Abstract
Objectives.
This study expands on previous research by examining the effects of prolonged grief disorder (PGD) symptoms and bereavement on diurnal cortisol patterns above and beyond depressive symptomatology.
Methods.
Drawing on information from 56 depressed older adults, 3 groups were compared: (1) a depressed nonbereaved group, (2) a depressed bereaved without elevated PGD symptoms group, and (3) a depressed bereaved with elevated PGD symptoms group. Multilevel modeling was used to examine differences in diurnal cortisol profiles between these 3 groups, controlling for demographic factors and depressive symptoms.
Results.
Results revealed that those who were bereaved had more dysregulated cortisol patterns, but PGD symptomatology seemed to have little effect. Subsidiary analysis with just the bereaved participants suggests that those who were recently widowed may have had greater cortisol dysregulation compared with other bereaved individuals in the sample.
Discussion.
These findings suggest that the circumstance of being bereaved may be associated with more dysregulated cortisol, regardless of PGD symptomatology. This pattern of results might reflect greater disturbance in daily routines among bereaved individuals and acute stress in the case of those experiencing the recent loss of a spouse, which leads to disruption in circadian rhythms and the diurnal cycle of cortisol.
Key Words: Biomarkers, Death and dying, Widowhood, Salivary cortisol, Complicated grief.
The loss of a loved one can have serious health consequences (Jones, Bartrop, Forcier, & Penny, 2010; Stroebe, Schut, & Stroebe, 2007). Most notably, widowed and other bereaved individuals are at significantly higher risk for mortality—a trend which is often referred to as the “broken-heart” phenomenon (Moon, Kondo, Glymour, & Subramanian, 2011). However, the precise mechanisms by which this phenomenon occurs are poorly understood. Studies have documented a number of more intermediate health outcomes among bereaved individuals, including depressed immune functioning (Bartrop, Luckhurst, Lazarus, Kiloh, & Penny, 1994), cardiovascular risk factors (Buckley, McKinley, Tofler, & Bartrop, 2010), physical pain (Bradbeer, Helme, Yong, Kendig, & Gibson, 2003), and increased medication usage (Thompson, Breckenridge, Gallagher, & Peterson, 1984). Although these studies certainly point to the gravity of loss for health and well-being, it remains unclear the extent to which differences in physical health outcomes reflect increased depressive symptoms, severe grief reactions, or the stress of the bereavement itself. Thus, the purpose of this study is to disentangle the unique effects of bereavement and grief on cortisol—an objective biomarker of stress and physical health.
Cortisol is regarded by many as a crucial biological intermediary by which chronic stress gets “underneath the skin” and leads to disease (Sapolsky, Romero, & Munck, 2000). In particular, stressful life circumstances, like bereavement, often stimulate hypothalamic–pituitary–adrenocortical (HPA) axis functioning, and activation of this hormonal response system ultimately results in increased blood levels of cortisol, frequently referred to as the “stress hormone” (Lovallo, 2005). A daily pattern characterized by peak levels of cortisol at wake and a steep negative slope is generally regarded as “normal”; whereas, a shallower or flatter cortisol slope is typically considered an indication of dysregulation. The recent meta-analysis of Miller, Chen, and Zhou (2007) supported this idea and found that, compared with nonstressed controls, chronically stressed groups generally had a dysregulated pattern of hormone secretion, with lower than normal morning output but higher than expected secretion across the rest of the day, yielding a flattened diurnal pattern. These prolonged elevations in cortisol can increase allostatic load and compromise the immune system, ultimately increasing one’s risk for autoimmune-related and metabolic disorders (Sapolsky et al., 2000).
With regard to bereavement and cortisol in particular, previous studies generally suggest that the loss of a loved one is associated with more dysregulated cortisol patterns (Miller et al., 2007; Stroebe et al., 2007). Among these studies, most have compared cortisol patterns between bereaved and nonbereaved individuals, sometimes matching for demographic factors (e.g., age, sex; Gerra et al., 2003) and sometimes not matching (Luecken, 1998). Despite the methodological advantages of many of these studies (e.g., using a controlled design), uncertainty remains about whether the observed differences are due to increased distress or bereavement per se because groups likely differed on factors other than bereavement status (e.g., depression or anxiety symptoms). Two additional studies have compared bereaved individuals who are particularly distressed with less distressed bereaved individuals, and generally speaking these studies suggest that bereaved individuals who are depressed or show complications in their grieving process have more dysregulated cortisol patterns (O’Connor, Wellisch, Stanton, Olmstead, & Irwin, 2012; Roy, Gallucci, Avgerinos, Linnoila, & Gold, 1998). However, even in these studies it is unclear to what extent grief and depressive symptoms uniquely contributed to cortisol dysregulation.
