Abstract
We have previously generatedconvincing evidence that combinations of N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine (PEITC-NAC, 3 μmol/g diet) and myo-inositol (MI, 56 μmol/g diet) were significantly more effective than the individual compounds as inhibitors of tobacco smoke carcinogen-induced lung tumorigenesis in A/J mice. In this study, we further investigated the efficacy of combinations of PEITC-NAC (9 or 15 μmol/g diet) and MI (56 μmol/g diet). Female A/J mice were treated with a mixture of the tobacco smoke carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and benzo[a]pyrene (BaP) by gavage once weekly for eight weeks. PEITC-NAC plus MI was given in the diet beginning at one day after the 4th of eight carcinogen treatments (temporal sequence A) or one week after the last carcinogen treatment (temporal sequence B). Regardless of the dose of carcinogen or PEITC-NAC plus MI, or temporal sequence, administration of PEITC-NAC plus MI significantly reduced the multiplicity of gross tumors and, in most instances, adenocarcinoma. PEITC-NAC plus MI was particularly effective against bigger tumors. The observed inhibition of lung tumorigenesis by PEITC-NAC plus MI was attributed, at least partly, to inhibition of cell proliferation and induction of apoptosis. These results clearly demonstrate the efficacy of PEITC-NAC plus MI in the prevention of tobacco carcinogen-induced lung adenocarcinoma in A/J mice and provide a basis for future evaluation of PEITC-NAC plus MI in clinical trials as a chemopreventive agent for current and former smokers.
Keywords: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, benzo[a]pyrene, lung tumorigenesis, chemoprevention, N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine, myo-inositol
Introduction
Lung cancer is the leading cause of cancer death in the U.S. and worldwide, annually killing about 160,000 people in the U.S. and 1.2 million in the world (1, 2). Approximately 90% of this mortality is caused by cigarette smoking (3). Prevention of initiation of smoking and cessation of smoking are the best approaches to prevent lung cancer. However, tobacco control has not been uniformly successful and there are still 45 million smokers in the U.S. and over 1200 million worldwide (4). An alternate approach to lung cancer prevention is chemoprevention but there are no agents with proven efficacy available at the present time (5).
Our goal is to develop chemopreventive agents for smokers who are unable to quit in spite of their best efforts, and for ex-smokers. Unfortunately, even the best smoking cessation programs have poor success rates, seldom exceeding 25% cessation after one year (6). Smokers who fail in these programs are certainly logical candidates for chemoprevention as are ex-smokers, whose risk for lung cancer is elevated for many years after quitting (7). Our target for chemoprevention is cigarette smoke carcinogens, the cause of lung cancer in smokers. While there are over 60 established carcinogens in cigarette smoke, it is widely agreed that two types- tobacco-specific nitrosamines, typified by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), and polycyclic aromatic hydrocarbons, typified by benzo[a]pyrene (BaP)- are among the chief causes of lung cancer (3, 8). Therefore, we have devoted our studies to neutralizing the carcinogenic effects of NNK and BaP, using animal models of lung tumor induction by these compounds (9–12). While it could be argued that induction of lung cancer by tobacco smoke itself would be a better model, there are severe practical limitations of such an approach, and all models that have been developed have problems of various types (13). The complexity of tobacco smoke carcinogenesis strongly suggests that a mixture of chemopreventive agents will be necessary for successful inhibition of lung cancer. Therefore, the development of an efficacious mixture has been our priority. We have focused on naturally occurring compounds present in commonly consumed vegetables, as it appears logical that these would have limited toxicity when used at appropriate doses. Furthermore, many studies indicate that vegetable consumption is associated with decreased risk for lung cancer (14).
Beginning in the 1980s, in collaborative studies led by Chung and others, we discovered that 2-phenethyl isothiocyanate (PEITC), a naturally occurring compound found as a conjugate in watercress and several other cruciferous vegetables, was a powerful inhibitor of lung cancer induction by NNK in rats and mice, but it had little effect on carcinogenesis by BaP (11, 15–19). The major mechanism by which PEITC inhibits NNK carcinogenesis is blocking of the metabolic activation of NNK, and this can be traced mainly to inhibition by PEITC of specific cytochrome P450 enzymes (19, 20). While PEITC was shown to be effective only when administered before or during NNK treatment, later studies by Chung et al demonstrated that a major metabolite of PEITC, N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine (PEITC-NAC), was as effective as PEITC as an inhibitor of lung tumorigenesis by NNK (21) while being less irritating and pungent than PEITC. PEITC-NAC inhibited lung tumor development when given to mice following BaP treatment (22), and was also an effective inhibitor of lung tumor progression in mice treated with NNK plus BaP (23). Collectively, these results indicated that PEITC-NAC, which dissociates to PEITC in vivo, might have even superior chemopreventive qualities to those of PEITC.
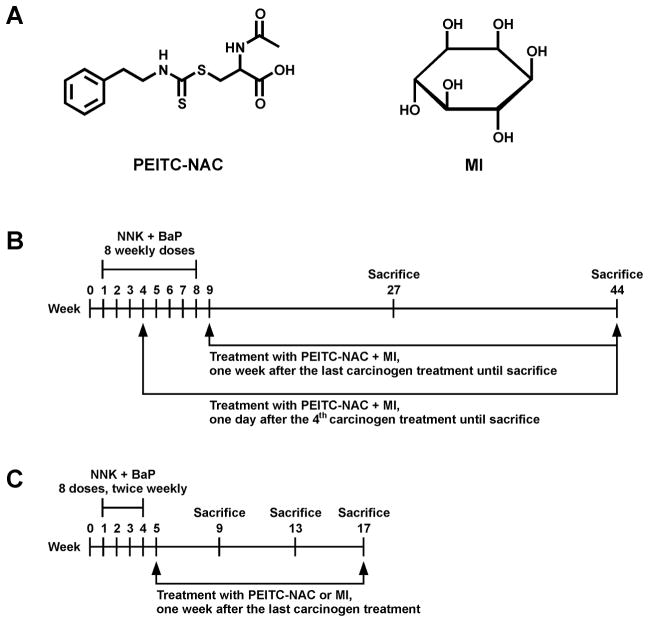
Wattenberg was the first to demonstrate that myo-inositol (MI) is an effective chemopreventive agent against lung tumor development in mice treated with BaP or NNK (24–26). MI inhibits tumor development when given either with or after the carcinogen, and it has the additional advantage of having virtually no toxicity, a feature recently confirmed in a Phase I clinical trial (27). While the mechanism by which MI prevents tumors is not clear, its broad activity and low toxicity appeared to us as very attractive features for its inclusion as one component of a mixture designed to inhibit lung tumorigenesis by NNK and BaP. We carried out dose-response studies which demonstrated that 56 μmol/g diet (1% in the diet) MI was an effective inhibitor of mouse lung tumorigenesis induced by NNK and BaP, when given either during carcinogen treatment or after the carcinogens( 28). We then examined the efficacy of a mixture of PEITC-NAC (3 μmol/g diet) and MI (56 μmol/g diet) against lung tumor induction in A/J mice by a mixture of NNK and BaP (29). We found that this mixture was superior to the compounds alone when administered during the carcinogen treatment phase or afterward. We also examined the effects of PEITC-NAC plus MI in various temporal sequences, including beginning treatment at the 50% point of carcinogen administration (after 4 weeks of the 8 week total). This was intended to model to some extent the smoker transitioning to quitting (in whom extensive carcinogen damage to the lung has already occurred) and this model has also been used in the present study. In the study reported here, we extended our investigation of the chemopreventive activity of PEITC-NAC plus MI, using higher doses of the former and carrying out the experiment for a longer period of time, sufficient for the development of adenocarcinoma of the lung. We demonstrate that this mixture significantly inhibits adenocarcinoma of the lung in mice treated with NNK plus BaP, when given either from the 50% stage of carcinogen treatment or after the completion of carcinogen treatment, and furthermore that PEITC-NAC plus MI inhibits cell proliferation and Akt phosphorylation in the post-carcinogen phase of treatment. The chemical structures of PEITC-NAC and MI are shown in Fig. 1A.
Figure 1.
Experimental design. A, Chemical structures of PEITC-NAC and MI. B, Experimental protocol to assess efficacy of PEITC-NAC plus MI to inhibit NNK plus BaP-induced lung tumorigeneis in A/J mice (Experiment 3). Mice were given a diet supplemented with PEITC-NAC plus MI beginning at 50% in the carcinogen treatment phase (the 4th of eight carcinogen treatments) or one week after the last dose of the carcinogens until sacrifice at week 19 or 36 after the last carcinogen treatment. C, Experimental design to harvest tissues for Western studies (Experiment 4). Mice received eight carcinogen treatments, biweekly, and were given a diet supplemented either with PEITC-NAC or MI beginning from one week after the last carcinogen dose until sacrifice at week 9, 13 or 17 of the experiment. Lung tissues from these mice were used for Western immunoassays.
