Abstract
Aim:
The present study attempts to explore the oral hygiene practices and oral health status in autistic patients as compared to nonaffected, same aged healthy individuals.
Materials and Methods:
The oral hygiene practices, prevalence of caries and periodontal status were evaluated in 117 autistic patients and 126 healthy individuals. The test and control groups were divided into three categories, based on the type of dentition as Primary dentition (Category 1), Mixed dentition (Category 2) and Permanent dentition (Category 3). Plaque and gingival status was recorded by plaque index (Loe, 1967) and gingival index (Loe, 1967), periodontal status by community periodontal index of treatment needs and dental caries by DMFT/DEF index. Statistical analysis was done using descriptive statistics, independent sample t-test, contingency coefficient test and one-way ANOVA test by SPSS 14 software.
Results:
There was no statistically significant difference in the brushing habits between autistics and controls (P = 0.573); however, Autistics required assistance in brushing. Prevalence of caries was significantly lower in autistic patients (P = 0.000). Plaque and gingival scores were significantly higher in autistic patients (P = 0.000) and prevalence of periodontal disease was significantly higher in autistic patients (P = 0.000). Greater number of autistic patients required professional scaling and root planing (P = 0.000).
Conclusion:
The present study suggests that autistic patients have a higher rate of periodontal disease and lower caries compared to controls. Attempts should be made by parents, general dentists and periodontists to teach oral hygiene methods to these patients by constant repetition and patience, as autistic individuals can develop skills over a period of time and lead a more productive and independent life.
Keywords: Autism, dental caries, oral hygiene, periodontal status
INTRODUCTION
Autism is a neurodevelopmental condition characterized by impairments in social interaction, impaired communication and restricted, repetitive, or stereotyped behaviors.[1] Autism specifically affects brain function in the areas responsible for the development of communication and social interaction skills. The hallmark of autism is the lack of communication skills. Affected children also have problems with language, behavior and social skills. Early diagnosis, early intensive remedial education and behavioral therapy significantly enhance the child's social functioning.[2]
Autism was first described in 1943 by US psychologist, Leo Kanner, affecting boys 3-4 times more often than girls.[2] Common etiological factors proposed include post-encephalitic infection or sepsis, genetic and autoimmune factors and vitamin D deficiency. Family income, education, and lifestyle do not seem to affect the risk of autism.[3]
The risk of dental caries and gingivitis is expected to be higher in these patients due to improper brushing and flossing because of the difficulties the trainers and parents encounter when they brush the children's teeth. It could also be due to a lack of necessary manual dexterity of autistic children. In general, children with autism prefer soft and sweetened foods, and they tend to pouch food inside the mouth instead of swallowing it due to poor tongue coordination, thereby increasing the susceptibility to caries. Also, children diagnosed with autism spectrum disorder (ASD) are prescribed psychoactive drugs or anticonvulsants, and the presence of generalized gingivitis might be the side-effects of these medications.[4] Children with autism have multiple medical and behavioral problems, making their dental treatment extremely difficult. Several studies show that autistic children demonstrate self-injurious behavior (SIB), aggression, odd responses to sensory stimuli, unusual food likes or dislikes. They also have abnormalities of mood and excessive fear, causing injuries to their head, neck or mouth.[5]
In general, relatively little has been written about autism in developing countries as compared with North America and Europe. While this disorder is not rare, majority of people in India have not been diagnosed and do not receive the services they need due to lack of awareness among medical professionals.[6]
Information on the patterns of development of the disease in the population is important because it acts as a foundation for the planing of public oral health policies.[4] The present study would attempt to explore the oral hygiene practices and oral health status in such patients as compared to the control group.
MATERIALS AND METHODS
Approval to conduct this study was obtained from the Ethical Committee of our institution. One hundred and seventeen patients diagnosed with autism were screened for the study from Academy for Severely Handicapped and Autistics, ranging from the age 5 to 22 years. A total of 126 healthy individuals of the same age group were screened from a school of our town to form the control group. The Directors of these institutions signed the informed consent.