This point is particularly salient, given findings related to prolonged grief disorder (PGD)—a diagnostic label proposed for International Classification of Diseases, volume 11 characterized by intense separation distress, a sense of purposelessness and meaninglessness, and difficulties with day-to-day functioning (Prigerson, Vanderwerker, & Maciejewski, 2008). Notably, PGD symptoms have been found to uniquely predict other physical health outcomes, such as blood pressure, changes in eating/smoking habits, and incidence of cancer, even after controlling for depressive and anxiety symptoms (Prigerson et al., 1997). In a large population-based study in Sweden, unresolved grief reactions were also shown to be associated with increased health care usage, greater sleep problems, and more sick days taken at work (Lannen, Wolfe, Prigerson, Onelov, & Kreicbergs, 2008). Thus, it stands to reason that PGD symptoms may also uniquely predict cortisol, above and beyond depressive symptoms. Such a distinction could have important clinical implications because past research suggests that different types of bereavement interventions may differentially affect depressive and PGD symptoms (Holland, Currier, & Gallagher-Thompson, 2009).
The purpose of this study is to disentangle the unique effects of bereavement and grief (above and beyond depression) on cortisol by comparing three groups of older adults: (1) a depressed nonbereaved group, (2) a depressed bereaved without elevated PGD symptoms group, and (3) a depressed bereaved with elevated PGD symptoms group. This study is unique in several different ways. First, to our knowledge, this is the first study to examine such a research question in an exclusively older adult sample. It is notable that when compared with nondepressed controls, depressed elders are characterized by greater HPA activation, more so than for younger adults (Stetler & Miller, 2011)—highlighting the relevance of cortisol as a crucial biomarker for older individuals in particular. In addition, no previous study has simultaneously examined the effect of depressive symptoms and PGD symptoms on cortisol, limiting the conclusions that can be made about the unique impact of specific psychiatric symptoms. Finally, we also examine diurnal cortisol trajectories using multilevel modeling, which is a more methodologically sound way of examining cortisol data compared with correlation or other traditional statistical approaches (for a discussion of the advantages of this methodology, see Hruschka, Kohrt, & Worthman, 2005). In this study, it is hypothesized that both bereavement and grief will uniquely contribute to dysregulated cortisol patterns (i.e., lower than normal waking cortisol and flatter diurnal slopes; Miller et al., 2007), with Group 3 having the worst outcomes, followed by Group 2 —both of which will show greater dysregulation than Group 1.
Method
Participants
This study draws upon data collected from 60 depressed older adults recruited as part of a larger study on predictors of response to cognitive–behavioral therapy for late-life depression. In the present analysis, 56 participants were included, given that 4 participants did not provide one or more reliable saliva samples. These four excluded participants did not significantly differ from the rest of the sample in terms of demographic factors or depressive symptoms.
Following Institutional Review Board approval, participants were recruited throughout the San Francisco Bay Area, via radio, newspaper and Internet advertisements, flyers, free talks in senior centers, and through referrals from other professionals. Participants were eligible for the study if they were English speaking, had a score of 16 or higher on the Center for Epidemiologic Studies Depression scale (Radloff, 1977), and met diagnostic criteria for some type of current depressive disorder (e.g., major depression, dysthymic disorder, adjustment disorder with depressed mood), as assessed by the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998). Those who reported active suicidal ideation or plan, a history of psychosis or mania, or active substance abuse at baseline were excluded from the study, as well as any individuals who showed frank evidence of some type of dementia. Participants’ demographic and background information are presented in Table 1. Two participants reported being on hormone replacement therapy, and 17 were taking antidepressant medications. In addition, participants had on average experienced 6.0 (SD = 3.2) stressful life events in the past year (e.g., major personal illness or injury, deterioration of financial state), as indicated on the Elders Life Stress Inventory (Aldwin, 1991).
Table 1.
Demographic and Background Information (N = 56)
| Overall | Depressed nonbereaved | Depressed bereaved without elevated PGD | Depressed bereaved with elevated PGD | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Gender | ||||||||
| Men | 22 | 39.3 | 12 | 37.5 | 8 | 53.3 | 2 | 22.2 |
| Women | 34 | 60.7 | 20 | 62.5 | 7 | 46.7 | 7 | 77.8 |
| Ethnicity/race | ||||||||
| Caucasian | 40 | 71.4 | 25 | 78.1 | 8 | 53.3 | 7 | 77.8 |
| African American | 2 | 3.6 | 0 | 0.0 | 2 | 13.3 | 0 | 0.0 |
| Asian American | 6 | 10.7 | 4 | 12.5 | 2 | 13.3 | 0 | 0.0 |
| Latino/Hispanic | 5 | 8.9 | 2 | 6.3 | 2 | 13.3 | 1 | 11.1 |
| Native American | 3 | 5.4 | 1 | 3.1 | 1 | 6.7 | 1 | 11.1 |
| Antidepressants | ||||||||
| Yes | 14 | 25.0 | 11 | 34.4 | 0 | 0.0 | 3 | 33.3 |
| No | 42 | 75.0 | 21 | 65.6 | 15 | 100 | 6 | 66.7 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 69.9 | 7.6 | 70.4 | 7.5 | 69.4 | 7.5 | 68.0 | 7.3 |
| Years of education | 15.0 | 2.6 | 15.3 | 2.8 | 15.4 | 1.8 | 13.8 | 2.6 |
| HRSD | 14.2 | 5.6 | 14.4 | 4.9 | 12.7 | 6.3 | 15.5 | 6.6 |
| ELSI | 6.0 | 3.2 | 5.5 | 3.1 | 6.4 | 3.6 | 7.2 | 3.1 |
Notes. ELSI = Elders Life Stress Inventory; HRSD = Hamilton Rating Scale for Depression; PGD = prolonged grief disorder.
