Abstract
Tumour mutations corrupt cellular pathways, and accumulate to disrupt, dysregulate, and ultimately avoid mechanisms of cellular control. Yet the very changes that tumour cells undergo to secure their own growth success also render them susceptible to viral infection. Enhanced availability of surface receptors, disruption of antiviral sensing, elevated metabolic activity, disengagement of cell cycle controls, hyperactivation of mitogenic pathways, and apoptotic avoidance all render the malignant cell environment highly supportive to viral replication. The therapeutic use of oncolytic viruses (OVs) with a natural tropism for infecting and subsequently lysing tumour cells is a rapidly progressing area of cancer research. While many OVs exhibit an inherent degree of tropism for transformed cells, this can be further promoted through pharmacological interventions and/or the introduction of viral mutations that generate recombinant oncolytic viruses adapted to successfully replicate only in a malignant cellular environment. Such adaptations that augment OV tumour selectivity are already improving the therapeutic outlook for cancer, and there remains tremendous untapped potential for further innovation.
The Tumour: A Unique Niche for Virus Growth
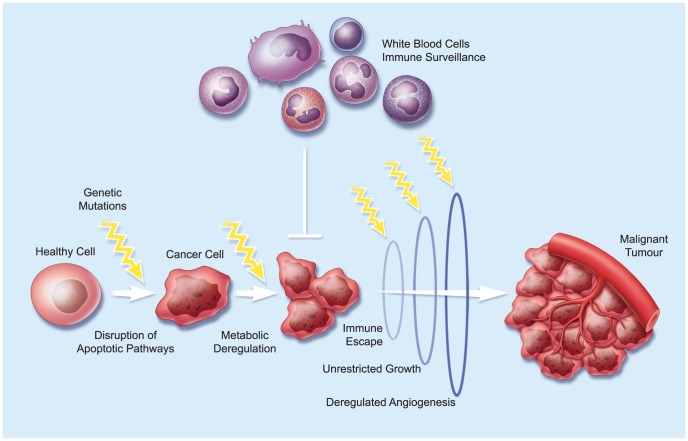
Tumour progression is generally considered a stochastic process, but is nevertheless associated with a series of hallmark changes that include, among others, resistance to apoptosis, metabolic deregulation, immune escape, growth independence, and enhanced angiogenic capacity [1] (Figure 1). Either taking certain pathways offline, or boosting their activity, disrupts cellular homeostasis and creates a supportive environment that facilitates exponential cancer cell growth. While such changes allow the malignant cell a competitive survival and growth advantage over “normal” cells, they also render it susceptible to infection, since many of the pathways subverted by the tumour are also necessary for effective antiviral responses.
Figure 1. Tumour Evolution.
A hypothetical pathway of tumour evolution from a normal cell to an advanced-stage cancer. Mutations in key regulatory genes lead to changes in cell physiology that favour tumour growth. Over time, these genetic defects accumulate to confer many of the known hallmarks of cancer [131]. The sequence of these events and the timing represented here is only one example of how this might occur.
Once a virus penetrates a tumour cell, the malignant metabolic infrastructure provides abundant support for viral replication. Since the same pathways that are already boosted during cancer cell transformation are also engaged by viral replication, tumour cells are attractive targets for OVs, a class of cancer biotherapeutics that includes such diverse virus families as rhabdoviridae (e.g., vesicular stomatitis virus [VSV], Maraba virus), poxviridae (e.g., vaccinia [VV], myxoma [MYXV]), adenoviridae (e.g., adenovirus serotype 5 [Ad5], Colo-Ad1), paramyxoviridae (e.g., Newcastle disease virus [NDV], measles virus [MV]), togaviridae (e.g., Sindbis virus [SV]), herpesviridae (e.g., herpes simplex virus-1 [HSV-1]), reoviridae (e.g., reovirus type III), picornaviridae (e.g., poliovirus, coxsackievirus), and parvoviridae (e.g., H1-parvovirus). The current status of clinical trials for such therapeutic OVs has recently been reviewed by Russell and colleagues [2].
In this review, we highlight the similarities between the requirements for optimal cancer cell growth and successful viral replication, and the mounting evidence that tumours with altered metabolic and signaling networks provide a unique niche for OV propagation. While some viruses are inherently oncophilic, it is also possible to direct selective growth in cancer cells by inactivating or deleting certain viral virulence genes whose lost functions are complemented by mechanisms that drive malignancies and distinguish tumours from normal healthy tissues. The application of such genetic interventions and/or the potential for coadministering pharmacological compounds to enhance OV activity specifically in tumours will be discussed.
