,* Comments: A patent application for Quarc, a method to quantify anatomical regional change developed at UCSD, has been filed through the UCSD Technology Transfer Office (“Longitudinal registration of anatomy in magnetic resonance imaging”; Application number: 12/742,642; Publication number: US 2010/0259263 A1). Rahul Desikan—RELATED: Grant: T32 EB005970,* Comments: NIH Training Grant. Anders Dale—RELATED: Grant: UCSD,* Comments: Numerous NIH grants as PI and Co-I; UNRELATED: Grants/Grants Pending: UCSD*; Patents (planned, pending or issued): UCSD,* MGH,* Comments: I am an inventor of a provisional patent on Restriction Spectrum Imaging, and other issued patents in the areas of MRI acquisition and post-processing; Stock/Stock Options: CorTechs Labs Inc, Comments: I am a founder and hold equity in CorTechs Labs, Inc, and also serve on its Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by UCSD in accordance with its conflict-of-interest policies; Other: UCSD* Comments: I am the principal investigator of a research agreement between UCSD and General Electric Medical Systems. Linda McEvoy—RELATED: Grant: National Institute on Aging,* Comments: Grant support from K01AG029218, R01AG22381; Support for Travel to Meetings for the Study or Other Purposes: National Institute on Aging,* Comments: Grants listed above used to support presentation of data at 2012 Alzheimer's Association International conference (*money paid to institution).
A.M.D. is a founder and holds equity in CorTechs Labs, Inc, and also serves on its Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies. McEvoy's spouse is President of CorTechs Labs, Inc.
Data used in preparation of this article were obtained from the ADNI database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
This research was supported by NIH grants R01AG031224, R01AG22381, U54NS056883, P50NS22343, and P50MH081755 (A.M.D.); NIA K01AG029218 (L.K.M.); NIH-NIBIB T32 EB005970 (R.S.D.).
REFERENCES
- 1. Duker AP, Espay AJ, Wszolek ZK, et al. Atypical motor and behavioral presentations of Alzheimer disease: a case-based approach. Neurologist 2012;18:266–72 [DOI] [PubMed] [Google Scholar]
- 2. Murray ME, Graff-Radford NR, Ross OA, et al. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 2011;10:785–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holland D, Desikan RS, Dale AM, et al. Rates of decline in Alzheimer disease decrease with age. PLoS One 2012;7:e42325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Flier WM, Pijnenburg YA, Fox NC, et al. Early-onset versus late-onset Alzheimer's disease: the case of the missing APOE varepsilon4 allele. Lancet Neurol 2011;10:280–88 [DOI] [PubMed] [Google Scholar]
- 5. Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers Dement 2013;9:208–45 [DOI] [PubMed] [Google Scholar]
- 6. Miech RA, Breitner JC, Zandi PP, et al. Incidence of AD may decline in the early 90s for men, later for women: the Cache County study. Neurology 2002;58:209–18 [DOI] [PubMed] [Google Scholar]
- 7. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis: APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997;278:1349–56 [PubMed] [Google Scholar]
- 8. Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer's disease: the impact of mortality on risk estimates in the Framingham Study. Neurology 1997;49:1498–504 [DOI] [PubMed] [Google Scholar]
- 9. Andersen K, Launer LJ, Dewey ME, et al. Gender differences in the incidence of AD and vascular dementia: the EURODEM Studies: EURODEM Incidence Research Group. Neurology 1999;53:1992–97 [DOI] [PubMed] [Google Scholar]
- 10. Plassman BL, Langa KM, McCammon RJ, et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol 2011;70:418–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology 1999;53:321–31 [DOI] [PubMed] [Google Scholar]
- 12. Barnes LL, Wilson RS, Schneider JA, et al. Gender, cognitive decline, and risk of AD in older persons. Neurology 2003;60:1777–81 [DOI] [PubMed] [Google Scholar]
- 13. Hebert LE, Scherr PA, McCann JJ, et al. Is the risk of developing Alzheimer's disease greater for women than for men? Am J Epidemiol 2001;153:132–36 [DOI] [PubMed] [Google Scholar]
- 14. Ghebremedhin E, Schultz C, Thal DR, et al. Gender and age modify the association between APOE and AD-related neuropathology. Neurology 2001;56:1696–701 [DOI] [PubMed] [Google Scholar]
- 15. Irvine K, Laws KR, Gale TM, et al. Greater cognitive deterioration in women than men with Alzheimer's disease: a meta analysis. J Clin Exp Neuropsychol 2012;34:989–98 [DOI] [PubMed] [Google Scholar]
- 16. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–23 [DOI] [PubMed] [Google Scholar]