Measures
Loss status.—
For the present analyses, participants were divided into three groups based on their bereavement status and the severity of their grief. Thirty-two participants indicated that they were not “actively grieving” the loss of a loved one, and these individuals were considered depressed non-bereaved individuals (Group 1). Twenty-four individuals responded that they were actively grieving the loss of a loved one. Among the bereaved, the loss of a spouse or partner was most commonly reported (n = 8; 33.0%), followed by the loss of a parent (n = 4; 16.7%), sibling (n = 3; 12.5%), friend (n = 3; 12.5%), or child (n = 1; 4.2%). The vast majority of deaths were due to natural causes (n = 21; 87.5%) and on average losses occurred 3.1 years ago.
Bereaved individuals were divided into two subgroups: depressed bereaved individuals without elevated PGD symptoms (Group 2) and depressed bereaved individuals with elevated PGD symptoms (Group 3). PGD symptoms were assessed with a self-report version of the PG-13 (Prigerson & Maciejewski, 2008), which is based on a set of empirically derived criteria that have demonstrated internal consistency (Cronbach’s α = .82 to .93) and incremental validity (Delalibera, Coelho, & Barbosa, 2011; Prigerson et al., 2009). For the purposes of this study, if a participant reported experiencing a particular PGD symptom “at least once a week” or more frequently (i.e., at least a 3 on a 5-point scale), the symptom was considered to be present for that individual. Bereaved participants who met at least two of the three symptom-based PGD criteria (i.e., separation distress; cognitive, emotional, and behavioral symptoms; and functional impairment) were considered to have elevated PGD symptoms. Using this scoring procedure, 15 participants were categorized as depressed bereaved individuals without elevated PGD symptoms, and 9 participants were categorized as depressed bereaved individuals with elevated PGD symptoms. No significant differences were found between Groups 1, 2, and 3 in terms of depressive symptoms, number of stressful life events in the past year, or demographic factors (see Table 1). However, depressed bereaved individuals without elevated PGD symptoms were significantly less likely to be taking antidepressant medications compared with the other two groups. (The multivariate analysis was run controlling for antidepressant medication and hormone replacement therapy usage, and an identical pattern of results was obtained as that reported in this article.)
Depression.—
Depressive symptoms were assessed with the Hamilton Rating Scale for Depression (HRSD). The HRSD is an interviewer-rated tool composed of 17 questions, 8 on a 5-point scale from 0 to 4, and 9 on a 3-point scale from 0 to 2, with higher scores representing greater severity of depressive symptoms. A recent meta-analytic study showed that the HRSD has good test–retest reliability (mean r = 0.87), internal consistency (mean α = .79), and inter-rater reliability (mean r = 0.87; Trajković et al., 2011), in addition to correlating highly with other established depression measures (Whisman et al., 1989).
Cortisol.—
Salivary cortisol served as our primary dependent variable in this study. Saliva was collected using an oral swab kit at wake, 5:00 p.m., and 9:00 p.m. across 2 consecutive days. Participants were instructed to freeze each sample immediately after collection and return it to research staff at their next appointment, where it was then stored in a laboratory freezer before being assayed. They were also told not to eat, drink, smoke, brush their teeth, or use mouthwash in the 30min before collection and not to drink alcohol during the 8–10hr before collecting samples or during the 2 days of collection. Participants were asked to record the exact time of saliva collection and any events that might have influenced the sample (e.g., recent smoking or alcohol use) in a sample collection log. Samples that were collected improperly were not used in this analysis.
All samples were assayed for salivary cortisol using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 25 μL of saliva per determination, has a lower limit of sensitivity of 0.003 μg/dL, standard curve range from 0.012 to 3.0 μg/dL, an average intraassay coefficient of variation of 3.5%, and an average interassay coefficient of variation of 5.1%. Method accuracy determined by spike and recovery averaged 100.8%, and linearity determined by serial dilution averaged 91.7%. Values from matched serum and saliva samples show the expected strong linear relationship, r(47) = 0.91, p < .0001. To correct for positive skewness, cortisol values were log transformed. (This log transformation was performed by adding 1 to the raw cortisol values and then taking the natural logarithm of that value. We added a constant (i.e., 1) beforehand because many of the raw cortisol values, measured in micrograms per deciliter, were less than 1. In these cases, a log transformation can yield very large negative numbers, thereby skewing the distribution further.)