Overexpressed Tumour Antigens: Entangling Viruses
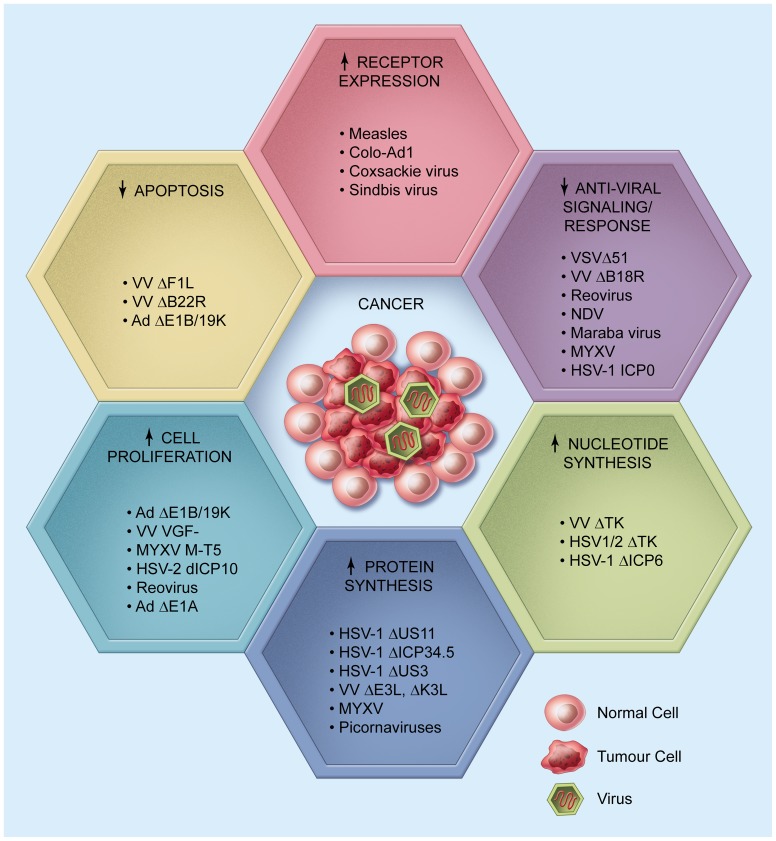
Host cell entry is one of the first challenges that viruses must overcome to access intracellular replication sites, and the availability of cell surface receptors for viral attachment and uptake is of paramount importance. Malignant cells undergo tremendous changes in the profile of cell surface receptors they display, and the tumour specificity of many oncolytic viruses often begins with engaging these overexpressed antigens at the cell surface (Figure 2). For example, poliovirus binds the cell surface receptor, nectin-like molecule 5 (NECL-5) [3], which is expressed at very low levels in normal tissues, but is broadly overexpressed in several solid tumours and in the proliferating vasculature that supports them, including glioblastoma multiforme and ovarian, prostate, colorectal, and lung carcinomas [4]–[8]. This natural biological observation has been exploited to create a potent chimeric OV that selectively targets a variety of tumours of neuroectodermal origin [9]. Similarly, measles virus (MV) and the chimeric adenovirus ColoAd1 both bind the cell surface receptor, CD46, which is commonly upregulated by cancer cells [10],[11]. MV gene expression, cytopathic effect, and oncolysis have all been correlated with density of CD46 on the cell surface [12]. Nectin-4 can also be exploited for MV entry [13], and is highly expressed in lung, breast, colon, and ovarian carcinomas [14]–[16]. The natural oncolytic capacity of coxsackievirus A21 (CVA21) is based on high expression of intracellular adhesion molecule 1 (ICAM-1) and/or decay acceleration factor (DAF) on the surface of malignant cells [17], while the uptake of coxsackievirus B3 (CVB3) and certain oncolytic adenoviruses is particularly enhanced in medulloblastoma, neuroblastoma, and endometrial carcinomas overexpressing the host cell coxsackievirus–adenovirus receptor (CAR) [18],[19]. Oncolytic Sindbis virus (SV) binds the Laminin receptor (LamR) [20], which is modestly expressed in almost every mammalian cell, but highly upregulated in many solid tumours [21]–[24]. In normal cells, LamRs are occupied by the laminin ligand and SV attachment is outcompeted, while in cancer cells, the overexpression of LamRs exposes a significant number of unoccupied SV target sites [25].
Figure 2. Oncolytic Viruses Are Designed to Grow in the Tumour Niche.
There are at least six key critical features of tumour cell growth that can be targeted by oncolytic viruses. These include changes in the expression of viral-host cell receptors, the antiviral response, nucleotide and protein synthesis, cell proliferation, and apoptosis. A number of engineered or selected oncolytic viruses exist that can exploit one or more of these malignant characteristics.
OVs have also been reprogrammed to bind specific tumour surface antigens through the display of single-chain antibodies or polypeptide-binding ligands on the virion surface, or by fusing scaffolding moieties within viral surface proteins (reviewed in [26]). For example, several ligands have been successfully displayed on the surface of MV particles, including single-chain antibodies against EGFR [27],[28], CD20 [29], CD38 [27], and the folate receptor alpha [30]. Similarly, herpes simplex virus (HSV) and various adenoviruses have been genetically redirected toward tumours through the incorporation of cancer-specific receptor binding ligands into viral surface proteins [31]–[35]. Finally, increased oncotropism of VV has been achieved by fusing targeting moieties against the Human Epidermal Growth Factor Receptor 2 or Mucin-1 to virus envelope resident proteins [36],[37].
A Dysfunctional Cellular Antiviral Defense: The Achille's Heel of Cancer
An estimated 65–70% of cancer cell lines have defective interferon (IFN) responses [38]. However, both the cancerous and stromal compartments of a tumour integrate antiviral signals to establish a scale of IFN responsiveness, which is different for each malignancy. The biological drivers of cancer evolution that lead to the loss of interferon responsiveness are not completely understood, but it is thought that physiological processes that favour antiviral activity are incompatible with efficient tumour growth. For instance, interferon and interferon-responsive genes are anti-angiogenic [39], and are known to induce apoptosis [40], cell growth arrest [41], and immune stimulation [42], all of which cancer cells strive to evade. Not surprisingly, one of the most common genetic changes in the tumour as it transitions to a stealth phenotype is the loss of expression of genes from the interferon pathway, and the establishment of further rounds of immune editing that allow the cancer to become invisible to the host immune system [43]. As tumours evolve to full malignancy, this elimination or inactivation of certain interferon gene products may allow a growth advantage, but also compromises cellular antiviral responses to varying degrees.
The IFN pathway is part of the innate immune response triggered upon pathogen entry by a limited number of cellular immune receptors recognizing broadly conserved pathogen-associated molecular patterns (PAMPs). Most wild-type viruses encode gene products that antagonize interferon response signaling, and can robustly infect tumour and normal cells alike. Some “natural” OVs have evolved to infect nonmammalian organisms (e.g., Newcastle disease virus (NDV) in avian hosts) and thereby lack virulence genes that can effectively antagonize mammalian antiviral responses; such viruses only prosper in tumour cells that acquire mutations in interferon response genes [44]. For other viruses, it is necessary to engineer defects in virulence gene products that normally antagonize interferon responses to confer a robust growth response in cancer cells but an inability to productively infect normal tissues [45]–[47] (Figure 2).