The selection criteria for the sample were: Patients diagnosed to have autism for the test group / cases, Normal patients of similar age group as controls. Exclusion criteria were Dental treatment in the last 6 months, any other systemic disease known to cause dental problems and uncooperative patient. Age-matched controls with similar socioeconomic status were selected from a regular school.
The test and control groups were divided into three categories, based on the type of dentition present as primary dentition (Category 1), mixed dentition (Category 2) and permanent dentition (Category 3). In test group, Category 1 consisted of 11 patients, Category 2 of 48 patients and Category 3 of 58 patients. In the control group category 1 consisted of 13 patients, Category 2 of 58 patients and Category 3 of 53 patients.
Following a complete medical history and drug history if any and personal history regarding oral hygiene practices, all patients were examined by one examiner using a dental mirror, explorer, straight explorer and a CPITN Probe under artificial light. Patients were questioned about their frequency of brushing, method of brushing, if any other dental aids were used and whether they could brush themselves or needed assistance in brushing. Plaque and gingival status was recorded using plaque index (Loe, 1967)[7] and gingival index (Loe, 1967)[7] respectively for the entire dentition. Periodontal status was assessed by community periodontal index of treatment needs and dental caries by DMFT/DEF index.
The periodontal status was recorded using the CPITN. Due to the evidences of higher periodontal disease in autistics, the CPITN was slightly modified and pocket depths were also recorded in children below 15 years of age in both cases and control groups. However, probing was not done on erupting teeth and they were excluded. According to the CPITN, the dentition was divided into six sections (left/right maxillary/mandibular posterior teeth, maxillary/mandibular anterior teeth). Each section was examined only if two or more teeth were present and not scheduled for extraction. Code Zero for indicates healthy periodontium, code one indicates bleeding on probing, code two indicates plaque or other retentive factors, code three indicates pathological pocket 4-5 mm in depth and code four indicates periodontal pocket 6mm or more in depth. TN1 indicates a need for improving the oral hygiene only, TN2 indicates scaling and root planing and TN3 indicates complex periodontal treatment.
The dental caries was measured using the decayed, missing, and filled teeth index (DMFT) for primary (0-5 years) and early-mixed dentitions (6-10 years). Decayed, missing, and filled teeth index (DMFT) was used for late-mixed (11-15 years) and permanent dentitions (16 years and older). According to the codes and criteria established by the World Health Organization, a tooth was considered decayed when there was frank carious cavitation, as missing if it was extracted due to caries and as filled if it had a restoration for a carious lesion. Exfoliated teeth in the primary and mixed dentition, unerupted, and those extracted for other reasons apart from caries were not included in the indices.
Statistical analysis
It was done using descriptive statistics, independent sample t-test, contingency coefficient test and one way ANOVA test using SPSS 14 software. Statistical significance was accepted at P < 0.05.
RESULTS
Oral hygiene practices
A total of 117 autistic patients and 126 healthy individuals were examined. Table 1 shows that around 66% (65.81%) of the cases and around 68% (68.25%) of the controls brushed their teeth once daily. Hence, there was no statistically significant difference in the brushing habits between cases and controls (P = 0.573). Table 2 shows that on intra group comparison, no statistically significant difference in the brushing habits was seen in between categories in both the cases (P = 0.235) and controls (P = 0.265). Figure 1 shows that autistic children require assistance in brushing their teeth, and self-brushing by these children increased with an increase in age which is statistically significant (P = 0.001). While the controls showed self-brushing in all the categories.
Table 1.
Intergroup comparison of brushing habits
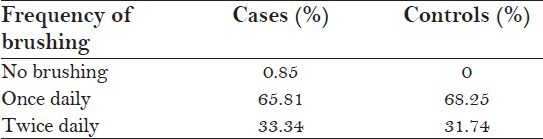
Table 2.
Intragroup comparison of brushing habits
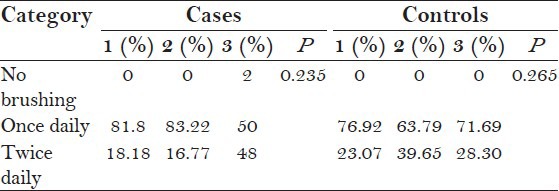
Figure 1.