Plan of Analysis
Multilevel modeling was used to evaluate differences between the nonbereaved and two bereaved groups (with and without elevated PGD symptoms) in terms of their cortisol patterns across the day (Singer & Willett, 2003). This statistical procedure allows parameters to vary at more than one level—in this case, both between and within individuals. All analyses were conducted using the SPSS Mixed program, and parameters were estimated using maximum likelihood. Log-transformed cortisol (log-cortisol) values were used as the dependent variable.
In these analyses, the passage of time was measured as the number of hours since wake, and wake was considered initial status (i.e., time zero). Data collected from the saliva samples across the 2 consecutive days were combined in order to allow for a more reliable estimate of each participants typical diurnal pattern (Kraemer et al., 2006).
Loss status was treated as a dummy-coded variable with depressed nonbereaved individuals serving as the reference group. In addition to examining loss status as an independent variable, demographic factors and depressive symptoms (as measured by the HRSD) were also included as statistical controls. Continuous independent variables (i.e., age, years of education, HRSD scores) were centered by subtracting the sample mean. Dichotomous variables were coded as follows: gender (−1 = women, 1 = men), race/ethnicity (−1 = ethnic/racial minority, 1 = Caucasian). In order to examine the relationship of each of these variables to initial levels of log-cortisol at wake as well as to the slope of diurnal log-cortisol trajectories across the day, all independent variables were entered as main effects and as interaction effects with time.
Results
Main Analysis
Findings from the main multilevel modeling analysis are presented in Table 2. This analysis revealed that the overall mean for log-cortisol at wake was significantly greater than zero (coefficient = .305, p < .001), and that log-cortisol values tended to significantly decrease across the day (coefficient = −.015, p < .001), which is consistent with cortisol’s known diurnal pattern. It should be noted that the coefficients in this model can be interpreted similar to beta coefficients in standard linear regression. Specifically, these estimates represent the degree of change in the dependent variable (in this case log-cortisol) for every one unit increase in the independent variable. For example, since time was measured in hours, an estimate of −.015 means that, on average, for every passing hour log-cortisol levels decreased by .015.
Table 2.
Multilevel Model Examining the Association Between Loss Status and Diurnal Cortisol Controlling for Depressive Symptoms and Demographic Factors (N = 56)
| Coefficient | Standard error | p | |
|---|---|---|---|
| Intercept (cortisol at wake) | .305 | 0.024 | <.001 |
| Age | .001 | 0.002 | .429 |
| Gender | .041 | 0.018 | .026 |
| Race/ethnicity | −.003 | 0.020 | .864 |
| Education | −.006 | 0.007 | .395 |
| HRSD | −.007 | 0.003 | .039 |
| Bereaved group without elevated PGD symptoms | −.114 | 0.040 | .006 |
| Bereaved group with elevated PGD symptoms | −.013 | 0.047 | .788 |
| Time | −.015 | 0.002 | <.001 |
| Time × age | .000 | 0.000 | .450 |
| Time × gender | −.003 | 0.001 | .064 |
| Time × race/ethnicity | −.001 | 0.001 | .621 |
| Time × education | .000 | 0.001 | .543 |
| Time × HRSD | .000 | 0.000 | .082 |
| Time × bereaved group without elevated PGD symptoms | .007 | 0.003 | .020 |
| Time × bereaved group with elevated PGD symptoms | .001 | 0.004 | .791 |
Notes. HRSD = Hamilton Rating Scale for Depression; PGD = prolonged grief disorder.
Dichotomous variables were coded as follows: Gender (−1 = women, 1 = men), race/ethnicity (−1 = ethnic/racial minority, 1 = Caucasian). The loss status variable was dummy coded, and nonbereaved individuals were treated as the comparison group.