The recognition that interferon signaling is in large part critical to OV selectivity made it clear that manipulation of this cellular response could be a target for therapeutic intervention. Tumour cells that have completely lost their interferon response provide an ideal substrate for OV replication. Such tumours can easily be cured with a “single shot” of virus [48]. However, most human tumours are genetically and architecturally heterogeneous, and thus the extent of their antiviral responsiveness can be variable and provide a significant barrier to OV therapy [49]. This problem can be biologically addressed by enhancing the potency of existing OVs [50],[51], selecting inherently more potent virus backbones (such as Maraba, which outperforms several related OVs [e.g. VSV] in terms of replication speed, viral productivity, and lytic capacity across multiple cancer cell lines [52]), or coinfecting tumours with distinct attenuated OVs that can complement each other's growth in a hostile tumour environment [53]. Coinfection of tumours with an oncolytic vaccinia virus (VV) that expresses the IFN-scavenging protein, B18R, can significantly enhance the ability of attenuated, interferon-sensitive vesicular stomatitis virus (VSV) to spread through tumours exhibiting partial defects in their antiviral defenses, resulting in improved oncolytic activity compared to either virus alone [53].
Several pharmacological strategies have aimed to improve OV therapy by disrupting IFN responses in resistant tumour cells. Our group, and others, have previously shown that histone deacetylase inhibitors (HDIs) can improve the oncolytic activity of OVs both in vitro and in vivo, including VSVΔ51, several strains of VV, and herpes simplex virus (HSV) [54]–[56]. HDIs profoundly impact cellular epigenetics, and inhibit the IFN response by blocking the transcriptional upregulation of IFN-stimulated genes following viral infection or IFN signalling [57],[58]. A related strategy has been to coadminister small molecules, selected on the basis of their ability to enhance viral oncolysis. Using this method, we recently identified a class of viral sensitizer compounds (VSe1–15) specifically selected for their superior activity in comparison with the clinically approved HDI, Vorinostat. Such viral sensitizers robustly enhance VSVΔ51 growth in IFN-resistant tumour cells [59]. Importantly, the “proviral” effects of both HDIs and novel viral sensitizers only occur in tumour cells, which may be attributable to the tumour microenvironment already being conducive to viral growth.
Dysregulated Tumour Metabolism: Fueling the OV Fire
Exploiting Increased Nucleotide Pools
As tumours expand and develop, metabolic activity on a per-cell basis increases to sustain cellular output and fuel biosynthetic processes. Many cancer cells meet this demand by reprogramming energy production from mitochondrial phosphorylation to aerobic glycolysis, a phenomenon known as the “Warburg effect” [60]. Since efficient virus replication and virion assembly is a similarly energy demanding process fueled by the host cell, certain viruses may act to skew cellular metabolism toward glycolysis to divert glycolytic intermediates into biomachinery programs that synthesize viral macromolecules. For example, the VV N1L gene encodes a multifunctional protein that targets different cellular kinases, one of which influences ATP levels during virus replication [61].
While a metabolic boost increases overall levels of biomolecules in host cells, several viruses also directly impact the size of these biosynthetic pools. Large DNA viruses achieve replicative self-sufficiency by encoding enzymes involved in the de novo synthesis of deoxynucleotides (dNTPs). For example, herpes- and orthopoxviruses encode both large (R1) and small (R2) subunits of ribonucleotide reductase (RR), a key enzyme involved in catalyzing the conversion of ribonucleotides to dNTPs [62]. Other oncolytic poxviruses encode at least one RR subunit. In the absence of virally supplied RR proteins, successful virus replication depends on host cell RR activity, which is reportedly enhanced in malignant cells [63] and acts together with elevated levels of dNTPs to enable viral tumour selectivity. Selective deletions in viral genes encoding RR subunits serve to enhance the oncotropism of OVs. For example, HSV-1 and HSV-2 mutants with deletions in the ICP6 gene, which encodes the large subunit of RR, often preferentially replicate in actively dividing cells [64], although some reports have shown replication in quiescent cells with mutations in p16 [65]. Herpes- and poxviruses also encode the enzyme thymidine kinase (TK), which is primarily responsible for phosphorylating thymidine, a key step in DNA synthesis. Deletion or inactivation of TK in VV or HSV-1 leads to preferential viral replication in host cells with high levels of TK, such as tumour cells (Figure 2). Inactivating viral products involved in nucleotide metabolism has been a widely utilized and successful strategy to generate tumour-selective viruses: two of the top three current clinical OV candidates, OncoVEX GM-CSF and JX-594, contain such deletions and/or mutations [66],[67].
Relatively few attempts have been made to pharmacologically exploit the nucleotide synthesis pathway and simultaneously address the issue of tumour heterogeneity for OV growth. One example comes from Passer et al. [68], who identified inhibitors of equilibrative nucleoside transporter 1 (ENT1), a glycoprotein that mediates cellular uptake of nucleosides, from a small library screen of approved pharmacological agents as capable of augmenting oncolytic HSV infection. The ENT1 inhibitors, dilazep and dipyridamole, increased the spread and subsequent oncolytic ability of HSV by increasing ribonucleoside activity in many cancer cell lines but not in normal epithelial cells, an effect that required defective viral ICP6.