Materials and Methods
Chemicals, reagents and diets
BaP, MI and protease inhibitor cocktail were obtained from Sigma (St. Louis, MO). PEITC-NAC and NNK were synthesized following the methods described elsewhere (30, 31). Mouse diets (AIN-93G and AIN-93M) were purchased from Harlan Teklad (Madison, WI). AIN-93G diet, high in protein and fat, was used to support rapid growth of the mice from the time they arrived until they were 15 weeks old, whereas AIN-93M diet, low in protein and fat, was given to maintain growth of the animals beginning from the time the mice were 15 weeks old until the termination of the experiment (32). Reagents for Western assays were obtained from Invitrogen (Carlsbad, CA), whereas primary and secondary antibodies were from Cell Signaling Technology (Beverly, MA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Chemiluminescent immunodetection reagents and autoradiography films were purchased from Pierce (Rockford, IL) and Denville Scientific (Metuchen, NJ), respectively.
Animal experiments
Four animal studies were carried out. In Experiments 1 and 2, we tested the toxicity of PEITC-NAC and PEITC-NAC plus MI, respectively. Experiment 3 was an efficacy test of PEITC-NAC plus MI for chemoprevention of lung tumorigenesis, and Experiment 4 was performed to harvest tissues for Western assays.
Experiment 1
Female A/J mice, 5–6 weeks of age, were purchased fromJackson Laboratory (Bar Harbor, ME) and housed in the specific pathogen-free animal quarters of Research Animal Resources (RAR), at the University of Minnesota Academic Health Center. The mice were randomized into six groups of 20 mice each and maintainedon AIN-93G pelleted diet (32) for one week. One week after arrival, the mice wereswitched to AIN-93G powdered diet without (group 1) or with additions of different doses of PEITC-NAC (3, 6, 9, 12 and 15 μmol PEITC-NAC/g diet, groups 2–6, respectively). At week nine of the experiment, the diet was changed from AIN-93G to AIN-93M (32) and dietary supplementation with PEITC-NAC was continued. During the entire period of the experiment, the mice were checked daily for signs of toxicity (lethargy, hyper-excitability, abnormal discharges or roughness of the hair coat). At week 13, the mice were killed by CO2 asphyxiation. Blood samples were collected by cardiac puncture and urine samples were obtained directly from the bladder. Lung, liver, heart, spleen, urinary bladder, kidney, small and large intestine, forestomach and glandular stomach, bone marrow, esophagus, pancreas and salivary glands were harvested, the weight of liver and kidney recorded and all organs were fixed in 10% buffered formalin. Blood samples were used for hematology and clinical chemistry studies. Hematological measurements included hemoglobin concentration, hematocrit, erythrocyte counts, total and differential leukocyte counts and platelet count. Clinical chemistry determinations included glucose, calcium, potassium, urea nitrogen, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltranspeptidase, alanine phosphatase, creatinine, bilirubin, triglycerides, cholesterol, albumin, globulin, and total protein. Urine analysis included determination of specific gravity, osmolarity, pH, proteins, glucose, ketones, bilirubin, and urobilinogen as well as microscopic examination of formed elements.
Experiment 2
Female A/J mice were maintained on diet supplemented with a mixture of PEITC-NAC (6, 9 or 12 μmol PEITC-NAC/g diet) and MI (56 μmol/g diet) for 13 weeks and the same tissues and samples as in Experiment 1 were harvested and analyzed. In Experiment 1, we observed the formation of eosinophilic granules in the cytoplasm of bladder mucosa cells of mice treated with PEITC-NAC. Therefore, parallel to the toxicity study, we assessed prevention of the PEITC-NAC-induced eosinophilic granules in bladder mucosa cells by N-acetylcysteine or 2-mercaptoethane sulfonate, known cytoprotective agents (33). Mice were maintained on PEITC-NAC-supplemented diet (15 μmol PEITC-NAC/g diet) without or with N-acetylcysteine (160 μmol/g diet) or 2-mercaptoethane sulfonate (1 mg/ml drinking water) for 30 weeks, sacrificed and the urinary bladders were harvested and kept in 10% buffered formalin.
Experiment 3
Female A/J mice, 5–6 weeks of age, were weighed and randomly allocated into 10 groups. Groups 1 and 2 were treated with NNK plus BaP only, whereas groups 3–7 were treated with NNK plus BaP and given PEITC-NAC plus MI in the diet. Groups 8 and 9 were given PEITC-NAC plus MI only. Group 10 served as a vehicle control. The treatment protocol and sacrifice schedule are shown in Fig. 1B and Table 1. The mixture of NNK and BaP (1 or 2 μmol of each) was administered by gavage as eight weekly doses in0.1 ml cottonseed oil. Mice in the vehicle control group (group 10) were given 0.1 ml cottonseed oil. PEITC-NAC plus MI was administered in the diet beginning either one day after the 4th of eight carcinogen treatments (temporal sequence A) or one week after the last dose of the carcinogens (temporal sequence B). The dose levels of the chemopreventive mixture were 9 μmol/g diet PEITC-NAC plus 56 μmol /g diet (1% in the diet) MI or 15 μmol/g diet PEITC-NAC plus 56 μmol/g diet MI (Table 1). Temporal sequence A could reflect the situation in smokers transitioning to quitting while temporal sequence B could mirror the situation in former smokers who may use chemopreventive agents beginning early in their tobacco smoke-free lives. The doses of PEITC-NAC and MI were chosen on the basis of the toxicity studies (Experiment 1 and 2) and earlier chemoprevention studies (28, 29). Fresh diet wasprovided twice a week. Food consumption was monitoredtwice a week and body weight and water consumption were recorded every week. At week 19 after the last carcinogen treatment, some of the mice were killed using an overdose of CO2 (see Table 1). Lungs were perfused with cold PBS, removed from the thoracic cavity and separated into the 5 lobes (left, right cranial, right middle, right caudal and accessory). Tumor count was performed by examining all surfaces of each lung lobe under a dissecting microscope at 10 x magnification. Tumors from the left lung lobe of each mouse were excised from the rest of the parenchyma and immediately frozen on dry ice. The remaining 4 lung lobes were placed in 10% buffered formalin. All gross evaluations were carried out by the same board certified anatomic veterinary pathologist (I.M.) who was blind to the treatment of the mice. At week 36 after the last carcinogen treatment, the rest of the mice were sacrificed and the lungs harvested as described above. Tumor size was estimated using the calibrated scale in the eyepiece of a dissecting microscope. Each tumor was assigned to one of the following categories: < 2 mm, 2–4 mm and > 4 mm. Tumors from the left lobe of each mouse were excised and kept on dry ice whereas all right lung lobes were placed in 10% formalin.
Table 1.
Effects of PEITC-NAC plus MI on lung tumor induction by a mixture of NNK plus BaP in A/J mice (Macroscopic observations)
| Group | No. of mice at beginning of the study | Temporal sequence | Compound (dose, μmol/g diet) | Carcinogen (dose, μmol) | No. of mice at termination | Mean body weight at termination (g ± SD) | Lung tumors |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of mice with lung tumors | Tumors/mouse (mean ±
SD) |
|||||||||||||
| Reduction (%) | ||||||||||||||
|
| ||||||||||||||
| Week 27 | Week 44 | 27 Weeks | 44 Weeks | 27 Weeks | 44 Weeks | 27 Weeks | 44 Weeks | 27 Weeks | 44 Weeks | |||||
| 1 | 80 | none | NNK (2) +BaP (2) | 23 (25)b | 22 (55)b | 23.8 ± 2.9 | 24.8 ± 2.2 | 100 | 100 | 19.3 ± 9.1 | 22.5 ± 6.7 | |||
| 2 | 80 | none | NNK (1) +BaP (1) | 25 (25) | 53 (55) | 24.1 ± 3.0 | 26.5 ± 1.8 | 90 | 100 | 4.1 ± 2.4 | 7.4 ± 3.4 | |||
| 3 | 65 | A | PEITC-NAC (15) +MI (56) | NNK (2) +BaP (2) | 17 (25) | 19 (40) | 21.0 ± 1.9 | 22.3 ± 2.1 | 94 | 100 | 4.4 ± 3.0 | 6.2 ± 2.6 | 77** | 72** |
| 4 | 65 | A | PEITC-NAC (15) +MI (56) | NNK (1) +BaP (1) | 18 (25) | 40 (40) | 21.7 ± 2.5 | 23.3 ± 2.6 | 72 | 88 | 1.7 ± 1.4 | 3.0 ± 2.0 | 58* | 59* |
| 5 | 65 | A | PEITC-NAC (9) +MI (56) | NNK (2) +BaP (2) | 19 (25) | 23 (40) | 22.0 ± 2.0 | 22.8 ± 1.6 | 95 | 96 | 5.8 ± 3.2 | 6.9 ± 3.2 | 70** | 69** |
| 6 | 65 | A | PEITC-NAC(9) +MI (56) | NNK (1) +BaP (1) | 19 (25) | 39 (40) | 21.3 ± 2.4 | 23.2 ± 2.4 | 68 | 95 | 1.8 ± 1.5 | 4.0 ± 2.2 | 56* | 46* |
| 7 | 65 | B | PEITC-NAC (15) +MI (56) | NNK (2) +BaP (2) | 18 (25) | 21 (40) | 20.7 ± 1.8 | 22.1 ± 2.8 | 100 | 100 | 8.8 ± 1.5 | 15.2 ± 6.3 | 55** | 32** |
| 8 | 20 | – | PEITC-NAC (15) +MI (56) | none | 10 (10) | 10 (10) | 22.2 ± 2.1 | 24.8 ± 2.2 | 20 | 0 | 0.2 ± 0.4 | 0.2 ± 0.4 | ||
| 9 | 20 | – | PEITC-NAC(9) +MI (56) | none | 10 (10) | 10 (10) | 23.0 ± 1.9 | 25.8 ± 2.0 | 30 | 20 | 0.3 ± 0.5 | 0.2 ± 0.4 | ||
| 10 | 25 | none | none | 10 (10) | 14 (15) | 25.1 | 26.7 ± 2.4 | 10 | 64 | 0.1 ± 0.3 | 0.7 ± 0.6 | |||
Beginning at age 7–8 weeks, groups of female A/J mice were treated by gavage weekly for 8 weeks with a mixture of NNK + BaP (1 or 2 μmol of each) in 0.1 ml cottonseed oil. The mice were maintained on AIN-93G diet from age 5–6 weeks until 1 week after the end of carcinogen treatment, then shifted to AIN-93M diet for the duration of the experiment. Chemopreventive agents were added to the diet according to the temporal sequences shown in the Table. The mice were killed 19 weeks (short-term) or 36 weeks (long-term) after the last carcinogen treatment.