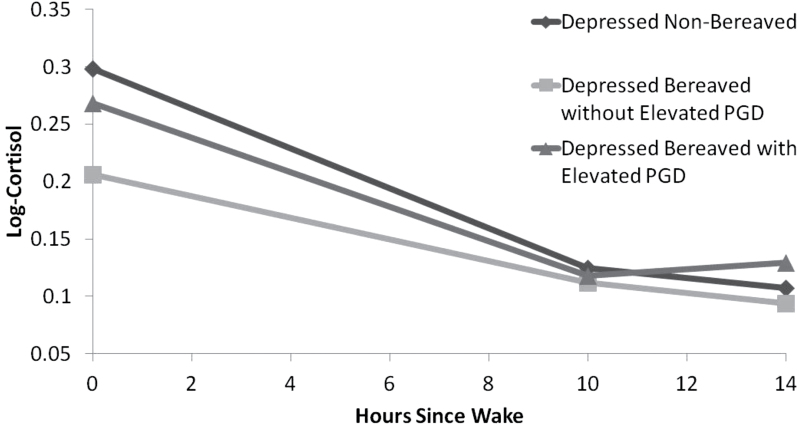
Consistent with our hypothesis, findings revealed that those classified as depressed bereaved without elevated PGD symptoms had significantly lower levels of log-cortisol at wake (coefficient = −.114, p = .006) and flatter diurnal slopes (coefficient = .007, p = .02), compared with those categorized as depressed nonbereaved. This pattern is consistent with a more dysregulated cortisol profile. Surprisingly, no significant differences were found between the depressed bereaved with elevated PGD symptoms and the depressed nonbereaved groups although the depressed bereaved with elevated PGD symptoms group trended toward having more dysregulated cortisol profiles (see Figure 1 for a graphical depiction of the cortisol profiles for the three groups). Considering this surprising result, the main multilevel modeling analysis was rerun using the depressed bereaved with elevated PGD symptoms group as the reference category, and in this reanalysis no significant differences were observed between bereaved individuals with and without elevated PGD symptoms in terms of waking cortisol (coefficient = −.101, p = .07) or diurnal slope (coefficient = .006, p = .14). Taken together, these results suggest that the loss of a loved one is predictive of more dysregulated cortisol, irrespective of one’s level of PGD symptoms. It should be noted that in both of these analyses, demographic factors (e.g., age and ethnicity/race) and depressive symptoms were included as statistical controls.
Figure 1.
Mean cortisol profiles for individuals grouped as either depressed nonbereaved, depressed bereaved without elevated PGD symptoms, or depressed bereaved with elevated PGD symptoms.
Though not a central focus of our study, women and those who had higher levels of depressive symptoms trended toward having more dysregulated cortisol profiles, characterized by significantly lower log-cortisol levels at wake and somewhat flatter diurnal slopes. However, differences in diurnal slope for gender and HRSD scores were not statistically significant.
Subsidiary Analysis
Given the unexpected finding that bereavement, but not necessarily elevated PGD symptoms, was the strongest predictor of participants’ log-cortisol patterns, an unplanned subsidiary analysis was performed to aid in the interpretation of this surprising result. Specifically, a multilevel modeling analysis was performed with the subset of 24 individuals who reported “actively grieving” the loss of a loved one. In this analysis, we examined the impact of three independent variables (as well as all possible two-way interactions between these independent variables) on log-cortisol at wake and its diurnal slope. Specifically, PGD symptoms were included as a continuous variable (created by summing PG-13 items), given evidence that these symptoms may be best conceptualized as dimensional rather than categorical (Holland, Neimeyer, Boelen, & Prigerson, 2009). In addition, because there was a fair amount of variability in the sample in terms of relationship with the deceased and time since loss, these variables were also included. Relationship with the deceased was dichotomized to distinguish between those who lost a spouse/partner and those who lost another type of relationship.
Overall, a significant interaction effect between relationship with the deceased and time since loss was found, whereby individuals who more recently lost a spouse had greater cortisol dysregulation, characterized by significantly lower log-cortisol levels at wake (coefficient = −.016, p = .011) and somewhat flatter diurnal slopes (coefficient = .001, p = .113). PGD symptoms (both as a main effect and interaction term with other variables) did not significantly predict log-cortisol in this analysis.
Discussion
Consistent with our hypothesis, the bereaved older adults in this study tended to have more dysregulated cortisol patterns, and this finding fits with previous research on HPA-axis functioning among bereaved individuals and other chronically stressed populations (Miller et al., 2007; Stroebe et al., 2007). Surprisingly, PGD symptoms appeared to be a relatively weak predictor of cortisol, regardless of whether it was conceptualized as a categorical or continuous variable. Taken together, these findings suggest that there may be something about the circumstance of being bereaved that puts older adults at-risk for cortisol dysregulation, even though the severity of grief symptoms may play little role.
Although preliminary, the subsidiary analysis conducted in this study points to one possible explanation for this pattern of results. Specifically, older adults who had lost a spouse in the more recent past were found to have the most dysregulated cortisol patterns compared with other bereaved individuals in the sample. The recent loss of a spouse, irrespective of whether this loss is anticipated, is one of the most acutely stressful events that can occur during one’s life. Further, it seems likely that those who lost a cohabitating spouse and had not had time to adjust to this life transition experienced the most disruptions in daily activities, especially if the deceased fulfilled important instrumental functions in the relationship (e.g., transportation, monitoring health, cooking; Hansson & Stroebe, 2007).
