Abstract
A radioimmunoassay capable of measuring 1 pmole of human fibrinopeptide A has been developed, and should prove useful to detect the release of this peptide from fibrinogen during the coagulation process. Antibodies to fibrinopeptide A were produced by injecting New Zealand white rabbits with a mixture of Freund's adjuvant and native fibrinopeptide coupled to human albumin. N-Tyrosyl fibrinopeptide A was synthesized by the solid-phase method, and was iodinated with 125I by the Chloramine-T method. 48-73% of the radiolabeled peptide could be bound by the serum of a rabbit immunized with the fibrinopeptide-albumin preparation. Antibody-bound peptide was precipitated by dioxane and was thus separated from unbound peptide. The addition of excess native fibrinopeptide to the radiolabeled material prevented its binding to serum. Native fibrinopeptide A and synthetic fibrinopeptide A were identical in their ability to prevent binding, whereas fibrinogen was from 1/25,000 to 1/50,000 as effective on a weight basis. Plasma filtered through a membrane relatively impermeable to molecules larger than a molecular weight of 34,000 showed no fibrinopeptide reactivity, whereas a similar filtrate of serum gave quantitative recovery of fibrinopeptide reactivity.
Keywords: fibrinogen, blood coagulation, peptide synthesis, pmoles detected, dysfibrinogenemia
Full text