The numbers in parentheses indicate the number of mice scheduled for sacrifice; differences indicate death prior to termination time.
P < 0.05, compared to group 2.
P < 0.05, compared to group 1.
Experiment 4
Female A/J mice were randomly assigned to four groups, 18 mice each, and maintained on AIN-93G diet: group 1, NNK plus BaP-treated; group 2, NNK plus BaP and PEITC-NAC-treated; group 3, NNK plus BaP and MI-treated; group 4, vehicle (cottonseed oil)-treated (Fig. 1 C). A combination of NNK plus BaP (2 μmol of each) was administered to mice in the first three groups by gavage, twice a week for four consecutive weeks. Beginning one week after the last dose of the carcinogens, the diet was changed to AIN-93M and the diets of the mice in groups 2 and 3 were supplemented with 15 μmol/g diet PEITC-NAC or 56 μmol/g diet MI, respectively. At week 9, 13 or 17 of the experiment, 6 mice/group were sacrificed and the lungs harvested and snap frozen in liquid nitrogen.
Histopathological analysis
Randomly selected formalin-fixed lung tissues were processed through a series of graded alcohols, embedded in paraffin, cut in 4 μm thick sections and stained with hematoxylin and eosin (HE). For the assessment of pulmonary tumor multiplicity and types of proliferative lesions, three step sections (each 200 μm apart) were cut and stained with HE for mice in groups treated with the carcinogens or carcinogens and chemopreventive agents. Proliferative lesions were counted in each step section and the total number of each type of lesion per mouse was expressed as an average number of each lesion per section (sum of each lesion in 3 step sections divided by 3). One section was cut and stained with HE for mice given the chemopreventive agent alone or maintained on non-supplemented diet.
Proliferative lesions in the lungs were classified as hyperplasia, adenoma, and adenocarcinoma based on recommendations published by the Mouse Models of Human Cancers Consortium (34). The category “adenoma with cellular pleomorphism” was added based on our experience and previously published literature (23, 35). The following criteria were used to diagnose adenoma with cellular pleomorphism (also known as adenoma with dysplasia and adenoma with progression): an adenoma in which 10 or more cells are pleomorphic, characterized by large cell and/or nuclear size; increased cytoplasmic-to-nuclear ratio, prominent nucleoli, nuclear crowding and increased numbers of mitotic figures with no evidence of parenchymal invasion by pleomorphic cells. The assessment of cellular pleomorphism was independent from and in addition to morphologic type of adenoma (solid, papillary or mixed).
Immunohistochemical analysis
To assess the rate of cell proliferation in lung tissues by Ki-67 staining, 4 μm formalin-fixed paraffin sections were deparaffinized and antigen retrieved by incubating the slides in a pressure cooker in citrate buffer (pH 6.0) for 30 sec at 121 °C and 10 sec at 90 °C followed by cooling for 15 min. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 15 min at room temperature. The sections were incubated with a universal protein block (DAKO, Carpinteria, CA) for 10 min, followed by a 60 min, room temperature incubation with rat monoclonal antibody against mouse Ki-67 (DAKO, Carpinteria, CA) diluted at 1:50. Sections were then incubated for 30 min at room temperature with biotinylated anti-rat secondary antibody (Vector, Burlingame, CA) diluted at 1:300. Binding was detected by incubating sections with streptavidin/HRP (DAKO, Carpinteria, CA) for 20 min at room temperature followed by diaminobenzidine chromagen application for 5 min at room temperature. Sections were counterstained with Mayer’s Hematoxylin (DAKO, Carpinteria, CA). For negative control slides, the primary antibody was substituted by Super Sensitive Negative Control Rat serum (Biogenix, San Ramon, CA). The percentage of cells with Ki-67-positive nuclear staining (dense brown precipitate restricted to the nuclei) was determined using a Nikon Eclipse E800 microscope. Images were captured with an attached camera linked to a computer. Ten randomly selected fields were counted and each field corresponded to a total number of cells ranging from 700–1,000.
Western immunoblot analyses
Aliquots of lung tissues from six mice (30 mg/mouse) were pooled and homogenized in ice-cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM, 1% Triton X-100, pH 7.4) containing protease inhibitors [aprotinin (1 μg/ml), leupeptin(1 μg/ml), pepstatin (1 μM) and phenylmethylsulfonylfluoride (0.1 mM)] and the phosphatase inhibitors Na3VO4 (1mM) and NaF (1 mM). After the homogenates had been centrifuged (14,000 x g for 25 min at 4 °C), the supernatants were collected, aliquoted and stored at −80 °C. For Western immunoblotting, 60 μg of protein per sample were loaded onto a 4–12% Novex Tris-glycine gel (Invitrogen, Carlsbad, CA) and run for 60 min at 200 V. The proteins were then transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA) for 1 h at 30 V. Protein transfer was confirmed by staining membranes with BLOT-FastStain (Chemicon, Temecula, CA). Subsequently, membranes were blocked in 5% Blotto non-fat dry milk in Tris buffer containing 1% Tween-20 for 1 h and probed overnight with the following primary antibodies: anti-Akt (1:1,000), anti-phospho Akt ser 473 (1:1,000), anti-phospho BAD ser 155 (1:1,000), anti-proliferating cell nuclear antigen (PCNA, 1:1000) and anti-poly (ADP-ribose) polymerase (PARP) (1:1,000). After incubating the membranes with a secondary antibody (goat anti-rabbit IgG, 1:20,000, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h, chemiluminescent immunodetection was employed. Signal was visualized by exposing membranes to HyBolt CL autoradiography film. Membranes were stripped and probed with anti-β-actin to check for differences in the amount of protein loaded in each lane.
Statistical analysis
The results of gross tumor counts and microscopic lesions per mouse were summarized as mean and standard deviation for the different groups defined by the dose of the carcinogens and chemopreventive agents and the duration of PEITC-NAC plus MI administration. The data were further divided according to the temporal sequence of PEITC-NAC plus MI administration. The effects of PEITC-NAC plus MI are reported as a percent change in tumor multiplicity in carcinogen and chemopreventive agent-treated groups relative to the groups treated with carcinogen only. Statistical comparisons between the different groups were performed using Poisson regression, which is specific for data representing counts or number of events and can handle cases where few or no events occur. The regression analyses data for microscopic lesions were adjusted for the number of sections and mice associated with each specimen. A p-value of less than 0.05 was used to judge statistical significance.