Indeed, past research suggests that widowhood in late-life is associated with poor eating behaviors and nutrient intake (Rosenbloom & Whittington, 1993), disrupted social rhythms (Brown et al., 1996), and sleep impairment (Richardson, Lund, Caserta, Dudley, & Obray, 2003). In fact one study showed that, compared with healthy controls, distressed grievers are more likely to have fewer social contacts, skip meals, stay indoors, miss work, and have evening snacks (Monk, Houck, & Shear, 2006). This abrupt change in daily routines may be responsible for disruption of circadian rhythms and dysregulation of cortisol’s normal diurnal pattern (Stetler, Dickerson, & Miller, 2004). Such an explanation could help account for past findings indicating that the mortality rate among widowed populations is at its highest in the first 6 months of bereavement (Martikainen & Valkonen, 1996; Moon et al., 2011). Notably, irregularity of daily activities at 3-months postloss has been shown to be associated with mental health symptoms at 1- and 2-years postloss among older bereaved spouses (Prigerson et al., 1996), and it stands to reason that these psychological difficulties might be accompanied by physical health problems as well.
If our findings are replicated in studies with larger, more diverse samples, researchers may begin exploring new treatments for bereaved older adults (particularly those who are recently widowed) that focus on re-establishing daily routines. For example, some adaptation of social rhythm therapy for bereavement that focuses on maintaining regular daily rhythms in activities such as sleeping, waking, eating, and exercise could help ameliorate the deleterious health effects of losing a loved one who may have helped to maintain a sense of stability and structure in life (Frank, Swartz, & Kupfer, 2000). In addition, research that focuses on other biomarkers that may detect disruptions in biological rhythms could help improve early identification of bereaved older adults at greatest risk for negative physical health outcomes. This study suggests that cortisol may be one such biomarker, and future studies might focus on other factors such as melatonin levels (Mirick & Davis, 2008) or genes associated with circadian rhythms (e.g., the “Clock” gene; Herzog, Takahashi, & Block, 1998).
It should be noted that this study is limited in several ways. First, our small sample size limited statistical power and also prevented higher order interactions (i.e., three- or four-way interactions) from being adequately tested. The associations and interactions between demographic factors (e.g., gender, ethnicity/race), circumstances of the loss (e.g., time since loss, relationship to the deceased, cause of death), psychiatric distress (e.g., PGD symptoms, depression) and cortisol are likely complex, and future studies with large sample sizes could help identify specific subgroups of individuals who are most at-risk for cortisol dysregulation and other negative health outcomes. In particular, closer examination of gender differences would be a fruitful area for further investigation. Although it was not a central focus in this study, some gender differences were observed in our larger analysis with the full sample, and other studies have noted gender differences in mortality rates among bereaved individuals (Moon et al., 2011), suggesting that men and women may have different physiological reactions to loss.
This study also included a broad range of bereaved individuals in terms of time since loss and relationship to the deceased. Although this allowed for some examination of how these factors might influence cortisol, it seems entirely possible that a different pattern of results might have been found had we only focused on widowed individuals or those who experienced recent losses. A more fine-grained analysis (e.g., comparing cortisol patterns for those who lost a spouse with those who lost a child or sibling) with a larger sample also seems warranted, given findings that the loss of more intimate relationships are associated with greater separation and traumatic distress (Holland & Neimeyer, 2011). In addition, most of the participants classified as having elevated PGD symptoms did not meet full criteria for the disorder, which could partly account for null findings related to the severity of grief symptoms. In addition, the vast majority of bereaved participants in this study had lost a loved one to natural causes. Considering that past research has found that loss by violent means may have a particularly profound negative impact on the bereaved (Holland & Neimeyer, 2011), researchers would do well to examine the impact of cause of death on cortisol among surviving family members. Finally, it should be noted that the distinction between bereaved and nonbereaved in this study was based on participants’ perception, rather than a more formalized assessment, which could have yielded a somewhat different categorization of participants.
Notwithstanding these limitations, this study is the first to simultaneously examine the effects of PGD symptoms and more general effects associated with losing a loved one on cortisol. The finding that the circumstance of being bereaved (regardless of PGD symptomatology) is associated with more dysregulated cortisol patters, particularly among those who recently lost a spouse, has important implications for future interventions and assessment practices with bereaved older adults.
Funding
This study was funded by an National Institute of Mental Health Exploratory/Developmental Research Grant Program (R21 MH091625-01).