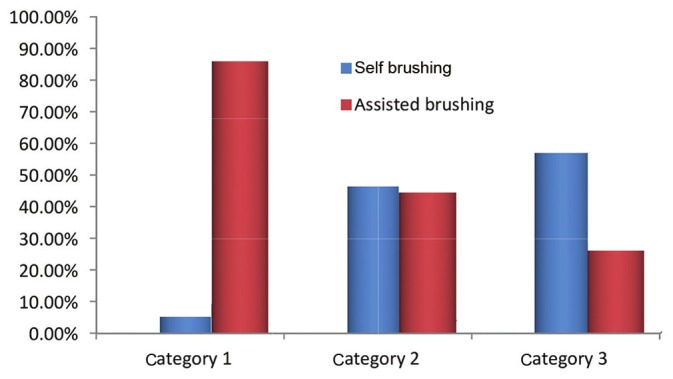
Method of brushing in autistic group
Oral health status
The mean DMFT score in cases was 1.2966 and in controls 3.736 [Figure 2]. The prevalence of caries was lower in autistic patients with a statistical significance of P = 0.000, the incidence of caries was increasing with age in both cases and controls [Table 3]. The mean PI and GI scores in cases were 1.3039 and 1.0015, respectively, and in controls were 1.0015 and 0.8542, respectively [Figure 2]. The scores were statistically higher in cases than in controls (P = 0.000 and P = 0.000, respectively), with the incidence increasing with age in both cases and controls [Table 3].
Figure 2.
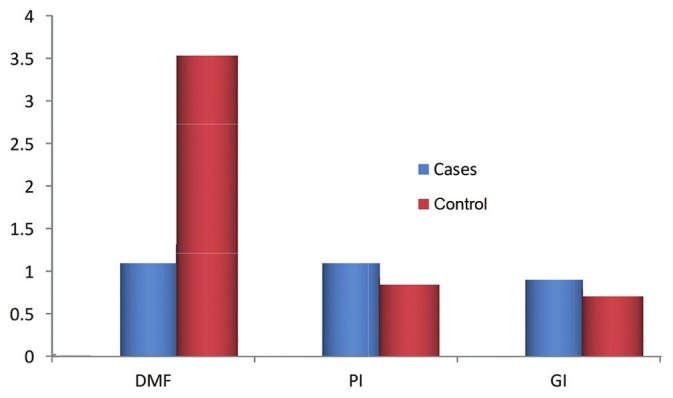
Intergroup distribution of oral diseases
Table 3.
Intragroup distribution of oral diseases
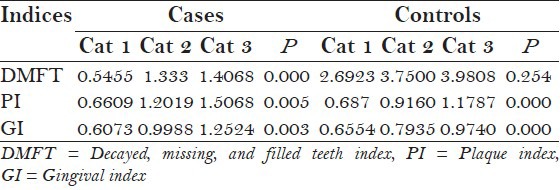
Periodontal status
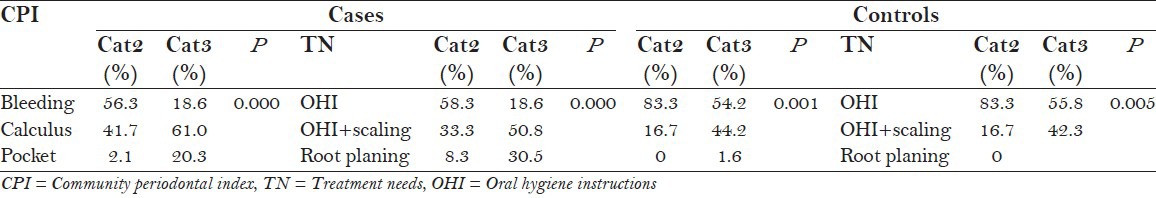
CPITN was recorded only in Category 2 and Category 3, which showed that prevalence of periodontal disease was significantly higher in autistic patients (P = 0.000) [Figure 3] and greater number of autistic patients required professional scaling and root planing (P = 0.000) [Figure 4]. The incidence of periodontal disease and need for periodontal treatment increases with an increase in age in both cases and controls which is statistically significant [Table 4].