Results
Toxicity studies (Experiments 1 and 2)
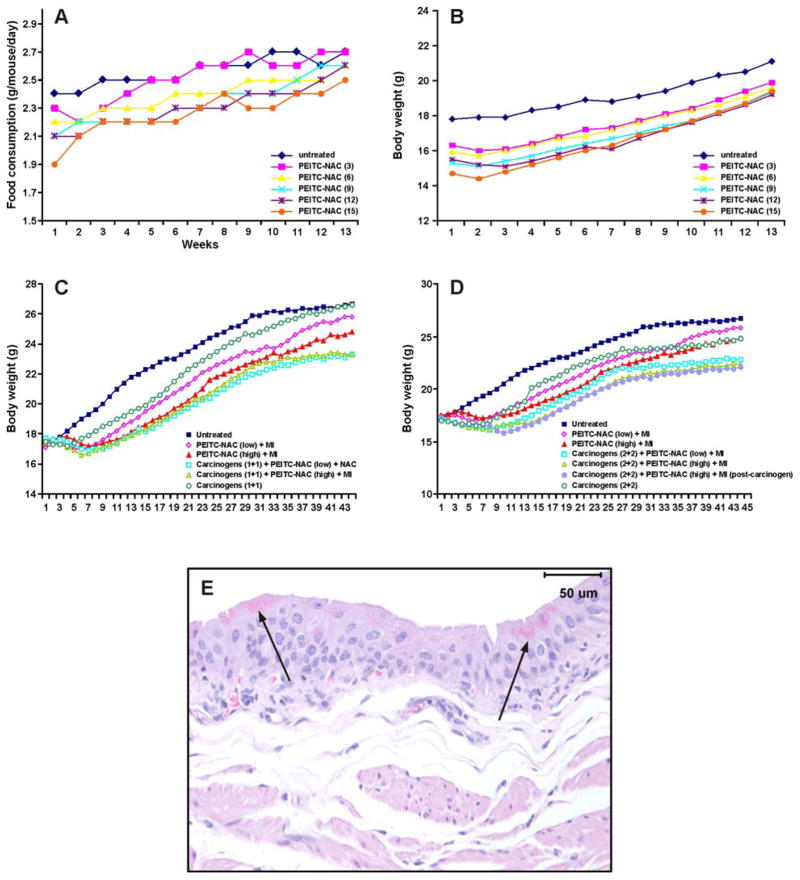
During the first six weeks of dietary supplementation, food consumption of mice given PEITC-NAC decreased significantly (P < 0.01) by 7–12%, compared to the group given untreated diet (Fig. 2A), presumably due to the poor palatability of the diet. As a result, the body weight gain of mice maintained on PEITC-NAC-supplemented diet decreased significantly (P < 0.01) by 10–14% in a dose-dependent manner (Fig. 2B). However, in the course of the study, the food consumption and body weight gain of the mice improved. During the last three weeks of the experiment (week 10–13), the food consumption and body weights of mice maintained on PEITC-NAC-supplemented diets were not significantly different (5–8% reduction) from that of the group given non-supplemented diet. The same result was obtained upon supplementation of the diet with combinations of PEITC-NAC (6, 9 and 12 μmol/g diet) plus MI (μmol/g diet) (Experiment 2, data not shown). The absolute and relative weights of liver and kidney of mice in all treatment groups were similar to those of the control group. Histopathological examination of the different organs did not reveal any abnormalities except a dose-dependent increase in the frequency of eosinophilic bodies within the cytoplasm of urinary bladder epithelial cells. The number of superficial bladder cells containing eosinophilic granules was estimated as none, rare-some, some-many, some-many, many-all and all at 0, 3, 6, 9, 12, and 15 μmol PEITC-NAC/g diet, respectively (data not shown). A representative photomicrograph of PEITC-NAC-induced eosinophoilic granules in urinary bladder mucosa cells is shown in Fig. 2 E. Neither N-acetylcysteine nor 2-mercaptoethane sulfonate decreased the formation of eosinophilic granules in bladder cells of mice treated with PEITC-NAC (15 μmol/g diet). All hematological, clinical chemistry and urine analysis determinations were within the normal range. Overall, other than a slight reduction in body weight gain and the appearance of eosinophilic granules in urinary bladder cells, the chemopreventive agents did not cause any signs of toxicity.
Figure 2.
Effect of PEITC-NAC, alone or in combination with MI, on food consumption, body weight gain and urinary bladder mucosa cells. Mean food consumption (A) and body weight curves (B) of mice given PEITC-NAC (3–15 μmol/g diet) in the diet for 13 weeks (Experiment 1, toxicity studies). C, Mean body weight curves of mice treated with the lower dose of NNK plus BaP (1 μmol of each) and PEITC-NAC plus MI (Experiment 3). D, Mean body weight curves of mice treated with the higher dose of NNK plus BaP (2 μmol of each) and PEITC-NAC plus MI (Experiment 3). E, Representative photomicrograph of PEITC-NAC- or PEITC-NAC plus MI-induced eosinophilic granules in the urinary bladder mucosa of A/J mice.
Tumor bioassay (Experiment 3)
Food consumption of mice treated with carcinogens and given PEITC-NAC plus MI was significantly lower than that of mice treated with carcinogens alone (data not shown). The average body weights of the mice treated with carcinogens and chemopreventive agents were significantly lower (8–13%) than those of the control group treated with the carcinogens alone (Figs. 2C and 2D). Similar to the results from the toxicity study (Experiment 1), food consumption and the body weights of mice in groups 8 and 9 (maintained on PEITC-NAC plus MI-supplemented diet but not treated with carcinogens) were significantly lower than those of mice in group 10 (the vehicle control group given non-supplemented conventional AIN-93 diet) during the early phase of the study only (Fig. 2C and D). The results of the lung tumor study, based on macroscopic observations, are presented in Table 1 and can be summarized as follows: (a) PEITC-NAC plus MI significantly reduced (P < 0.05) tumor multiplicity when administered in temporal sequences A (46–77%) and B (32–55%); (b) efficacy was greater in temporal sequence A than in temporal sequence B; (c) in mice given PEITC-NAC plus MI in temporal sequence A, for each treatment group, efficacy of the chemopreventive agent in the short-term study was similar to that found in the long-term study; (d) efficacy of the lower dose of PEITC-NAC (9 μmol/g diet) plus MI (56 μmol/g diet) was similar to that of the higher dose of PEITC-NAC (15 μmol/g diet) plus MI (56 μmol/g diet); (e) irrespective of the dose of PEITC-NAC plus MI, reductions in tumor multiplicity were higher in mice treated with the higher dose of the carcinogens compared to mice treated with the lower dose; (f) tumor incidence was not reduced in any of the treatment groups, and (g) lung tumor multiplicities in groups treated with PEITC-NAC plus MI alone (without carcinogens) were similar to those of the vehicle control group.
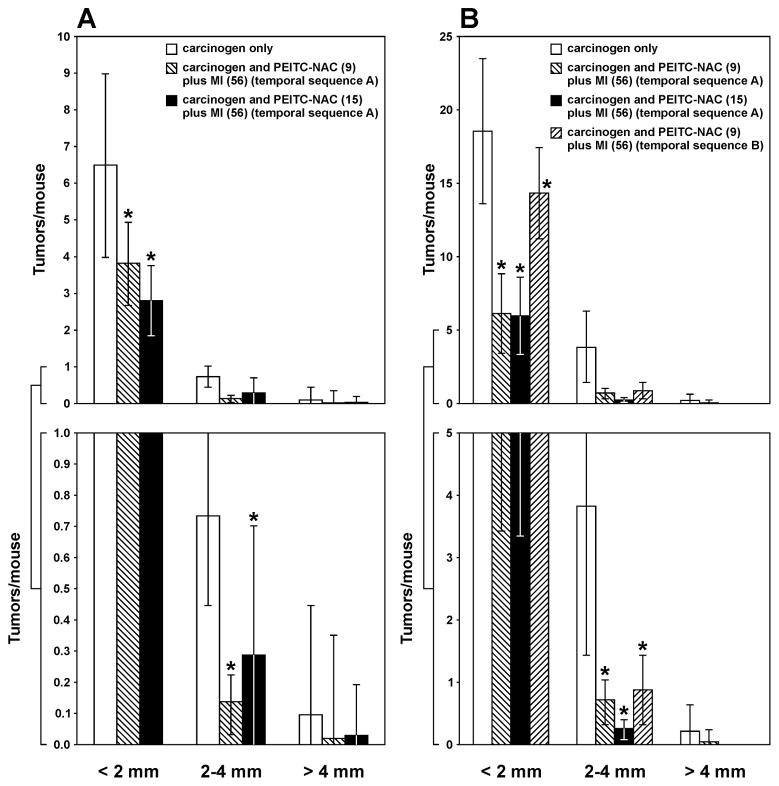
Upon counting of the lung tumors from the long-term study, the size of the tumors was categorized into three classes: < 2 mm, 2–4 mm and > 4 mm. Most of the tumors, regardless of treatment, were< 2 mm in diameter. In mice treated with the lower dose of the carcinogens, the multiplicities of tumors with a diameter of < 2 mm, 2 – 4 mm and > 4 mm were 6.49 ± 2.51, 0.74 ± 0.29, and 0.11 ± 0.34, respectively (Fig. 3A). The higher dose of PEITC-NAC (15 μmol/g diet) plus MI administered in temporal sequence A significantly reduced the multiplicity of tumors with a diameter of < 2 mm and 2–4 mm to 2.80 ± 0.96 (P < 0.001) and 0.28 ± 0.42 (P < 0.001), corresponding to reductions by 57% and 62%, respectively. Although the multiplicity of tumors with a diameter of > 4 mm was decreased to 0.03 ± 0.16, corresponding to a reduction by 73%, the effect was not significant (P = 0.162) due to a high standard deviation. The lower dose of PEITC-NAC (9 μmol/g diet) plus MI caused similar effects (Fig. 3A).
Figure 3.