Taking Over the Protein Synthesis Machine
While enhancing metabolic activity, suppressing antiviral signaling, and increasing nucleotide levels are all necessary and potentially rate-limiting steps for optimizing cancer growth or viral infection, the translation of proteins ultimately represents the final hurdle that ensures either cellular growth/division or the production of viral particle components. Dysregulation of translational control is one of the key events that promotes cellular transformation, and enhanced ribosome biogenesis, elevated levels of initiation factors, and changes in transcriptional repressors are found in a broad spectrum of cancers [69]. Since all eukaryotic viruses are fully dependent on host cell translational machinery to synthesize viral proteins, virus-host cell interactions that regulate translation, both globally and for specific mRNAs, contribute to the oncotropism of certain OVs (Figure 2). Generally, changes in the expression or availability of translational machinery components in cancer cells increase the overall rate of protein production (including viral polypeptides) [70],[71], and therefore enhance OV replication in tumours compared to normal tissues [72],[73].
Translation of most viral mRNA depends on the cellular cap-dependent program, where the rate-limiting initiation step represents a prime target for viral control. Viral dsRNA activates cellular protein kinase R (PKR), which inactivates the translation initiation factor eIF2 to limit host cell growth and initiate antiviral responses. As eIF2 phosphorylation dramatically reduces the efficiency and rate of viral translation, several viruses directly or indirectly prevent PKR activation. For example, HSV-1 encodes both US11, which directly inactivates PKR, and ICP34.5, which recruits cellular phosphatases to oppose PKR [74]. Similarly, VV encodes a PKR-binding protein (E3L) and a PKR pseudo-substrate (K3L) [74],[75]. There is evidence that eIF2 phosphorylation is dysregulated during tumourigenesis and, as a result, the inactivation of viral gene products involved in modulating PKR responses can generate an oncotropic virus. Most notably, a version of VSV expressing a catalytically inactive version of eIF2B-epsilon has a reduced ability to grow in benign cells compared to the parental VSV, but retains the ability to grow in malignant cells [76].
The mammalian target of rapamycin signalling complex 1 (mTORC1) is a master regulator of cell growth and metabolism; as such its activation promotes various anabolic processes in the cell, including protein biosynthesis. Notably, mTORC1 stimulates protein translation by inhibiting the translational repressor eIF4E-binding protein 1 (4EBP1) [77]. Herpes-, adeno-, and poxviruses encode viral products that ultimately inactivate 4EBP1, often through the mTORC1 pathway, and thus promote viral protein synthesis. For example, the HSV-1 protein kinase, US3, directly phosphorylates and inactivates 4EBP1 [78]. Since the mTORC1 pathway is almost always constitutively activated in malignant disease [79], US3-deficient HSV-1 mutants have a strong specificity for translationally active cancer cells [80]. The pharmacological manipulation of mTOR in combination with OVs has been explored using rapamycin, a well-tolerated and well-characterized mTOR inhibitor. Rapamycin and other “rapalogues” prevent the phosphorylation and activation of S6K and eIF4e, and retain the translational repressor, 4EBP1, in an active form. Rapamycin therefore hampers translation of both viral and cellular transcripts, but particularly negatively affects cellular mRNAs with complex secondary structures, such as those encoding the antiviral effectors, IFN and interferon regulatory factor 7 (IRF7), thereby favouring viral spread [81]–[84]. Inhibiting mTOR decreases activity of the downstream S6K, which in turn relieves its inhibitory effects on phosphatidylinositide 3-kinases (PI3K) and Akt signalling. For some viruses, such as myxoma virus (MYXV), increased activation of Akt improves replication and oncolytic activity in several mouse, rat, and human tumour models [85]–[87]. While systemic immunosuppressive effects of rapamycin or rapalogues also likely play a key role, such drugs have nevertheless been successfully used with several OVs, including adenovirus, HSV, VSV, MYXV, and VV, to improve their control of tumour progression [81],[86],[88]–[90].
Under conditions of cellular stress (e.g., hypoxia) where levels of eIF4E are low, tumours can also drive the expression of key malignancy-associated genes using a cap-independent (IRES-mediated) mechanism. Cap-independent translation often requires extremely high levels of eIF4G, which are typically found in transformed cells [91],[92]. Notably, several oncolytic picornaviruses (e.g., poliovirus and coxsackieviruses) lack the m7-cap required for normal translation, and thus encounter a strategic translational advantage in malignant cells [93]. Functional studies indicate that the rate of interaction between IRES and eIF4G may be an important factor that determines oncolytic poliovirus selectivity and enhanced propagation in certain tumour cells, such as glioblastoma [93]. In addition, recent studies showed that several noncanonical factors are recruited to bind the poliovirus IRES to modulate translation, and may play a role in cancer cell specificity [94].
Riding the Tumour Cell Cycle
Dysregulation of the cell cycle to allow unchecked proliferation is a critically important step in tumourigenesis. Many of the key checkpoint proteins that normally regulate and restrict cell cycle progression, such as p53, RB, and myc, are actively disengaged, destabilising cells and priming them for further genetic mutations [95]. The successful replication of many wild-type viruses, including adenovirus, HSV, VV, MYXV, and reovirus, also requires effective targeting of central hubs of the proliferative signaling circuitry (Figure 2). While many viruses are capable of entering into and even initiating virus transcription in quiescent and terminally differentiated cells, the host cell must often enter the cell cycle to complete the viral replication cycle [96]–[99]. Viral proteins have therefore evolved to induce cell cycling, ensuring the activation of cellular biosynthetic machinery and mobilization of substrates necessary for the production of viral progeny. For example, the adenovirus E1A protein plays a key role in activating proliferation and cell cycle progression by binding and abrogating the activity of the retinoblastoma (RB) protein family [100],[101]. Since cancer cells almost uniformly downregulate such tumour suppressive proteins to release proliferation control, partial deletion of the E1A viral gene generates a specific oncolytic adenovirus [102]. MYXV and reovirus are also able to efficiently replicate and lyse a wide variety of human tumour cell lines with dysfunctional tumour suppressor genes, such as RB and TP53 [103].