PDF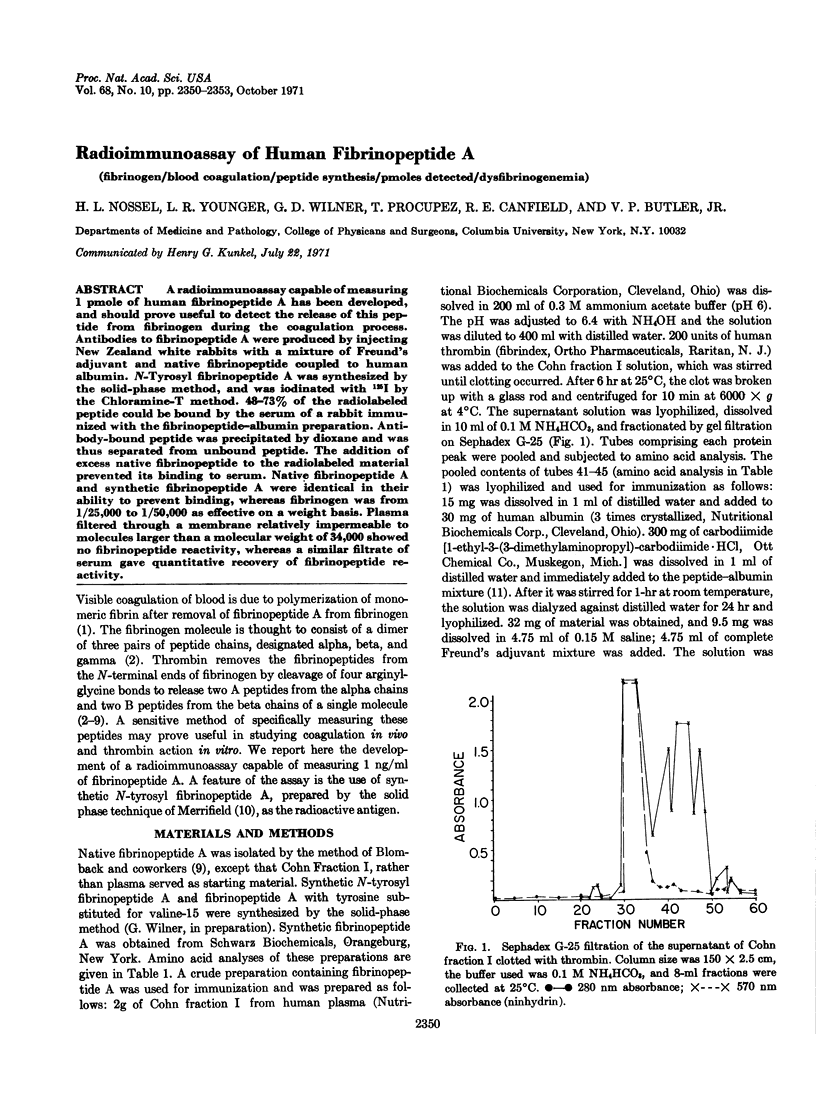
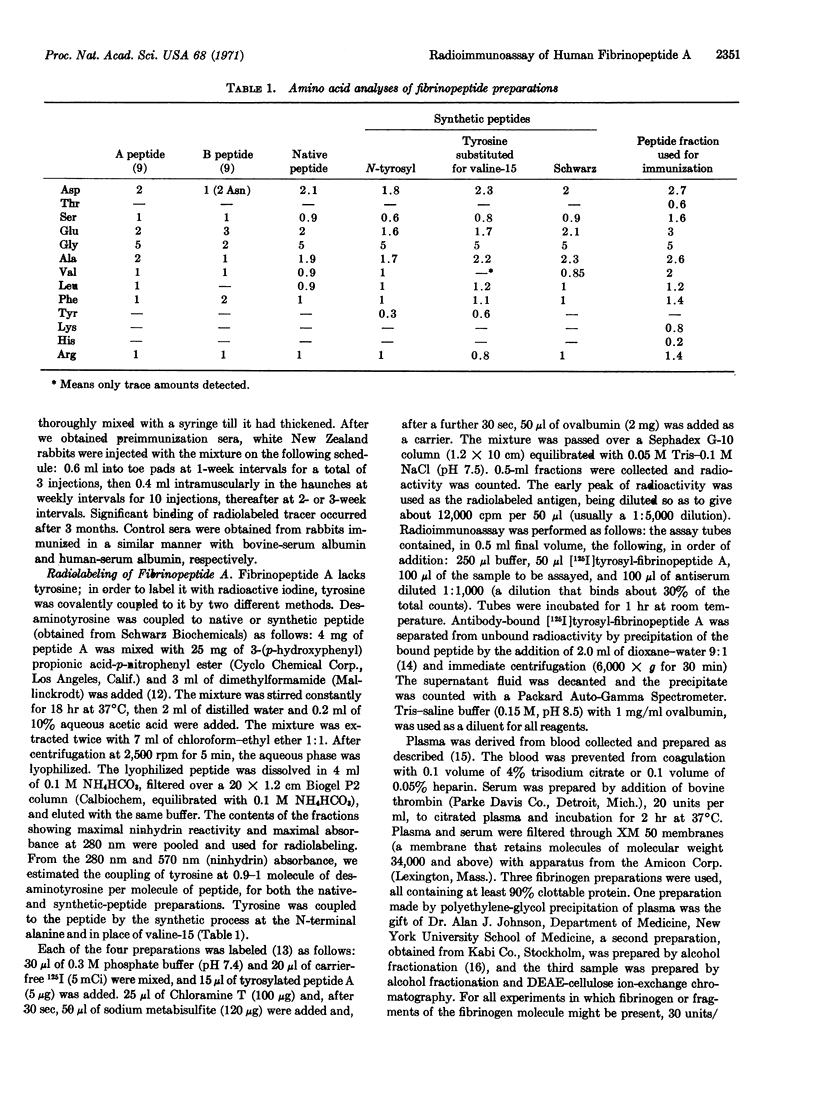
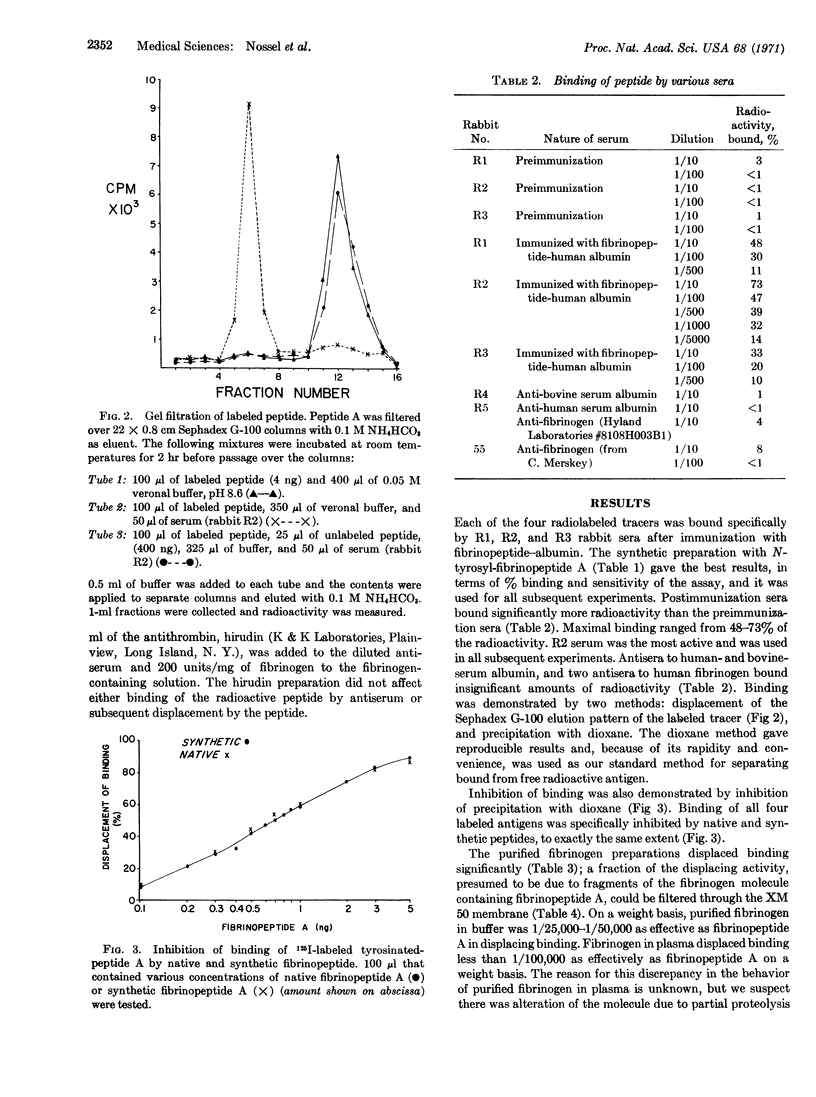

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY K., BETTELHEIM F. R., LORAND L., MIDDLEBROOK W. R. Action of thrombin in the clotting of fibrinogen. Nature. 1951 Feb 10;167(4241):233–234. doi: 10.1038/167233a0. [DOI] [PubMed] [Google Scholar]
- BETTELHEIM F. R., BAILEY K. The products of the action of thrombin on fibrinogen. Biochim Biophys Acta. 1952 Nov;9(5):578–579. doi: 10.1016/0006-3002(52)90213-8. [DOI] [PubMed] [Google Scholar]
- BETTELHEIM F. R. The clotting of fibrinogen. II. Fractionation of peptide material liberated. Biochim Biophys Acta. 1956 Jan;19(1):121–130. doi: 10.1016/0006-3002(56)90393-6. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Blombäck M., Edman P., Hessel B. Human fibrinopeptides. Isolation, characterization and structure. Biochim Biophys Acta. 1966 Feb 28;115(2):371–396. doi: 10.1016/0304-4165(66)90437-5. [DOI] [PubMed] [Google Scholar]
- Blombäck M., Blombäck B., Mammen E. F., Prasad A. S. Fibrinogen Detroit--a molecular defect in the N-terminal disulphide knot of human fibrinogen? Nature. 1968 Apr 13;218(5137):134–137. doi: 10.1038/218134a0. [DOI] [PubMed] [Google Scholar]