- 17. Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 2011;10:241–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sando SB, Melquist S, Cannon A, et al. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer's disease: a case control study from central Norway. BMC Neurol 2008;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khachaturian AS, Corcoran CD, Mayer LS, et al. Apolipoprotein E epsilon4 count affects age at onset of Alzheimer disease, but not lifetime susceptibility: the Cache County Study. Arch Gen Psychiatry 2004;61:518–24 [DOI] [PubMed] [Google Scholar]
- 20. Corder EH, Saunders AM, Strittmatter WJ, et al. Apolipoprotein E, survival in Alzheimer's disease patients, and the competing risks of death and Alzheimer's disease. Neurology 1995;45:1323–28 [DOI] [PubMed] [Google Scholar]
- 21. Holland D, McEvoy LK, Dale AM. Unbiased comparison of sample size estimates from longitudinal structural measures in ADNI. Hum Brain Mapp 2012;33:2586–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holland D, Dale AM. Nonlinear registration of longitudinal images and measurement of change in regions of interest. Med Image Anal 2011;15:489–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holland D, McEvoy LK, Desikan RS, et al. Enrichment and stratification for predementia Alzheimer disease clinical trials. PloS One 2012;7:e47739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology 1992;42:1681–88 [DOI] [PubMed] [Google Scholar]
- 25. Arnold SE, Hyman BT, Flory J, et al. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex 1991;1:103–16 [DOI] [PubMed] [Google Scholar]
- 26. Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 1997;18:351–57 [DOI] [PubMed] [Google Scholar]
- 27. Williams MM, Storandt M, Roe CM, et al. Progression of Alzheimer's disease as measured by Clinical Dementia Rating Sum of Boxes scores. Alzheimers Dement 2013;9(1 Suppl):S39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–14 [DOI] [PubMed] [Google Scholar]
- 29. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–64 [DOI] [PubMed] [Google Scholar]
- 30. Rockwood K, Fay S, Gorman M, et al. The clinical meaningfulness of ADAS-Cog changes in Alzheimer's disease patients treated with donepezil in an open-label trial. BMC Neurol 2007;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 32. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, New Jersey: Wiley; 2011 [Google Scholar]
- 33. Bernick C, Cummings J, Raman R, et al. Age and rate of cognitive decline in Alzheimer disease: implications for clinical trials. Arch Neurol 2012;69:901–05 [DOI] [PubMed] [Google Scholar]
- 34. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29:125–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Corder EH, Ghebremedhin E, Taylor MG, et al. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci 2004;1019:24–28 [DOI] [PubMed] [Google Scholar]
- 36. Yaffe K, Haan M, Byers A, et al. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology 2000;54:1949–54 [DOI] [PubMed] [Google Scholar]
- 37. Xu H, Gouras GK, Greenfield JP, et al. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med 1998;4:447–51 [DOI] [PubMed] [Google Scholar]
- 38. Prince JA, Zetterberg H, Andreasen N, et al. APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurology 2004;62:2116–18 [DOI] [PubMed] [Google Scholar]
- 39. Kester MI, Blankenstein MA, Bouwman FH, et al. CSF biomarkers in Alzheimer's disease and controls: associations with APOE genotype are modified by age. J Alzheimers Dis 2009;16:601–07 [DOI] [PubMed] [Google Scholar]
- 40. Walker LC, Pahnke J, Madauss M, et al. Apolipoprotein E4 promotes the early deposition of Abeta42 and then Abeta40 in the elderly. Acta Neuropathol 2000;100:36–42 [DOI] [PubMed] [Google Scholar]
- 41. Koffie RM, Hashimoto T, Tai HC, et al. Apolipoprotein E4 effects in Alzheimer's disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain 2012;135:2155–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol 1990;27:457–64 [DOI] [PubMed] [Google Scholar]
- 43. Van Hoesen GW, Parvizi J, Chu CC. Orbitofrontal cortex pathology in Alzheimer's disease. Cereb Cortex 2000;10:243–51 [DOI] [PubMed] [Google Scholar]
- 44. Saunders AM. Apolipoprotein E and Alzheimer disease: an update on genetic and functional analyses. J Neuropathol Exp Neurol 2000;59:751–58 [DOI] [PubMed] [Google Scholar]
- 45. Growdon JH, Locascio JJ, Corkin S, et al. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer's disease. Neurology 1996;47:444–48 [DOI] [PubMed] [Google Scholar]
- 46. Cosentino S, Scarmeas N, Helzner E, et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology 2008;70:1842–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Juva K, Verkkoniemi A, Viramo P, et al. APOE epsilon4 does not predict mortality, cognitive decline, or dementia in the oldest old. Neurology 2000;54:412–15 [DOI] [PubMed] [Google Scholar]
- 48. Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol 2011;68:1062–64 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.