Increases in the frequency of mitogenic signals received through receptors such as epidermal growth factor receptor (EGFR) and its main downstream signaling transduction partners, PI3K/AKT and RAS/Mitogen-Activated Protein Kinase Kinase/Mitogen-Activated Protein Kinase (RAS/MEK/MAPK), also drive transformed or infected cell proliferation. Both herpes- and poxviruses activate the EGFR during their replication cycle [104],[105]. For example, VV encodes vaccinia growth factor (VGF), an early secreted protein that binds EGFR and conditions surrounding cells for subsequent viral infection [106]. Since EGFR is often constitutively activated in gliomas and carcinomas of the lung, colon, head and neck, pancreas, and breast [107], such tumours are rendered hypersensitive to VV replication. Numerous OVs, including MYXV, VV, coxsackievirus B3, and HSV-1, target the PI3K/AKT signal transduction pathway [85],[108]–[110]. For example, MYXV encodes the M-T5 host range factor, which induces the phosphorylation of cellular AKT and creates a growth-favourable environment for virus replication [111]. While mutant MXYV viruses lacking M-T5 replicate poorly in most cells, tumour cells where AKT is constitutively activated are permissive to these viruses [112]. Several viruses also target the Ras signaling cascade. Replication of HSV-2 is facilitated by the viral ICP10 gene–encoded serine/threonine protein kinase (PK) domain, which activates the Ras/MEK/MAPK pathway. Deletion of this PK domain converts HSV-2 into a potent oncolytic agent, exhibiting preferential replication in and lysis of tumour cells with a constitutively activated Ras signaling pathway [113]. Exploitation of the Ras cascade is also a critical step for reovirus particle uncoating, infectivity, and release, and Ras transformation is necessary to realize potent oncolytic effects [114],[115]. Activation of the Ras pathway has also been linked to repression of the antiviral response by interfering with PKR, Retinoic Inducible Gene I (RIG-I), and IFN signaling [46],[115].
To Kill or Not to Kill: Balancing Apoptosis
The activation of the apoptotic pathway is a potent homeostatic tool for the early suppression of malignant cell outgrowth, while in infected cells, it represents a highly effective host response to curtail the infection cycle. Cellular changes that allow the avoidance of programmed cell death are essential for both tumour cell and virus host cell survival. Cancer cells employ multiple and diverse strategies to bypass the apoptotic death pathway normally induced by various stressors, and this evasive ability likely contributes to the survival and prolonged replication of naturally occurring or recombinant OVs. Yet while malignant apoptotic resistance supports optimal oncolytic virus growth, it can also compromise therapeutic efficacy, particularly given the importance of cytotoxic T cell–mediated killing of both infected and noninfected cancer cells for eliciting therapeutic responses following OV administration [116]–[119]. As such, a significant number of genetic strategies have aimed to improve direct and indirect cell killing upon OV infection. Multiple viruses encode viral proteins that target and regulate key steps in apoptotic pathways, balancing the maximum output of viral progeny with the necessity of keeping infected cells alive until successful transmission is achieved (Figure 2). For example, VV encodes several genes that modulate apoptosis, including F1L and SPI-1 (B22R), which directly inhibit pro-apoptotic Bcl-2–like proteins and caspase activation, respectively. Deletion of these viral genes enhances tumour selectivity compared with wild-type VV [120]. The adenoviral E1B-19K gene product performs a similar function, blocking apoptosis by sequestering and inhibiting numerous pro-apoptotic Bcl-2–like proteins. Multiple lines of evidence indicate that E1B-19K-deficient adenoviruses selectively replicate and kill tumour cells in vitro and in vivo, with no effects in normal cells [121].
The loading of OVs with suicide transgenes has also been employed by several groups to enhance killing of both infected and uninfected tumour cells. Successful virally encoded suicide payloads include TNF-related apoptosis-inducing ligand (TRAIL) [122], Fas ligand (FasL) [123], yeast cytosine deaminase (in combination with 5-FC) [124], HSV thymidine kinase (in combination with gancyclovir) [125], Drosophila melanogaster multisubstrate deoxyribonucleoside kinase [126], uracil phosphoribosyltransferase (in combination with 5-FU) [127], carboxypeptidase G2 (in combination with ZD2767P) [128], and carboxylesterase (in combination with irinotecan) [129].
Drugs can also be used to increase virus-induced tumour cell death. For example, while HDIs impact the cellular IFN response (as discussed above), they also increase virus-induced apoptosis [58]. Another example comes from an elegant study by Mahoney et al., who identified that knockdown of several proteins involved in the unfolded protein response significantly increased oncolytic Maraba virus–induced cell death following a genome-wide siRNA screen [130]. Knockdown of one of these genes, IRE-1, hampered the ability of cancer cells to cope with unfolded protein overload within the ER, effectively priming cancer but not benign cells to undergo virally induced apoptosis. A salicylaldehyde-based drug inhibitor of IRE-1 was subsequently synthesized and used to improve oncolytic Maraba efficacy in a resistant tumour model.
Future Perspectives
With OV cancer therapeutics entering advanced-stage trials and showing clinical efficacy, strategies that further broaden OV targeting and replication capacity to address the heterogeneous nature of tumours and their associated vascular and stromal architecture will be extremely useful. Since such heterogeneity not only exists between patients but also within a given tumour/patient, where the metabolism, signal transduction, and antiviral states of cancer cells can be variably abnormal and, therefore, variably support OV replication, combinatorial strategies will be essential to promoting reliable tumour control and regression. Finally, continued efforts to identify components innate to the complex tumour microenvironment that promote OV replication will be critical to further improving OVs and developing new engineering strategies.