References
- Aldwin C. M. (1991). The Elders Life Stress Inventory (ELSI): Research and clinical applications. In Keller P. A. (Ed.), Innovations in clinical practice: A source book (pp. 355–364). Sarasota, FL: Professional Resource Press [Google Scholar]
- Bartrop R. W., Luckhurst E. E., Lazarus L. L., Kiloh L. G., Penny R. R. (1994). Depressed lymphocyte function after bereavement.In Steptoe A., Wardle J. (Eds.), Psychosocial processes and health: A reader (pp. 166–170). New York, NY: Cambridge University Press [Google Scholar]
- Bradbeer M., Helme R. D., Yong H. H., Kendig H. L., Gibson S. J. (2003). Widowhood and other demographic associations of pain in independent older people. The Clinical Journal of Pain, 19, 247–254. 10.1097/00002508-200307000-00008 [DOI] [PubMed] [Google Scholar]
- Brown L. F., Reynolds C. F., Monk T. H., Prigerson H. G., Dew M. A., Houck P. R., … Kupfer D. J. (1996). Social rhythm stability following late-life spousal bereavement: Associations with depression and sleep impairment. Psychiatry Research, 62, 161–169. 10.1016/0165-1781(96)02914-9 [DOI] [PubMed] [Google Scholar]
- Buckley T., McKinley S., Tofler G., Bartrop R. (2010). Cardiovascular risk in early bereavement: A literature review and proposed mechanisms. International Journal of Nursing Studies, 47, 229–238. 10.1016/j.ijnurstu.2009.06.010 [DOI] [PubMed] [Google Scholar]
- Delalibera M., Coelho A., Barbosa A. (2011). [Validation of prolonged grief disorder instrument for Portuguese population]. Acta Médica Portuguesa, 24, 935–942 [PubMed] [Google Scholar]
- Frank E., Swartz H. A., Kupfer D. J. (2000). Interpersonal and social rhythm therapy: Managing the chaos of bipolar disorder. Biological Psychiatry, 48, 593–604. 10.1016/S0006-3223(00)00969-0 [DOI] [PubMed] [Google Scholar]
- Gerra G., Monti D., Panerai A. E., Sacerdote P., Anderlini R., Avanzini P., … Franceschi C. (2003). Long-term immune-endocrine effects of bereavement: Relationships with anxiety levels and mood. Psychiatry Research, 121, 145–158. 10.1016/S0165-1781(03)00255-5 [DOI] [PubMed] [Google Scholar]
- Hansson R. O., Stroebe M. S. (2007). Bereavement in late life. Washington, DC: American Psychological Association [Google Scholar]
- Herzog E. D., Takahashi J. S., Block G. D. (1998). Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nature Neuroscience, 1, 708–713. 10.1038/3708 [DOI] [PubMed] [Google Scholar]
- Holland J. M., Currier J. M., Gallagher-Thompson D. (2009). Outcomes from the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) program for bereaved caregivers. Psychology and Aging, 24, 190–202. 10.1037/a0014303 [DOI] [PubMed] [Google Scholar]
- Holland J. M., Neimeyer R. A., Boelen P. A., Prigerson H. G. (2009). The underlying structure of grief: A taxometric investigation of prolonged and normal reactions to loss. Journal of Psychopathology and Behavioral Assessment, 31, 190–201. 10.1007/s10862- 008-9113-1 [Google Scholar]
- Holland J. M., Neimeyer R. A. (2011). Separation and traumatic distress in prolonged grief: The role of cause of death and relationship to the deceased. Journal of Psychopathology and Behavioral Assessment, 33, 254–263. 10.1007/s10862-010-9214-5 [Google Scholar]
- Hruschka D. J., Kohrt B. A., Worthman C. M. (2005). Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology, 30, 698–714. 10.1016/j.psyneuen.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Jones M., Bartrop R. W., Forcier L., Penny R. (2010). The long-term impact of bereavement upon spouse health: A 10 year follow-up. Acta Neuropsychiatrica, 22, 212–217. 10.1111/j.1601- 5215.2010.00482.x [DOI] [PubMed] [Google Scholar]
- Kraemer H. C., Giese-Davis J., Yutsis M., Neri E., Gallagher-Thompson D., Taylor C. B., Spiegel D. (2006). Design decisions to optimize reliability of daytime cortisol slopes in an older population. The American Journal of Geriatric Psychiatry, 14, 325–333. 10.1097/01.JGP.0000201816.26786.5b [DOI] [PubMed] [Google Scholar]
- Lannen P. K., Wolfe J., Prigerson H. G., Onelov E., Kreicbergs U. C. (2008). Unresolved grief in a national sample of bereaved parents: Impaired mental and physical health 4 to 9 years later. Journal of Clinical Oncology, 26, 5870–5876. 10.1200/JCO.2007.14.6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W. R. (2005). Stress & health: Biological and psychological interactions. Thousand Oaks, CA: Sage [Google Scholar]
- Luecken L. J. (1998). Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosomatic Medicine, 60, 765–772 [DOI] [PubMed] [Google Scholar]
- Martikainen P., Valkonen T. (1996). Mortality after death of spouse in relation to duration of bereavement in Finland. Journal of Epidemiology & Community Health, 50, 264–268. 10.1136/jech.50.3.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Chen E., Zhou E. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenal axis in humans. Psychological Bulletin, 133, 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Mirick D. K., Davis S. (2008). Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiology, Biomarkers & Prevention, 17, 3306–3313. 10.1158/1055–9965.EPI-08-0605 [DOI] [PubMed] [Google Scholar]