Effect of PEITC-NAC plus MI on growth of lung tumors. The size of surface tumors on lungs of mice sacrificed at week 36 after the last carcinogen treatment was estimated using the calibrated scale in the eyepiece of a dissecting microscope. Each tumor was assigned to one of the following categories: < 2 mm, 2–4 mm and > 4 mm. The results show the percentage reduction in the multiplicity of tumors of the different size categories in mice treated with the lower (A) or higher (B) dose of the carcinogens and receiving PEITC-NAC plus MI in the diet. * P < 0.001
In mice treated with the higher dose of the carcinogens, the multiplicities of tumors with a diameter of < 2 mm, 2 – 4 mm and > 4 mm were 18.60 ± 4.96, 3.86 ± 2.41, and 0.23 ± 0.42, respectively (Fig. 3B). Upon administration of the higher dose of PEITC-NAC (15 μmol/g diet) plus MI in temporal sequence A, the multiplicity of tumors with a diameter of < 2 mm and 2–4 mm was decreased to 5.95 ± 2.63 (P < 0.001) and 0.26 ± 0.15 (P < 0.001), corresponding to reductions by 68% and 93%, respectively, whereas tumors with a diameter of > 4 mm were completely abolished (Fig. 3B). Similar effects were observed upon administration of the compound in temporal sequence B except that efficacy on the multiplicity of tumors with a diameter of < 2 mm and 2–4 mm was significantly less than that observed in temporal sequence A (reductions by 23% versus 68% for tumors with a size of < 2 mm and 77% versus 93% for tumors with a size of 2–4 mm, Fig. 3B). Also, the lower dose of PEITC-NAC (9 μmol/g diet) plus MI given in temporal sequence A caused a reduction in the multiplicity of all classes of tumors but only effects on tumors with a diameter of < 2 mm and 2–4 were significant (P < 0.001).
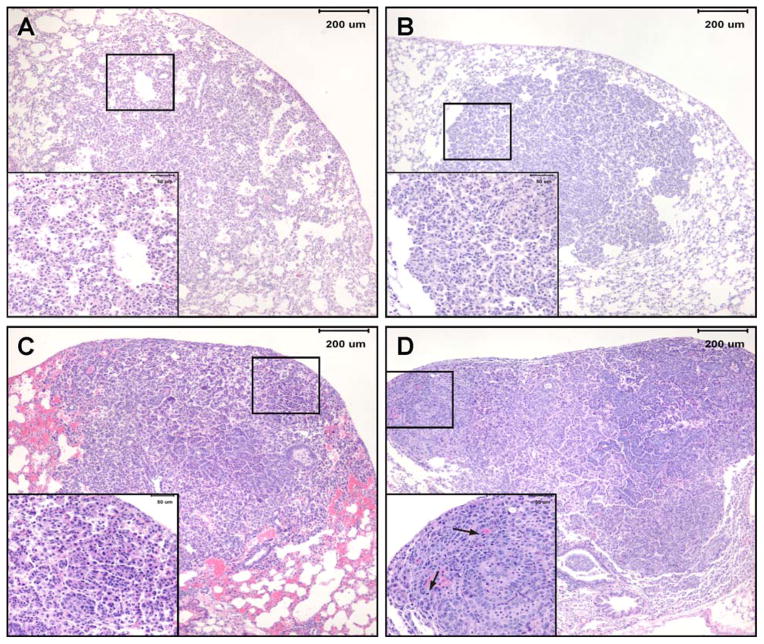
Microscopic lesions observed in lung tissues of mice sacrificed at week 36 after the last carcinogen treatment were classified as hyperplastic foci, adenoma, adenoma with cellular pleomorphism and adenocarcinoma (Figure 4) following established criteria (34). In mice treated with the higher dose of the carcinogens, the multiplicities of hyperplastic foci, adenoma, adenoma with cellular pleomorphism and adenocarcinoma were 2.22 ± 1.07, 1.07 ± 0.7, 3.94 ± 1.68 and 0.86 ± 0.59, respectively (Table 2). Upon administration of the higher dose of PEITC-NAC (15 μmol/g diet) plus MI following temporal sequence A, the multiplicities of hyperplastic foci, adenoma with cellular pleomorphism and adenocarcinoma were significantly reduced to 1.42 ± 0.52 (P = 0.003), 0.90 ± 0.81 (P < 0.001), and 0.23 ± 0.34 (P = 0.017), respectively, corresponding to reductions by 36%, 77% and 73%, respectively (Table 2). Adenoma multiplicity was not reduced. The lower dose of PEITC-NAC (9 μmol/g diet) plus MI not only showed the same level of efficacy as the higher dose but also marginally reduced the multiplicity of adenoma. Administration of PEITC-NAC plus MI in temporal sequence B significantly reduced the multiplicity of adenoma with cellular pleomorphism and adenocarcinoma to 1.64 ± 0.87 (P < 0.001) and 0.40 ± 0.41 (P = 0.026), respectively, corresponding to reductions by 58% and 53%, but the multiplicities of hyperplastic foci and adenoma were not significantly reduced (Table 2).
Figure 4.
Photomicrographs of lung lesions observed in NNK plus BaP-treated A/J mice lung. A, hyperplasia- a focal area of hypercellularity in the pulmonary parenchyma that consists of thickened alveolar walls covered by a single row of epithelial cells while maintaining alveolar architecture; B, adenoma- moderately or well-delineated, nodular area of pulmonary hypercellularity that is composed of a uniform population of cells; C, adenoma with cellular pleomorphism (aka adenoma with dysplasia and adenoma with progression)- adenoma in which a proportion of the tumor (at least 10 cells) or the entire tumor is composed of cells that have large cell and/or nuclear size, increased cytoplasmic-to-nuclear ratio, prominent nucleoli, and exhibit nuclear crowding and increased numbers of mitotic figures with no evidence of parenchymal invasion by pleomorphic cells; D, adenocarcinoma- variably delineated, usually highly cellular nodular or multinodular area in pulmonary parenchyma consisting of cells with large nuclei, increased cytoplasmic-to-nuclear ratio, anisocytosis, anisokaryosis, prominent nucleoli, moderate to high mitotic figure rate (including bizarre mitoses) and evidence of focal or multifocal invasion of parenchyma, airways or blood vessels.
Table 2.
Effect of PEITC-NAC plus MI on the multiplicity of NNK plus BaP-induced microscopic lung lesionsa
| Group | Temporal sequence | Compound (dose, μmol/g diet) | Carcinogen (dose, μmol) | No. of mice used for the analysis | Hperplastic foci/mouse | Reduction (%) | Adenoma/mouse | Reduction (%) | Adenoma with cellular pleomorphism/mouse | Reduction (%) | Adeno-carcinoma/mouse | Reduction (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NNK (2) +BaP (2) | 19 | 2.22 ± 1.07 | 1.07 ± 0.70 | 3.94 ± 1.68 | 0.86 ± 0.59 | ||||||
| 2 | NNK (1) +BaP (1) | 18 | 0.87 ± 0.38 | 0.20 ± 0.19 | 1.31 ± 0.44 | 0.24 ± 0.15 | ||||||
| 3 | A | PEITC-NAC (15) +MI (56 | NNK (2) +BaP (2) | 14 | 1.42 ± 0.52 | 36%** | 0.71 ± 0.32 | 35% | 0.90 ± 0.81 | 77%** | 0.23 ± 0.34 | 73%** |
| 4 | A | PEITC-NAC (15) +MI (56 | NNK (1) +BaP (1) | 17 | 0.49 ± 0.52 | 42%* | 0.16 ± 0.19 | 20% | 0.39 ± 0.30 | 70%* | 0.18 ± 0.19 | 25% |
| 5 | A | PEITC-NAC (9) +MI (56) | NNK (2) +BaP (2) | 22 | 1.23 ± 0.57 | 45%** | 0.61 ± 0.36 | 42% | 0.98 ± 0.51 | 75%** | 0.31 ± 0.24 | 64%** |
| 6 | A | PEITC-NAC (9) +MI (56) | NNK (1) +BaP (1) | 21 | 0.46 ± 0.21 | 47%* | 0.24 ± 0.10 | none | 0.30 ± 0.11 | 77%* | 0.06 ± 0.11 | 75%* |
| 7 | B | PEITC-NAC (15) +MI (56) | NNK (2) +BaP (2) | 25 | 2.56 ± 1.05 | none | 1.27 ± 0.92 | none | 1.64 ± 0.87 | 58%** | 0.40 ± 0.41 | 53%** |
| 8 | – | PEITC-NAC (15) +MI (56) | none | 10 | 0.25 ± 0.50 | – | – | – | ||||
| 9 | – | PEITC-NAC (9) +MI (56) | none | 10 | 0.13 ± 0.25 | – | – | – | ||||
| 10 | none | none | 5 | 0.33 ± 0.55 | 0.33 ± 0.55 | – | – |
Groups of female A/J mice received by gavage eight weekly doses of a mixture of NNK plus BaP in 0.1 ml cottonseed oil. The mice were maintained on AIN-93G diet from age 5–6 weeks until 1 week after the end of carcinogen treatment and then shifted to AIN-93M diet for the duration of the experiment. PEITC-NAC + MI was added to the diet in temporal sequence A or B.
P < 0.05, compared to group 2.
P < 0.05, compared to group 1.
Similar to our observations with the gross tumors, the multiplicity of microscopic lesions induced by the lower dose of the carcinogens was less than that induced by the higher dose. The multiplicities of hyperplastic foci, adenoma, adenoma with cellular pleomorphism and adenocarcinoma were 0.87 ± 0.38, 0.20 ± 0.19, 1.31 ± 0.44 and 0.24 ± 0.15, respectively (Table 2). The higher dose of PEITC-NAC (15 μmol/g diet) plus MI reduced the multiplicity of hyperplastic foci and adenoma with cellular pleomorphism to 0.49 ± 0.52 (P = 0.006) and 0.39 ± 0.30 (P < 0.001), respectively. However, adenoma and adenocarcinoma multiplicities were not significantly reduced. The effect of the lower dose of PEITC-NAC (9 μmol/g diet) plus MI was similar to that of the higher dose except that the multiplicity of adenocarcinoma was also significantly reduced to 0.06 ± 0.11, corresponding to a reduction by 75%.