Funding Statement
CSI is supported by a fellowship from Alberta Innovate Health Solutions. JSD and JCB are supported by the Canadian Institute for Health Research and the Terry Fox Research Institute. JCB is supported by the Ontario Institute for Cancer Research and the Ottawa Regional Cancer Foundation. JSD is supported by the Canadian Cancer Research Society and the Ottawa Hospital Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 2. Russell SJ, Peng KW, Bell JC (2012) Oncolytic virotherapy. Nat Biotechnol 30: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mendelsohn CL, Wimmer E, Racaniello VR (1989) Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 56: 855–865. [DOI] [PubMed] [Google Scholar]
- 4. Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, et al. (2009) Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol 183: 4921–4930. [DOI] [PubMed] [Google Scholar]
- 5. Enloe BM, Jay DG (2011) Inhibition of Necl-5 (CD155/PVR) reduces glioblastoma dispersal and decreases MMP-2 expression and activity. J Neurooncol 102: 225–235. [DOI] [PubMed] [Google Scholar]
- 6. Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, et al. (2001) Overexpression of the CD155 gene in human colorectal carcinoma. Gut 49: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakai R, Maniwa Y, Tanaka Y, Nishio W, Yoshimura M, et al. (2010) Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci 101: 1326–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suzuki K, Nakamura K, Kato K, Hamada H, Tsukamoto T (2007) Exploration of target molecules for prostate cancer gene therapy. Prostate 67: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 9. Goetz C, Dobrikova E, Shveygert M, Dobrikov M, Gromeier M (2011) Oncolytic poliovirus against malignant glioma. Future Virol 6: 1045–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaggar A, Shayakhmetov DM, Lieber A (2003) CD46 is a cellular receptor for group B adenoviruses. Nat Med 9: 1408–1412. [DOI] [PubMed] [Google Scholar]
- 11. Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G, et al. (2003) Adenovirus type 11 uses CD46 as a cellular receptor. J Virol 77: 9183–9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson BD, Nakamura T, Russell SJ, Peng KW (2004) High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res 64: 4919–4926. [DOI] [PubMed] [Google Scholar]
- 13. Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, et al. (2011) Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480: 530–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, et al. (2010) Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol 134: 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fabre-Lafay S, Garrido-Urbani S, Reymond N, Goncalves A, Dubreuil P, et al. (2005) Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J Biol Chem 280: 19543–19550. [DOI] [PubMed] [Google Scholar]
- 16. Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, et al. (2009) Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res 69: 6694–6703. [DOI] [PubMed] [Google Scholar]
- 17. Skelding KA, Barry RD, Shafren DR (2009) Systemic targeting of metastatic human breast tumor xenografts by Coxsackievirus A21. Breast Cancer Res Treat 113: 21–30. [DOI] [PubMed] [Google Scholar]
- 18. Giaginis CT, Zarros AC, Papaefthymiou MA, Papadopouli AE, Sfiniadakis IK, et al. (2008) Coxsackievirus and adenovirus receptor expression in human endometrial adenocarcinoma: possible clinical implications. World J Surg Oncol 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shafren DR, Williams DT, Barry RD (1997) A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J Virol 71: 9844–9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang KS, Kuhn RJ, Strauss EG, Ou S, Strauss JH (1992) High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol 66: 4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basolo F, Pollina L, Pacini F, Fontanini G, Menard S, et al. (1996) Expression of the Mr 67,000 laminin receptor is an adverse prognostic indicator in human thyroid cancer: an immunohistochemical study. Clin Cancer Res 2: 1777–1780. [PubMed] [Google Scholar]
- 22. Castronovo V, Colin C, Claysmith AP, Chen PH, Lifrange E, et al. (1990) Immunodetection of the metastasis-associated laminin receptor in human breast cancer cells obtained by fine-needle aspiration biopsy. Am J Pathol 137: 1373–1381. [PMC free article] [PubMed] [Google Scholar]
- 23. Cioce V, Castronovo V, Shmookler BM, Garbisa S, Grigioni WF, et al. (1991) Increased expression of the laminin receptor in human colon cancer. J Natl Cancer Inst 83: 29–36. [DOI] [PubMed] [Google Scholar]
- 24. Menard S, Tagliabue E, Colnaghi MI (1998) The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res Treat 52: 137–145. [DOI] [PubMed] [Google Scholar]
- 25. Jamieson KV, Hubbard SR, Meruelo D (2011) Structure-guided identification of a laminin binding site on the laminin receptor precursor. J Mol Biol 405: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verheije MH, Rottier PJ (2012) Retargeting of viruses to generate oncolytic agents. Adv Virol 2012: 798526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, et al. (2005) Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol 23: 209–214. [DOI] [PubMed] [Google Scholar]
- 28. Paraskevakou G, Allen C, Nakamura T, Zollman P, James CD, et al. (2007) Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther 15: 677–686. [DOI] [PubMed] [Google Scholar]
- 29. Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Johnston PB, et al. (2007) Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res 67: 10939–10947. [DOI] [PubMed] [Google Scholar]
- 30. Hasegawa K, Nakamura T, Harvey M, Ikeda Y, Oberg A, et al. (2006) The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res 12: 6170–6178. [DOI] [PubMed] [Google Scholar]
- 31. Coughlan L, Vallath S, Saha A, Flak M, McNeish IA, et al. (2009) In vivo retargeting of adenovirus type 5 to alphavbeta6 integrin results in reduced hepatotoxicity and improved tumor uptake following systemic delivery. J Virol 83: 6416–6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta V, Wang W, Sosnowski BA, Hofman FM, Chen TC (2006) Fibroblast growth factor-2-retargeted adenoviral vector for selective transduction of primary glioblastoma multiforme endothelial cells. Neurosurg Focus 20: E26. [PubMed] [Google Scholar]