- Monk T. H., Houck P. R., Shear M. K. (2006). The daily life of complicated grief patients—What gets missed, what gets added? Death Studies, 30, 77–85. 10.1080/07481180500348860 [DOI] [PubMed] [Google Scholar]
- Moon J. R., Kondo N., Glymour M. M., Subramanian S. V. (2011). Widowhood and mortality: A meta-analysis. PLoS ONE, 6, e23465. 10.1371/journal.pone.0023465 21858130 [Google Scholar]
- O’Connor M., Wellisch D. K., Stanton A. L., Olmstead R., Irwin M. R. (2012). Diurnal cortisol in complicated and non-complicated grief: Slope differences across the day. Psychoneuroendocrinology, 37, 725–728. 10.1016/j.psyneuen.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson H. G., Bierhals A. J., Kasl S. V., Reynolds C. F., III, Shear M. K., Day N. … Jacobs S. (1997). Complicated grief as a risk factor for mental and physical morbidity. American Journal of Psychiatry, 154, 616–623 [DOI] [PubMed] [Google Scholar]
- Prigerson H. G., Horowitz M. J., Jacobs S. C., Parkes C. M., Aslan M., Goodkin K. … Maciejewski P. K. (2009). Prolonged grief disorder: Psychometric validation of criteria proposed for DSM-V and ICD-11. PLoS Medicine, 6, e1000121. 10.1371/journal.pmed.1000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson H., Maciejewski P. (2008). Prolonged Grief Disorder (PG-13) scale. Boston: Dana-Farber Cancer Institute [Google Scholar]
- Prigerson H. G., Monk T. H., Reynolds C. F., Begley A., Houck P. R., Bierhals A. J., Kupfer D. J. (1996). Lifestyle regularity and activity level as protective factors against bereavement-related depression in late-life. Depression, 3, 297–302. 10.1002/depr.3050030607 [Google Scholar]
- Prigerson H. G., Vanderwerker L. C., Maciejewski P. K. (2008). A case for inclusion of prolonged grief disorder in DSM-V.In Stroebe M., Hansson R., Schut H., Stroebe W. (Eds.), Handbook of bereavement research and practice: Advances in theory and intervention (pp. 165–186). Washington, DC: American Psychological Association [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306 [Google Scholar]
- Richardson S. J., Lund D. A., Caserta M. S., Dudley W. N., Obray S. J. (2003). Sleep patterns in older bereaved spouses. Omega, 47, 361–383. 10.2190/0D4F-EPMW-3YUL-C1JK [Google Scholar]
- Rosenbloom C. A., Whittington F. J. (1993). The effects of bereavement on eating behaviors and nutrient intakes in elderly widowed persons. Journal of Gerontology: Social Sciences, 48, S223–S229. 10.1093/geronj/48.4.S223 [DOI] [PubMed] [Google Scholar]
- Roy A., Gallucci W., Avgerinos P., Linnoila M., Gold P. (1998). The CRH stimulation test in bereaved subjects with and without accompanying depression. Psychiatry Research, 25, 145–156. 10.1016/0165-1781(88)90045-5 [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Romero L. M., Munck A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21, 55–89. 10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Sheehan D. V., Lecrubier Y., Sheehan K. H., Amorim P., Janavs J., Weiller E., … Dunbar G. C. (1998). The mini-international neuropsychiatric interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59, 22–33 [PubMed] [Google Scholar]
- Singer J. D., Willett J. B. (2003). Applied longitudinal data analysis. New York, NY: Oxford University Press [Google Scholar]
- Stetler C., Dickerson S. S., Miller G. E. (2004). Uncoupling of social zeitgebers and diurnal secretion in clinical depression. Psychoneuroendocrinology, 29, 1250–1259. 10.1016/j.psyneuen. 2004.03.003 [DOI] [PubMed] [Google Scholar]
- Stetler C., Miller G. E. (2011). Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosomatic Medicine, 73, 114–126. 10.1097/PSY.0b013e31820ad12b [DOI] [PubMed] [Google Scholar]
- Stroebe M., Schut H., Stroebe W. (2007). Health outcomes of bereavement. Lancet, 370, 1969–1973. 10.1016/S0140- 6736(07)61816–9 [DOI] [PubMed] [Google Scholar]
- Thompson L. W., Breckenridge J. N., Gallagher D., Peterson J. (1984). Effects of bereavement on self-perceptions of physical health in elderly widows and widowers. Journal of Gerontology, 39, 309–314. 10.1093/geronj/39.3.309 [DOI] [PubMed] [Google Scholar]
- Trajković G., Starčević V., Latas M., Leštarević M., Ille T., Bukumirić Z., Marinković J. (2011). Reliability of the hamilton rating scale for depression: A meta-analysis over a period of 49 years. Psychiatry Research, 189, 1–9. 10.1016/j.psychres.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Whisman M. A., Strosahl K., Fruzzetti A. E., Schmaling K. B., Jacobson N. S., Miller D. M. (1989). A structured interview version of the hamilton rating scale for depression: Reliability and validity. Psychological Assessment, 1, 238–241. 10.1037/1040-3590.1.3.238 [Google Scholar]