The incidence of microscopic lesions in groups treated with the carcinogens and PEITC-NAC plus MI was not significantly different from that of the carcinogen-treated groups with the exception of adenocarcinoma in groups treated with the lower dose of the carcinogens and receiving both the lower and higher doses of PEITC-NAC plus MI and the group treated with the higher dose of carcinogens and given the higher dose of PEITC-NAC plus MI in temporal sequence A (reduction by 29–53%, data not shown).
A problem encountered during Experiment 3 was high mortality due to forestomach tumors that arose from repeated intragastric treatment with BaP. Mortality of mice treated with the higher dose of NNK and BaP and scheduled to be sacrificed at week 44 (long-term study) was about 50% (see Table 1).
Inhibition of cell proliferation
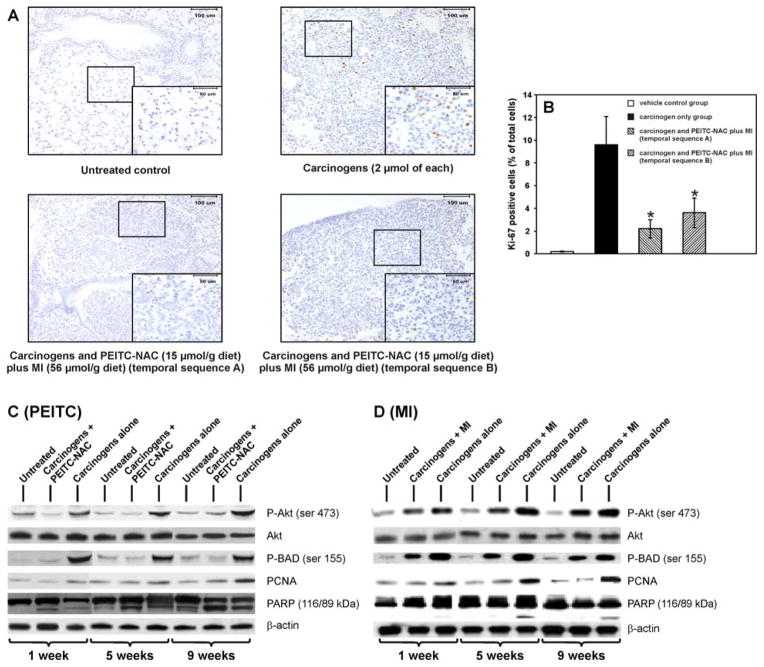
Representative micrographs of Ki-67-stained lung tissues from the different groups of mice (short-term study of Experiment 3) are shown in Fig. 5A. The number of Ki-67-positive nuclei was significantly higher in lung lesions (hyperplastic foci and adenoma) of mice treated with the carcinogens alone compared to the level in normal lungs from vehicle-treated mice (9.6 ± 2.5 % versus 0.2 ± 0.03%, P < 0.01). In mice treated with the carcinogens and given PEITC-NAC plus MI in temporal sequence A or B, the frequencies of Ki-67-positive nuclei were significantly reduced compared to the level in mice treated with the carcinogens alone [2.2 ± 0.8% versus 9.6 ± 2.5 % (P < 0.01) and 3.6 ± 1.3% versus 9.6 ± 2.5 % (P < 0.01)] in temporal sequences A and B, respectively, Fig. 5B). The results of the Ki-67 expression assay were further verified by determining, using Western assays, the level of PCNA expression in lung tissues harvested in Experiment 4. As shown in Fig. 5C and 5D, the expression of PCNA was higher in lung tissues of mice treated with the carcinogens alone relative to the levels in untreated mice. PEITC-NAC (15 μmol/g diet) or MI (56 μmol/g diet) administration to carcinogen-treated mice reduced PCNA expression levels in lungs harvested at all three time points.
Figure 5.
Effect of PEITC-NAC plus MI on cell proliferation and apoptosis. A, photomicrographs (x 20) of Ki-67-stained lung tissues from week 27 of the tumor bioassay (Experiment 3). Lung tissues were cut into 4 μm sections and stained with Ki-67 antibody and counterstained with hematoxylin. Images were captured with a camera attached to a Nikon Eclipse E800 microscope. B, Quantification of data, representing the percentage of Ki-67 positive nuclei based on counting at least 700 nuclei. Columns, mean (n = 10); bars, SD; * P < 0.001. C, D, Western immunoblots of lung tissues. Mice received eight carcinogen treatments, biweekly (Experiment 4), and were given a diet supplemented either with 15 μmol/g diet of PEITC-NAC (C) or 56 μmol/g diet of MI (D) beginning from one week after the last carcinogen dose until sacrifice at week 1, 5 or 9 after the last carcinogen treatment. Equal amounts of protein from lung homogenates of each group of mice (pooled lungs, 6/group) were loaded onto a 4–12% SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin.
Akt and BAD phosphorylation and PARP cleavage
NNK plus BaP treatment caused increased phsophorylation of both Akt and BAD and induced a slight cleavage of PARP. Supplementation of the diet with PEITC-NAC inhibited NNK plus BaP-induced phosphorylation of Akt and BAD and caused a marked cleavage of PARP (Fig. 5C). MI weakly inhibited phosphorylation of Akt and BAD but did not cause PARP cleavage (Fig 5D).
Discussion
The results of this study clearly demonstrate the efficacy of PEITC-NAC plus MI in preventing lung tumors in A/J mice treated with the tobacco smoke carcinogens NNK and BaP. Among mice in the same treatment group, with the exception of the post-carcinogen treatment group, the magnitude of reduction of tumor multiplicity in the short-term study was similar to that observed in the long-term study, suggesting efficacy of PEITC-NAC in inhibiting the development of lung tumors in the early as well as later stages of lung tumorigenesis. In addition to reducing tumor multiplicity, PEITC-NAC plus MI inhibited the growth of tumors, virtually eliminating tumors > 4mm in size. Consistent with this, histopathological analyses of tumors obtained from the long-term study revealed a significant reduction in the multiplicity of adenocarcinoma. There was a temporal-dependency in the efficacy of PEITC-NAC plus MI, with temporal sequence A being consistently more effective than temporal sequence B. Collectively, the results of this study provide compelling evidence supporting the combination of PEITC-NAC plus MI as a chemopreventive agent against lung cancer in smokers transitioning to quitting, and in former smokers.
Before assessing inhibition of lung tumorigenesis by PEITC-NAC plus MI, the potential toxicity of the chemopreventive agent was examined. The major findings of the toxicity study were moderate but significant reductions in body weight gain and the formation of eosinophilic inclusion bodies in urinary bladder epithelial cells. The reductions in body weight gain, which were observed during the tumor bioassay as well, could be attributed to lower food consumption resulting from low palatability of the PEITC-NAC plus MI-supplemented diet. Although calorie restriction is the most potent cancer prevention regimen (36), effects of calorie restriction and reduced body weight gain on lung tumor development have not been extensively studied. In one study, restriction of food intake by 40 and 60% reduced body weight by about 23 and 34%, respectively, but lung tumor multiplicity was reduced only by 25% in both groups (37). Therefore, it is unlikely that PEITC-NAC plus MI-induced reduction in food consumption (15–20% at the beginning of the study and 7–10% thereafter) and body weight gain (about 12–15% and 10% at the beginning and termination of the study, respectively) could contribute to the reduction in lung tumor multiplicity.
The eosinophilic granules observed in urinary bladder mucosa cells of PEITC-NAC plus MI-treated mice may be degradation products resulting from damage to this tissue. In earlier studies, treatment of male F344 rats with PEITC (6.1 μmol/g diet) for 14 days induced preneoplastic lesions (38) and extension of the treatment to 32 or 48 weeks caused the formation of urinary bladder carcinoma (39, 40). In the present study, continuous feeding of mice with PEITC-NAC (15 μmol/g diet) for 32 weeks did not induce any pathological lesions other than eosinophilic granules. The differential susceptibility of rat urinary bladder to PEITC as compared to that of mice might be related to differences in the metabolism of the compound between the two species (41,42).
Several chemopreventive agents have been studied for their inhibitory activity against lung tumorigenesis induced by tobacco smoke carcinogens in A/J mice. However, only a few of them have been examined for their effects on lung adenocarcinoma since most of the studies were terminated at the adenoma stage. Among studies in which chemoprevnetive agents were administered throughout the experiment, Wang et al. (43) studied inhibition by budesonide of BaP-induced lung adenocarcinoma in mutant and wild type A/J mice. Administration of budesonide to wild type mice beginning before carcinogen treatment or at week 18 of the experiment until termination of the study at week 40 decreased tumor multiplicity and volume by 70% and 94% and 53% and 82%, respectively. Agents that have been assessed for their inhibitory effects on the progression of tobacco smoke carcinogen-induced lung adenoma to adenocarcinoma include sulforaphane and their N-acetylcysteine conjugates (23), MI and budesonide (35), farnesyl transferase inhibitors (44), black tea (45), tea polyphenols and caffeine (46), and tea and tea polyphenols (47). With the exception of MI, all agents decreased the multiplicity/incidence of adenocarcinoma. In the present study, we followed a unique approach to examine the chemopreventive efficacy of PEITC-NAC plus MI on NNK and BaP-induced lung tumorigenesis in A/J mice: (a) mixtures of chemopreventive agents were used [PEITC-NAC (9 or 15 μmol/g diet) and MI (56 μmol/g diet)]; (b) lung tumorigenesis was induced with two doses of the carcinogens (1 or 2 μmol each of NNK and BaP) to assess if chemopreventive efficacy of PEITC-NAC plus MI would be related to tumor load; (c) PEITC-NAC plus MI was given in the diet following temporal sequence A or B to mirror the situation in smokers who are transitioning to quitting or former smokers who use chemopreventive agents beginning a short time after quitting smoking, respectively; (d) tumor burden was examined 19 weeks (short-term study) or 36 weeks (long-term study) after the last carcinogen treatment, corresponding to the time points at which virtually all of the tumors are adenoma and some adenoma develop into adenocarcinoma, respectively.