- 33. Harvey TJ, Burdon D, Steele L, Ingram N, Hall GD, et al. (2010) Retargeted adenoviral cancer gene therapy for tumour cells overexpressing epidermal growth factor receptor or urokinase-type plasminogen activator receptor. Gene Ther 17: 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oh IK, Mok H, Park TG (2006) Folate immobilized and PEGylated adenovirus for retargeting to tumor cells. Bioconjug Chem 17: 721–727. [DOI] [PubMed] [Google Scholar]
- 35. Wang W, Zhu NL, Chua J, Swenson S, Costa FK, et al. (2005) Retargeting of adenoviral vector using basic fibroblast growth factor ligand for malignant glioma gene therapy. J Neurosurg 103: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 36. Galmiche MC, Rindisbacher L, Wels W, Wittek R, Buchegger F (1997) Expression of a functional single chain antibody on the surface of extracellular enveloped vaccinia virus as a step towards selective tumour cell targeting. J Gen Virol 78 (Pt 11) 3019–3027. [DOI] [PubMed] [Google Scholar]
- 37. Paul S, Geist M, Dott K, Snary D, Taylor-Papadimitriou J, et al. (2007) Specific tumor cell targeting by a recombinant MVA expressing a functional single chain antibody on the surface of intracellular mature virus (IMV) particles. Viral Immunol 20: 664–671. [DOI] [PubMed] [Google Scholar]
- 38. Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, et al. (2003) VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4: 263–275. [DOI] [PubMed] [Google Scholar]
- 39. Indraccolo S (2010) Interferon-alpha as angiogenesis inhibitor: learning from tumor models. Autoimmunity 43: 244–247. [DOI] [PubMed] [Google Scholar]
- 40. Kotredes KP, Gamero AM (2013) Interferons as inducers of apoptosis in malignant cells. J Interferon Cytokine Res 33: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Subramaniam PS, Johnson HM (1997) A role for the cyclin-dependent kinase inhibitor p21 in the G1 cell cycle arrest mediated by the type I interferons. J Interferon Cytokine Res 17: 11–15. [DOI] [PubMed] [Google Scholar]
- 42. Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, et al. (2011) Direct effects of type I interferons on cells of the immune system. Clin Cancer Res 17: 2619–2627. [DOI] [PubMed] [Google Scholar]
- 43. Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, et al. (2005) A critical function for type I interferons in cancer immunoediting. Nat Immunol 6: 722–729. [DOI] [PubMed] [Google Scholar]
- 44. Fiola C, Peeters B, Fournier P, Arnold A, Bucur M, et al. (2006) Tumor selective replication of Newcastle disease virus: association with defects of tumor cells in antiviral defence. Int J Cancer 119: 328–338. [DOI] [PubMed] [Google Scholar]
- 45. Krishnamurthy S, Takimoto T, Scroggs RA, Portner A (2006) Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol 80: 5145–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shmulevitz M, Pan LZ, Garant K, Pan D, Lee PW (2010) Oncogenic Ras promotes reovirus spread by suppressing IFN-beta production through negative regulation of RIG-I signaling. Cancer Res 70: 4912–4921. [DOI] [PubMed] [Google Scholar]
- 47. Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, et al. (2000) Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 6: 821–825. [DOI] [PubMed] [Google Scholar]
- 48. Naik S, Nace R, Federspiel MJ, Barber GN, Peng KW, et al. (2012) Curative one-shot systemic virotherapy in murine myeloma. Leukemia 26: 1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Noll M, Berchtold S, Lampe J, Malek NP, Bitzer M, et al. (2013) Primary resistance phenomena to oncolytic measles vaccine viruses. Int J Oncol 43: 103–112. [DOI] [PubMed] [Google Scholar]
- 50. Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ, et al. (2007) Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther 15: 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le Boeuf F, Batenchuk C, Vaha-Koskela M, Breton S, Roy D, et al. (2013) Model-based rational design of an oncolytic virus with improved therapeutic potential. Nat Commun 4: 1974. [DOI] [PubMed] [Google Scholar]
- 52. Brun J, McManus D, Lefebvre C, Hu K, Falls T, et al. (2010) Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol Ther 18: 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Le Boeuf F, Diallo J-S, McCart JA, Thorne S, Falls T, et al. (2010) Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther 18: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alvarez-Breckenridge CA, Yu J, Price R, Wei M, Wang Y, et al. (2012) The histone deacetylase inhibitor valproic acid lessens NK cell action against oncolytic virus-infected glioblastoma cells by inhibition of STAT5/T-BET signaling and generation of gamma interferon. J Virol 86: 4566–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bridle BW, Chen L, Lemay CG, Diallo J-S, Pol J, et al. (2013) HDAC inhibition suppresses primary immune responses, enhances secondary immune responses, and abrogates autoimmunity during tumor immunotherapy. Mol Ther 21: 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. MacTavish H, Diallo J-S, Huang B, Stanford M, Le Boeuf F, et al. (2010) Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. PLoS ONE 5: e14462 doi:10.1371/journal.pone.0014462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chang HM, Paulson M, Holko M, Rice CM, Williams BR, et al. (2004) Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci U S A 101: 9578–9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nguyen TL, Abdelbary H, Arguello M, Breitbach C, Leveille S, et al. (2008) Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci U S A 105: 14981–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diallo J-S, Le Boeuf F, Lai F, Cox J, Vaha-Koskela M, et al. (2010) A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol Ther 18: 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Abrahams MR, Zhang Z, Chien S, Skerns T, Kotwal GJ (2005) The vaccinia virus N1L ORF may encode a multifunctional protein possibly targeting different kinases, one of which influences ATP levels in vivo. Ann N Y Acad Sci 1056: 87–99. [DOI] [PubMed] [Google Scholar]
