Abstract
The absolute configuration of the predominant Cecropia hormone, methyl 12,14-dihomojuvenate, has been determined to be methyl (E,E)-(10R,11S)-(+)-10,11-epoxy-7-ethyl-3,11-dimethyl-2, 6-tridecadienoate (I). The less abundant hormone, methyl 12-homojuvenate, can be presumed by analogy to have the corresponding 3,7,11-trimethyldienoate structure (II). The assignment has been established with microamounts of substance by applying Horeau's method to the glycol derivative (III) of the hormone.
The course of the perchloric acid-catalyzed epoxide ring opening of I was checked by conducting the conversion in 18O-labeled water. It has been ascertained that the configuration at the secondary hydroxyl group of the resulting III remained unchanged. On the other hand, the hydration proceeded with a surprisingly high rate of cis opening.
Keywords: Horeau's method, glycol, epoxide hydration, PMR, mass spectroscopy
Full text
PDF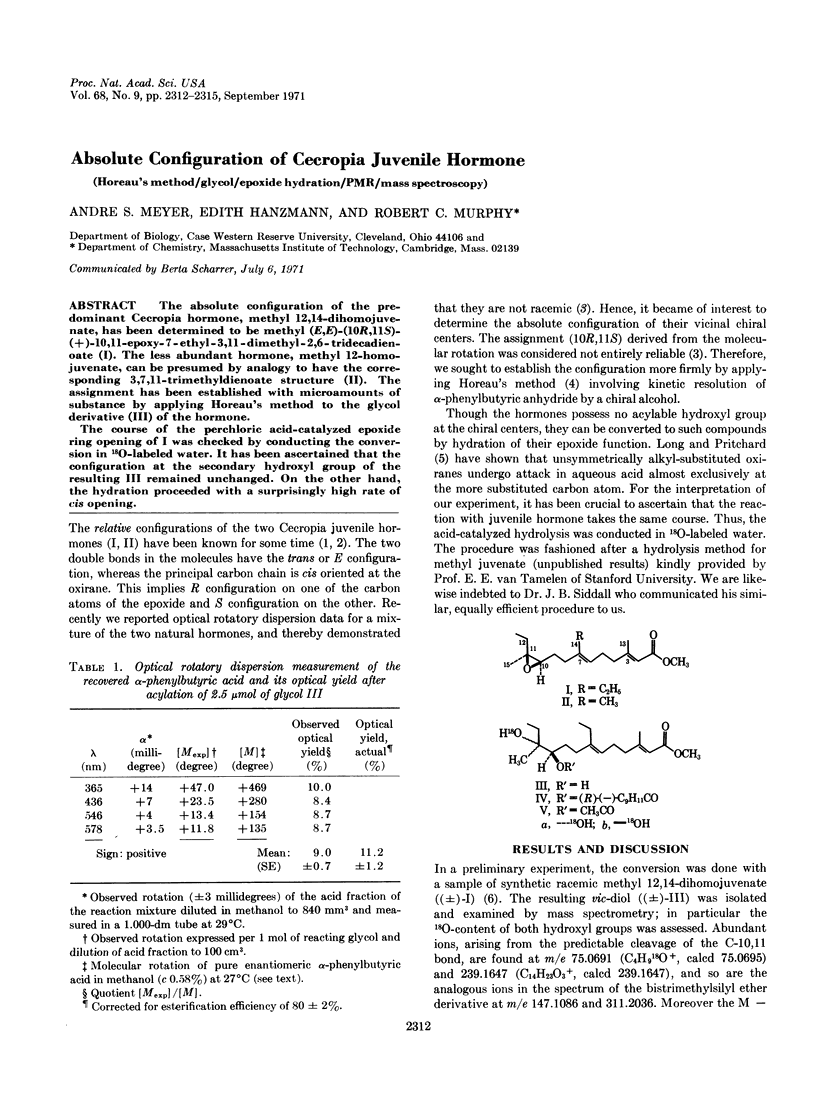



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaudhuri N. K., Nickolson R., Kimball H., Gut M. The synthesis and stereochemistry of (22R)-20 alpha, 22- and (22S)-20 alpha, 22-dihydroxycholesterol. Steroids. 1970 Apr;15(4):525–539. doi: 10.1016/s0039-128x(70)80081-2. [DOI] [PubMed] [Google Scholar]
- Horeau A., Kagan H. B. Détermination des configurations par "dédoublement partiel". 3. Alcools stéroides. Tetrahedron. 1964 Nov;20(11):2431–2441. doi: 10.1016/s0040-4020(01)90823-3. [DOI] [PubMed] [Google Scholar]
- Johnson W. S., Campbell S. F., Krishnakumaran A., Meyer A. S. Total synthesis of the racemic form of the second juvenile hormone (methyl 12-homojuvenate) from the cecropia silk moth. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1005–1009. doi: 10.1073/pnas.62.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew P., Siddall J. B., Spain V. L., Werthemann L. A short stereoselective synthesis of cecropia juvenile hormone. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1462–1464. doi: 10.1073/pnas.67.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. S., Hanzmann E., Schneiderman H. A., Gilbert L. I., Boyette M. The isolation and identification of the two juvenile hormones from the Cecropia silk moth. Arch Biochem Biophys. 1970 Mar;137(1):190–213. doi: 10.1016/0003-9861(70)90427-3. [DOI] [PubMed] [Google Scholar]
- Meyer A. S., Hanzmann E. The optical activity of the Cecropia juvenile hormones. Biochem Biophys Res Commun. 1970 Nov 25;41(4):891–893. doi: 10.1016/0006-291x(70)90167-1. [DOI] [PubMed] [Google Scholar]
- Meyer A. S., Schneiderman H. A., Hanzmann E., Ko J. H. The two juvenile hormones from the cecropia silk moth. Proc Natl Acad Sci U S A. 1968 Jul;60(3):853–860. doi: 10.1073/pnas.60.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mors W. B., dos Santos Filho M. F., Monteiro H. J., Gilbert B., Pellegrino J. Chemoprophylactic agent in schistosomiasis: 14,15-epoxygeranylgeraniol. Science. 1967 Aug 25;157(3791):950–951. doi: 10.1126/science.157.3791.950. [DOI] [PubMed] [Google Scholar]