The chemopreventive efficacy of PEITC-NAC plus MI, as measured by reduction in tumor multiplicity, was higher when administered following temporal sequence A than temporal sequence B. These results indicate that the primary mode of the inhibitory effect of PEITC-NAC plus MI is due to blocking of tumorigenesis. However, the following observations indicate that the combination of PEITC-NAC plus MI also possesses tumor suppressive activity. First, upon continuous treatment with PEITC-NAC plus MI following temporal sequence A, the percentage reduction in tumor multiplicity in the short-term study was similar to that of the long-term study, suggesting that the agents inhibited not only the earlier phases of lung tumorigenesis but also the latter stages. Second, reductions were observed in the multiplicity of larger (2–4 mm and > 4 mm size) tumors, suggesting inhibition of cell proliferation/apoptosis. Third, histopathological analysis of the tumors showed efficacy on adenoma with cellular pleomorphism and adenocarcinoma, but not on adenoma. When given following temporal sequence B, PEITC-NAC plus MI caused a weaker preventive activity in the long-term study compared to its effect in the short-term study (reduction by 32% versus 55%). This may be related to the higher number of adenoma in this group of mice (see Table 2), as revealed in histopathological analyses of tumors, which were not sensitive to PEITC-NAC plus MI treatment. This is consistent with the findings in other studies, in which adenomas not progressing to adenocarcinoma were found to be resistant to the effects of chemopreventive agents (23, 35, 46), and could be related to the lower cell proliferation in adenoma compared to adenocarcinoma (46). It is worthwhile to note that only about 14% of adenoma progress to adenocarcinoma (23).
The chemopreventive activity of PEITC-NAC appears to be due to the PEITC moiety since earlier studies in rats treated with NNK and given a high dose of N-acetylcysteine did not show tumor inhibitory effects (18). One of the main mechanisms through which PEITC prevents NNK–induced lung tumorigenesis in animal models is inhibition of cytochrome P450 enzymes that metabolically activate the carcinogen to promutagenic DNA adducts (48). Also, abundant data from in vitro studies (49) and a few reports from studies in mice (22, 23) indicate that PEITC inhibits cell proliferation and induces apoptosis in tumor cells. In the present study, we showed that treatment with NNK plus BaP increased the frequency of Ki-67-positive cells and the expression level of PCNA, the most common markers of cell proliferation. Administration of PEITC-NAC plus MI and PEITC-NAC after carcinogen treatment reduced the mouse lung tissue levels of Ki-67 positive cells and PCNA expression, respectively. We confirmed these results by showing inhibition by PEITC-NAC of NNK plus BaP-induced activation of Akt, a key protein for cell survival and proliferation (50), and phosphorylation of BAD, a proapoptotic protein and one of the downstream targets of Akt. Akt-induced phosphorylation of BAD results in dissociation of the protein from the Bcl-2/Bcl-X complex and loss of its proapoptotic function (51). On the other hand, inhibition by PEITC-NAC of phosphorylation of Akt and BAD could lead to induction of apoptosis. Indeed, PEITC-NAC treatment led to increased PARP cleavage, a marker of apoptosis.
The mode of chemopreventive activity of MI has not been extensively investigated. MI reversed the dedifferentiating effect of BaP-7,8-diol-9,10-epoxide in human lung cells (52). Also, MI reduced the mitotic rate of premalignant intestinal epithelial cells in rats treated with 1,2-dimethylhydrazine (53). In the present study, MI reduced NNK plus BaP-induced expression of PCNA and phosphorylation of Akt and BAD. However, the effects were not as strong as those caused by PEITC-NAC. To our knowledge, this is the first report associating inhibition of Akt activation with chemoprevention by MI. MI did not cause PARP cleavage, which is consistent with the lack of induction by MI of caspase-3 activity in prostate cancer cells (54). The present results as well as previous reports on the mode of chemopreventive activity of PEITC-NAC and MI indicate possible complementary effects between the two agents, a key factor influencing the efficacy of a mixture of chemoprevnetie agents.
Taken together, the results from this study clearly show that PEITC-NAC and MI inhibit NNK plus BaP-induced lung tumorigenesis in A/J mice through reduction of cell proliferation and induction of apoptosis. The chemopreventive agent was effective when given beginning one day after the 4th of eight carcinogen treatments or one week after the last carcinogen treatment, indicating the potential promise of PEITC-NAC plus MI for lung cancer chemoprevention in smokers transitioning to quitting and in former smokers.
Acknowledgments
Grant support: NIH/National Cancer Institute grant CA-102502 (SSH). We thank David Jewison for his help in the preparation of the diets and Bruce Lindgren for statistical analyses of the data.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon: IARC Press; 2004. Tobacco Smoke and Involuntary Smoking; pp. 53–119. [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Cigarette smoking among adults-United States, 2006. MMWR. 2007;56:1157–61. [PubMed] [Google Scholar]
- 5.Thanopolou E, Baltayiannis N, Lykogianni V. Nutritional aspects regarding lung cancer chemoprevention. J BUON. 2006;11:7–20. [PubMed] [Google Scholar]
- 6.Ranney L, Melvin C, Lux L, McClain E, Lohr KN. Systematic review: smoking cessation intervention strategies for adults and adults in special populations. Ann Intern Med. 2006;145:845–56. doi: 10.7326/0003-4819-145-11-200612050-00142. [DOI] [PubMed] [Google Scholar]
- 7.Blot WJ, Fraumeni JF. Cancers of the lung and pleura. In: Schottenfeld D, Fraumeni J, editors. Cancer epidemiology and prevention. New York: Oxford University Press; 1996. pp. 637–65. [Google Scholar]
- 8.Hecht SS. Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nature Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 9.Hecht SS, Isaacs S, Trushin N. Lung tumor induction in A/J mice by the tobacco smoke carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene: a potentially useful model for evaluation of chemopreventive agents. Carcinogenesis. 1994;15:2721–25. doi: 10.1093/carcin/15.12.2721. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS, Morse MA, Amin S, Stoner GD, Jordan KG, Choi CI, Chung FL. Rapid single-dose model for lung tumor induction in A/J mice by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the effect of diet. Carcinogenesis. 1989;10:1901–04. doi: 10.1093/carcin/10.10.1901. [DOI] [PubMed] [Google Scholar]
- 11.Hecht SS, Trushin N, Rigotty J, Carmella SG, Borukhova A, Akerkar SA, Rivenson A. Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol Biomarkers Prev. 1996;5:645–652. [PubMed] [Google Scholar]
- 12.Hecht SS, Kenney PMJ, Wang M, Upadhyaya P. Benzyl isothiocyanate: an effective inhibitor of polycyclic aromatic hydrocarbon tumorigenesis in A/J mouse lung. Cancer Lett. 2002;187:87–94. doi: 10.1016/s0304-3835(02)00410-x. [DOI] [PubMed] [Google Scholar]
- 13.Hecht SS. Carcinogenicity studies of inhaled cigarette smoke in laboratory animals: old and new. Carcinogenesis. 2005;26:1488–92. doi: 10.1093/carcin/bgi148. [DOI] [PubMed] [Google Scholar]
- 14.International Agency for Research on Cancer. Fruit and vegetables. Vol. 8. Lyon: IARC Printing Press; 2003. IARC Handbooks of Cancer Prevention; p. 323. [Google Scholar]
- 15.Morse MA, Wang CX, Stoner GD, Mandal S, Conran PB, Amin SG, Hecht SS, Chung FL. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989;49:549–53. [PubMed] [Google Scholar]
- 16.Morse MA, Amin SG, Hecht SS, Chung FL. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Res. 1989;49:2894–97. [PubMed] [Google Scholar]
- 17.Morse MA, Eklind KI, Amin SG, Hecht SS, Chung FL. Effects of alkyl chain length on the inhibition of NNK-induced lung neoplasia in A/J mice by arylalkyl isothiocyanates. Carcinogenesis. 1989;10:1757–59. doi: 10.1093/carcin/10.9.1757. [DOI] [PubMed] [Google Scholar]