Figure 3.
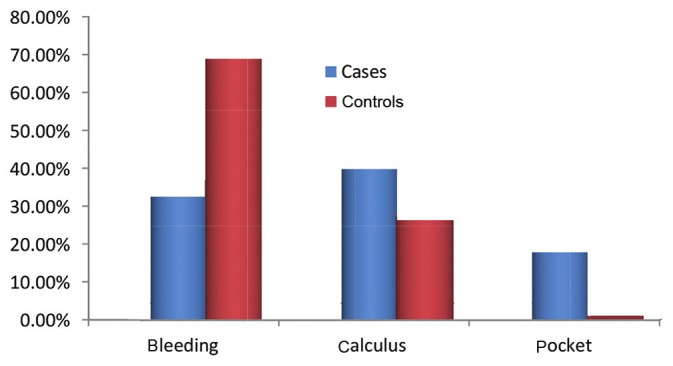
Intergroup comparison of CPI scores
Figure 4.
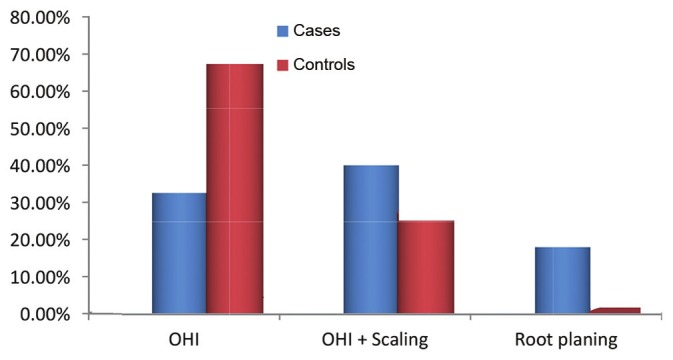
Intergroup comparison of TN scores
Table 4.
Intragroup comparison of CPI and TN scores
DISCUSSION
Over the past decade, autism has emerged as a major public health concern in many countries, characterized by a complex, behaviorally defined, static immature brain disorder.[8] Providing oral care to children with autism requires patience and a thorough understanding of the patient's degree of mental disability. Thus effective oral health promotion strategies need to be implemented to improve the oral health status of autistic children.
A total of 117 autistic patients and 126 healthy individuals were examined for the study in the age group of 5-22 years. In cases male-female ratio of 3.6:1 was observed, which was consistent with previously reported sex ratios of 2.8:1,[4] 4:1,[9] 3.7:1[10] and 4.3:1,[11] reflecting the higher prevalence of autism in males. The mean age among cases was 12.8 years, while in controls it was 12.3 years.
On examining the oral hygiene practices it was observed that the majority of the subjects from both groups brushed their teeth once daily. Patients with autism probably lacked manual dexterity and hence frequently needed assistance in brushing whereas in the controls all brushed by themselves. In autistics, as age increased more children could brush without assistance. Occasional use of mouthwash was seen in the autistic patients under supervision of care takers, flossing was not done in either of the groups. The authors did not come across many studies which recorded the oral hygiene practices in autistics. However, in a study by Pilebro et al., he quoted that parents usually assisted their autistic children in brushing their teeth at least once daily.[12]
In the present study, prevalence of caries was lower in autistics compared to, controls which was statistically significant. This is in accordance with Jaber et al.,[4] Loo et al.,[13] Namal et al.[12] However, studies by Tharapiwattananon et al.[14] have shown a higher caries incidence in autistics. Lower caries in autistics can be attributed to the good supervision by the parents and school teachers in the child's daily life activities like tooth brushing and lack of in-between snacking. The subjects in the case group for the present study were selected from a school that had a strict schedule for meals with lack of in between snacking. In a study with similar result Shapira et al.[15] concluded that the lower caries in autistics was due to less cariogenic diet, regular behavior at meals, and the autistics being less partial to sweets. Bassoukou[16] et al. in a study to evaluate saliva flow rate, buffer capacity and dental caries experience in autistics compared to healthy group found a lower caries incidence in autistics. In autistics neither a higher flow rate nor a better buffer capacity of saliva was found as compared to controls. In our study, even though overall the caries incidence was lower in autistics compared to controls, on intra group comparison it was found that DMFT score increased with increase in age in autistics whereas in control group no statistically significant increase in DMFT was seen with growing age.