- GOODFRIEND T. L., LEVINE L., FASMAN G. D. ANTIBODIES TO BRADYKININ AND ANGIOTENSIN: A USE OF CARBODIIMIDES IN IMMUNOLOGY. Science. 1964 Jun 12;144(3624):1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- Goodfriend T. L., Ball D. L. Radioimmunoassay of bradykinin: chemical modification to enable use of radioactive iodine. J Lab Clin Med. 1969 Mar;73(3):501–511. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Herzig R. H., Ratnoff O. D., Shainoff J. R. Studies on a procoagulant fraction of southern copperhead snake venom: the preferential release of fibrinopeptide B. J Lab Clin Med. 1970 Sep;76(3):451–465. [PubMed] [Google Scholar]
- LORAND L. Fibrino-peptide. Biochem J. 1952 Oct;52(2):200–203. doi: 10.1042/bj0520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen E. F., Prasad A. S., Barnhart M. I., Au C. C. Congenital dysfibrinogenemia: fibrinogen Detroit. J Clin Invest. 1969 Feb;48(2):235–249. doi: 10.1172/JCI105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Nossel H. L., Rubin H., Drillings M., Hsieh R. Inhibition of Hageman factor activation. J Clin Invest. 1968 May;47(5):1172–1180. doi: 10.1172/JCI105806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D. Hemostatic mechanisms in liver disease. Med Clin North Am. 1963 May;47:721–736. doi: 10.1016/s0025-7125(16)33574-x. [DOI] [PubMed] [Google Scholar]
- Thomas K., Ferin J. A new rapid radioimmunoassay for HCG (LH, ICSH) in plasma using dioxan. J Clin Endocrinol Metab. 1968 Nov;28(11):1667–1670. doi: 10.1210/jcem-28-11-1667. [DOI] [PubMed] [Google Scholar]