- 18.Chung FL, Kelloff G, Steele V, Pittman B, Zang E, Jiao D, Rigotty J, Choi CI, Rivenson A. Chemopreventive efficacy of arylalkyl isothiocyanates and N-acetylcysteine for lung tumorigenesis in Fischer rats. Cancer Res. 1996;56:772–78. [PubMed] [Google Scholar]
- 19.Hecht SS. Chemoprevention by Isothiocyanates. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer chemoprevention volume 1: Promising cancer chemopreventive agents. New Jersey: The Humana Press; 2004. pp. 21–35. [Google Scholar]
- 20.von Weymarn LB, Chun JA, Hollenberg PF. Effects of benzyl and phenethyl isothiocyanate on P450s 2A6 and 2A13: potential for chemoprevention in smokers. Carcinogenesis. 2006;27:782–790. doi: 10.1093/carcin/bgi301. [DOI] [PubMed] [Google Scholar]
- 21.Jiao D, Smith TJ, Yang CS, Pittman B, Desai D, Amin S, Chung FL. Chemopreventive activity of thiol conjugates of isothiocyanates for lung tumorigenesis. Carcinogenesis. 1997;18:2143–47. doi: 10.1093/carcin/18.11.2143. [DOI] [PubMed] [Google Scholar]
- 22.Yang YM, Conaway CC, Chiao JW, Wang CX, Amin S, Whysner J, Dai W, Reinhardt J, Chung FL. Inhibition of benzo[a]pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62:2–7. [PubMed] [Google Scholar]
- 23.Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–57. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 24.Estensen RD, Wattenberg LW. Studies of chemopreventive effects of myo-inositol on benzo[a]pyrene-induced neoplasia of the lung and forestomach of female A/J mice. Carcinogenesis. 1993;14:1975–77. doi: 10.1093/carcin/14.9.1975. [DOI] [PubMed] [Google Scholar]
- 25.Wattenberg LW, Estensen RD. Chemopreventive effects of myo-mositol and dexamethasone on benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced pulmonary carcinogenesis in female A/J mice. Cancer Res. 1996;56:5132–35. [PubMed] [Google Scholar]
- 26.Wattenberg LW. Chemoprevention of pulmonary carcinogenesis by myo-inositol. Anticancer Res. 1999;19:3659–61. [PubMed] [Google Scholar]
- 27.Lam S, McWilliams A, leRiche J, MacAulay C, Wattenberg L, Szabo E. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15:1526–31. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS, Kenney PMJ, Wang M, Upadhyaya P. Dose-response study of myo-inositol as an inhibitor of lung tumorigenesis induced in A/J mice by benzo(a)pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 2001;167:1–6. doi: 10.1016/s0304-3835(01)00454-2. [DOI] [PubMed] [Google Scholar]
- 29.Hecht SS, Upadhyaya P, Wang M, Bliss RL, McIntee EJ, Kenney PMJ. Inhibition of lung tumorigenesis in A/J mice by N-acetyl-S-(N-2-phenethylcarbamoyl)-L-cysteine and myo-inositol, individually and in combination. Carcinogenesis. 2002;23:1255–61. doi: 10.1093/carcin/23.9.1455. [DOI] [PubMed] [Google Scholar]
- 30.Mennicke WH, Gorler K, Krumbiegel G. Metabolism of some naturally occurring isothiocyanates in the rat. Xenobiotica. 1983;13:203–7. doi: 10.3109/00498258309052256. [DOI] [PubMed] [Google Scholar]
- 31.Hecht SS, Lin D, Castonguay A. Effects of α-deterium substitution on the mutagenicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Carcinogenesis. 1983;4:405–10. doi: 10.1093/carcin/4.3.305. [DOI] [PubMed] [Google Scholar]
- 32.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 33.Grdina DJ, Murley JS, Kataoka Y. Radioprotectants: current status and new directions. Oncology. 2002;63:2–10. doi: 10.1159/000067146. [DOI] [PubMed] [Google Scholar]
- 34.Nikitin AY, Alcaraz A, Anver MR, Bronson RT, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64:2307–16. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 35.Estensen RD, Jordan MM, Wiedmann TS, Galbraith AR, Steele VE, Wattenberg LW. Effect of chemopreventive agents on separate stages of progression of benzo[a]pyrene induced lung tumors in A/J mice. Carcinogenesis. 2004;25:197–201. doi: 10.1093/carcin/bgg196. [DOI] [PubMed] [Google Scholar]
- 36.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 37.Stinn W, Teredesai A, Kuhl P, Knoerr-Wittmann C, Kindt R. Mechanisms involved in A/J mouse lung tumorigenesis induced by inhalation of an environmental tobacco smoke surrogate. Inhal Toxicol. 2005;17:263–76. doi: 10.1080/08958370590922544. [DOI] [PubMed] [Google Scholar]
- 38.Akagi K, Sano M, Ogawa K, Hirose M, Goshima H, Shirai T. Involvement of toxicity as an early event in urinary bladder carcinogenesis induced by phenethyl isothiocyanate, benzyl isothiocyanate, and analogues in F344 rats. Toxicol Pathol. 2003;31:388–96. doi: 10.1080/01926230390202326. [DOI] [PubMed] [Google Scholar]
- 39.Hirose M, Yamaguchi T, Kimoto N, Ogawa K, Futakuchi M, Sano M, Shirai T. Strong promoting activity of phenylethyl isothiocyanate and benzyl isothiocyanate on urinary bladder carcinogenesis in F344 male rats. Int J Cancer. 1998;77:773–77. doi: 10.1002/(sici)1097-0215(19980831)77:5<773::aid-ijc17>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Sugiura S, Ogawa K, Hirose M, Takeshita F, Asamoto M, Shirai T. Reversibility of proliferative lesions and induction of non-papillary tumors in rat urinary bladder treated with phenylethyl isothiocyanate. Carcinogenesis. 2003;24:547–53. doi: 10.1093/carcin/24.3.547. [DOI] [PubMed] [Google Scholar]
- 41.Bollard M, Stribbling S, Mitchell S, Caldwell J. The disposition of allyl isothiocyanate in the rat and mouse. Food Chem Toxicol. 1997;35:933–43. doi: 10.1016/s0278-6915(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 42.Eklind KI, Morse MA, Chung FL. Distribution and metabolism of the natural anticarcinogen phenethyl isothiocyanate in A/J mice. Carcinogenesis. 1990;11:2033–36. doi: 10.1093/carcin/11.11.2033. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Zhang Z, Kastens E, Lubet RA, You M. Mice with alterations in both p53 and Ink4a/Arf display a striking increase in lung tumor multiplicity and progression: differential chemopreventive effect of budesonide in wild-type and mutant A/J mice. Cancer Res. 2003;63:4389–95. [PubMed] [Google Scholar]
- 44.Zhang Z, Wang Y, Lantry LE, Kastens E, Liu G, Hamilton AD, Sebti SM, Lubet RA, You M. Farnesyltransferase inhibitors are potent lung cancer chemopreventive agents in A/J mice with a dominant-negative p53 and/or heterozygous deletion of Ink4a/Arf. Oncogene. 2003;22:6257–65. doi: 10.1038/sj.onc.1206630. [DOI] [PubMed] [Google Scholar]
- 45.Yang GY, Wang ZY, Kim S, et al. Characterization of early pulmonary hyperproliferation and tumor progression and their inhibition by black tea in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model with A/J mice. Cancer Res. 1997;57:1889–94. [PubMed] [Google Scholar]
- 46.Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone–induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- 47.Yang CS, Yang GY, Landau JM, Kim S, Liao J. Tea and tea polyphenols inhibit cell hyperproliferation, lung tumorigenesis, and tumor progression. Exp Lung Res. 1998;24:629–39. doi: 10.3109/01902149809087391. [DOI] [PubMed] [Google Scholar]
- 48.Hecht SS, Kenney PM, Wang M, Trushin N, Upadhyaya P. Effects of phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 2000;150:49–56. doi: 10.1016/s0304-3835(99)00373-0. [DOI] [PubMed] [Google Scholar]
- 49.International Agency for Research on Cancer. Cruciferous vegetables, isothiocyanates and indoles. Vol. 9. Lyon: IARC Printing Press; 2004. IARC Handbooks of Cancer Prevention; pp. 171–76. [Google Scholar]
- 50.Crowell JA, Steele VE, Fay JR. Targeting the Akt protein kinsae for cancer chemoprevention. Mol Cancer Ther. 2007;6:2139–48. doi: 10.1158/1535-7163.MCT-07-0120. [DOI] [PubMed] [Google Scholar]
- 51.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 52.Jyonouchi H, Sun S, Iijima K, Wang M, Hecht SS. Effects of anti-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene on human small airway epithelial cells and the protective effects of myo-inositol . Carcinogenesis. 1999;20:139–45. doi: 10.1093/carcin/20.1.139. [DOI] [PubMed] [Google Scholar]
- 53.Shamsuddin AM, Ullah A, Chakravarthy A. Inositol and inositol hexaphospahte suppress cell proliferation and tumor formation in CD-1 mice. Carcinogenesis. 1989;10:1461–63. doi: 10.1093/carcin/10.8.1461. [DOI] [PubMed] [Google Scholar]
- 54.Kim HJ, Jang Y, Kim H, Kwon YH. Apoptotic effect of IP6 was not enhanced by co-treatment with myo-inositol in prostate cancer cells. Nutr Res Pract. 2007;1:195–99. doi: 10.4162/nrp.2007.1.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]