On examining the periodontal status, we found that autistics had statistically significant increase in PI, GI, CPI and TN scores which are in accordance with Shapira et al.[15] and Luppanapornlap et al.[5] It was very perplexing to know that autistics had a lower caries incidence and higher incidence of periodontal disease. Shapira et al.15 also quote a high periodontal disease and low caries incidence in autistic patients, which was unexplained. In our study, about 43% of the autistic patients required professional scaling and 20.6% required scaling and root planing whereas in the control group only 26% required professional scaling and 0.9% required professional scaling and root planing. This can be explained by the fact that autistic patients cannot brush as effectively as their normal counter parts. In a study with similar result, Luppanapornlap et al.5 stated that poor hand coordination leads to difficulty in maintaining good oral hygiene in autistics, thus increasing gingival diseases. This can be explained by various factors. Medina et al.17 has stated that self-injurious habits can also be the reason for increased gingival diseases. It was found in our study that around 28% of the autistic children had a history of bruxism and lip biting. Friedlander et al.18 stated that the changes in gingiva can be due to the side effects of medications given to autism patients. All the cases examined in the present study were under medication: Allopathic, ayurvedic or homeopathic. Around half of the autistic patients were on ayurvedic and homeopathic treatment, and the rest on allopathic medicines which included antidepressants and anticonvulsants. However, a complete, detailed drug history could not be recorded due to the complexity of the treatment and non-compliance of the parents, which we consider a drawback in our study. In the case group, two patients gave history of drug-induced gingival enlargement for which dental treatment was taken and the medication was substituted; the drug history, however, could not be elicited. However, two more patients presented with gingival enlargement. One patient was a 9-year-old male patient on homeopathic treatment and the other was a 14-year-old boy on Resperidone 0.5 mg twice daily. None in the control group had gingival enlargement.
In a recent study by Rashid et al.19 who assessed the serum and salivary oxidative stress biomarkers and evaluated the oral health status in autistics, it was shown that autistics had higher levels of serum and salivary melanodialdehyde (MDH) and lower levels of serum and salivary superoxide dismutase (SOD). Melanodialdehyde (MDH) is a product of lipid peroxidation which releases free radicals.[20] Free radical species have been implicated in the pathogenesis of periodontal diseases.[21] SOD is an antioxidant, which plays a vital role in the protective mechanism of periodontium.[22] Thus; these factors can be related to the increased periodontal disease in the autistics. However, the same has to be proved by further studies.
The prevalence of autism has been increasing drastically over the past few decades. Due to tremendous lack of awareness, this special group is often neglected. Few studies over the past show a higher rate of oral diseases among the autistic patients. However, the results of present study are that autistics have a lower caries rate and higher rate of periodontal diseases. This study also evaluates the oral hygiene practices and finds that autistic patients usually require assistance in brushing their teeth. The present study has a larger sample size compared to the previous studies and also the first among Indian population. However, we could not elaborate the reason for higher periodontal disease and lower caries. Thus, the future studies should be directed toward the reasoning of this along with a larger sample size and long-term follow-up.
CONCLUSIONS
The present study suggests that autistic patients have a higher rate of periodontal disease and lower caries compared to controls. Thus, children with autism require special dental management to improve their oral health by maintaining efficient oral hygiene. Attempts should be made by parents, general dentists and periodontists to teach oral hygiene methods to these patients by constant repetition and patience, as autistic individuals can develop skills over a period of time and lead a more productive and independent life.
ACKNOWLEDGMENT
A special thanks to Academy for Severly Handicapped and Autistics (ASHA), Mr. Lancy (statistician).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Barbaresi WJ, Katusic SK, Voigt RG. Autism: A review of the state of the science for pediatric primary health care clinicians. Arch Pediatr Adolesc Med. 2006;160:1167–75. doi: 10.1001/archpedi.160.11.1167. [DOI] [PubMed] [Google Scholar]
- 2.Daniel R. Dental management of children with autism. Pediatric Dental Health. 2005 [Google Scholar]
- 3.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–86. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 4.Jaber MA, Sayyab M, Abu Fanas SH. Oral health status and dental needs of autistic children and young adults. J Investig Clin Dent. 2011;2:57–62. doi: 10.1111/j.2041-1626.2010.00030.x. [DOI] [PubMed] [Google Scholar]
- 5.Luppanapornlarp S, Leelataweewud P, Putongkam P, Ketanont S. Periodontal status and orthodontic treatment need of autistic children. World J Orthod. 2010;11:256–61. [PubMed] [Google Scholar]
- 6. [Last accessed on 2012 March 19]. Available from: http://www.autism-india.org .
- 7.Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38:610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 8.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–86. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Mental health in the United States: Parental report of diagnosed autism in children aged 4-17 years–United States, 2003-2004. MMWR Morb Mortal Wkly Rep. 2006;55:481–6. [PubMed] [Google Scholar]
- 10.Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: An update. J Autism Dev Disord. 2003;33:365–82. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- 11.Pilebro C, Bäckman B. Teaching oral hygiene to children with autism. Int J Paediatr Dent. 2005;15:1–9. doi: 10.1111/j.1365-263X.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 12.Namal N, Vehit HE, Koksal S. Do autistic children have higher levels of caries? A cross-sectional study in Turkish children. J Indian Soc Pedod Prev Dent. 2007;25:97–102. doi: 10.4103/0970-4388.33457. [DOI] [PubMed] [Google Scholar]
- 13.Loo CY, Graham RM, Hughes CV. The caries experience and behavior of dental patients with autism spectrum disorder. J Am Dent Assoc. 2008;139:1518–24. doi: 10.14219/jada.archive.2008.0078. [DOI] [PubMed] [Google Scholar]
- 14.Tharapiwattananon T. Autistic child and dental management. CU Dent J. 1994;17:1–10. [Google Scholar]
- 15.Shapira J, Mann J, Tamari I, Mester R, Knobler H, Yoeli Y, et al. Oral health status and dental needs of an autistic population of children and young adults. Spec Care Dentist. 1989;9:38–41. doi: 10.1111/j.1754-4505.1989.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 16.Bassoukou IH, Nicolau J, dos Santos MT. Saliva flow rate, buffer capacity, and pH of autistic individuals. Clin Oral Investig. 2009;13:23–7. doi: 10.1007/s00784-008-0209-5. [DOI] [PubMed] [Google Scholar]
- 17.Medina AC, Sogbe R, Gómez-Rey AM, Mata M. Factitial oral lesions in an autistic paediatric patient. Int J Paediatr Dent. 2003;13:130–7. doi: 10.1046/j.1365-263x.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 18.Friedlander AH, Yagiela JA, Paterno VI, Mahler ME. The neuropathology, medical management and dental implications of autism. J Am Dent Assoc. 2006;137:1517–27. doi: 10.14219/jada.archive.2006.0086. [DOI] [PubMed] [Google Scholar]
- 19.Rashid MH, Al-Jubouri RH. Assessment of serum and salivary oxidative stress biomarkers with evaluation of oral health status in a sample of autistic male children. J Bagh Coll Dent. 2011;23:56–60. [Google Scholar]
- 20.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;(43):160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 21.Asman B, Engström PE, Olsson T, Bergström K. Increased luminol enhanced chemiluminescence from peripheral granulocytes in juvenile periodontitis. Scand J Dent Res. 1984;92:218–23. doi: 10.1111/j.1600-0722.1984.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 22.Brock GR. PhD Thesis. University of Birmingham; 2005. The role of antioxidants in the inflammatory periodontal diseases. [Google Scholar]