- 62. Gammon DB, Gowrishankar B, Duraffour S, Andrei G, Upton C, et al. (2010) Vaccinia virus–encoded ribonucleotide reductase subunits are differentially required for replication and pathogenesis. PLoS Pathog 6: e1000984 doi:10.1371/journal.ppat.1000984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Elford HL, Freese M, Passamani E, Morris HP (1970) Ribonucleotide reductase and cell proliferation. I. Variations of ribonucleotide reductase activity with tumor growth rate in a series of rat hepatomas. J Biol Chem 245: 5228–5233. [PubMed] [Google Scholar]
- 64. Chung RY, Saeki Y, Chiocca EA (1999) B-myb promoter retargeting of herpes simplex virus gamma34.5 gene-mediated virulence toward tumor and cycling cells. J Virol 73: 7556–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aghi M, Visted T, Depinho RA, Chiocca EA (2008) Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene 27: 4249–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim JH, Oh JY, Park BH, Lee DE, Kim JS, et al. (2006) Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther 14: 361–370. [DOI] [PubMed] [Google Scholar]
- 67. Liu BL, Robinson M, Han ZQ, Branston RH, English C, et al. (2003) ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 10: 292–303. [DOI] [PubMed] [Google Scholar]
- 68. Passer BJ, Cheema T, Zhou B, Wakimoto H, Zaupa C, et al. (2010) Identification of the ENT1 antagonists dipyridamole and dilazep as amplifiers of oncolytic herpes simplex virus-1 replication. Cancer Res 70: 3890–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ruggero D (2013) Translational control in cancer etiology. Cold Spring Harb Perspect Biol 5: a012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bjornsti MA, Houghton PJ (2004) Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell 5: 519–523. [DOI] [PubMed] [Google Scholar]
- 71. Holland EC, Sonenberg N, Pandolfi PP, Thomas G (2004) Signaling control of mRNA translation in cancer pathogenesis. Oncogene 23: 3138–3144. [DOI] [PubMed] [Google Scholar]
- 72. Barber GN (2005) VSV-tumor selective replication and protein translation. Oncogene 24: 7710–7719. [DOI] [PubMed] [Google Scholar]
- 73. Stanford MM, McFadden G (2007) Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opin Biol Ther 7: 1415–1425. [DOI] [PubMed] [Google Scholar]
- 74. Garcia MA, Meurs EF, Esteban M (2007) The dsRNA protein kinase PKR: virus and cell control. Biochimie 89: 799–811. [DOI] [PubMed] [Google Scholar]
- 75. Kawagishi-Kobayashi M, Silverman JB, Ung TL, Dever TE (1997) Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIF2alpha. Mol Cell Biol 17: 4146–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Balachandran S, Barber GN (2004) Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5: 51–65. [DOI] [PubMed] [Google Scholar]
- 77. Richter JD, Sonenberg N (2005) Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433: 477–480. [DOI] [PubMed] [Google Scholar]
- 78. Chuluunbaatar U, Roller R, Feldman ME, Brown S, Shokat KM, et al. (2010) Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev 24: 2627–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sabatini DM (2006) mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 6: 729–734. [DOI] [PubMed] [Google Scholar]
- 80. Liu TC, Wakimoto H, Martuza RL, Rabkin SD (2007) Herpes simplex virus Us3(-) mutant as oncolytic strategy and synergizes with phosphatidylinositol 3-kinase-Akt targeting molecular therapeutics. Clin Cancer Res 13: 5897–5902. [DOI] [PubMed] [Google Scholar]
- 81. Alain T, Lun X, Martineau Y, Sean P, Pulendran B, et al. (2010) Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci U S A 107: 1576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Colina R, Costa-Mattioli M, Dowling RJ, Jaramillo M, Tai LH, et al. (2008) Translational control of the innate immune response through IRF-7. Nature 452: 323–328. [DOI] [PubMed] [Google Scholar]
- 83. Costa-Mattioli M, Sonenberg N (2008) RAPping production of type I interferon in pDCs through mTOR. Nat Immunol 9: 1097–1099. [DOI] [PubMed] [Google Scholar]
- 84. Oliere S, Arguello M, Mesplede T, Tumilasci V, Nakhaei P, et al. (2008) Vesicular stomatitis virus oncolysis of T lymphocytes requires cell cycle entry and translation initiation. J Virol 82: 5735–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Correa RJ, Komar M, Tong JG, Sivapragasam M, Rahman MM, et al. (2012) Myxoma virus-mediated oncolysis of ascites-derived human ovarian cancer cells and spheroids is impacted by differential AKT activity. Gynecol Oncol 125: 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lun X, Alain T, Zemp FJ, Zhou H, Rahman MM, et al. (2010) Myxoma virus virotherapy for glioma in immunocompetent animal models: optimizing administration routes and synergy with rapamycin. Cancer Res 70: 598–608. [DOI] [PubMed] [Google Scholar]
- 87. Stanford MM, Shaban M, Barrett JW, Werden SJ, Gilbert PA, et al. (2008) Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo. Mol Ther 16: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fu X, Tao L, Rivera A, Zhang X (2011) Rapamycin enhances the activity of oncolytic herpes simplex virus against tumor cells that are resistant to virus replication. Int J Cancer 129: 1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Homicsko K, Lukashev A, Iggo RD (2005) RAD001 (everolimus) improves the efficacy of replicating adenoviruses that target colon cancer. Cancer Res 65: 6882–6890. [DOI] [PubMed] [Google Scholar]
- 90. Lun XQ, Jang JH, Tang N, Deng H, Head R, et al. (2009) Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res 15: 2777–2788. [DOI] [PubMed] [Google Scholar]
- 91. Brass N, Heckel D, Sahin U, Pfreundschuh M, Sybrecht GW, et al. (1997) Translation initiation factor eIF-4gamma is encoded by an amplified gene and induces an immune response in squamous cell lung carcinoma. Hum Mol Genet 6: 33–39. [DOI] [PubMed] [Google Scholar]
- 92. Braunstein S, Karpisheva K, Pola C, Goldberg J, Hochman T, et al. (2007) A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell 28: 501–512. [DOI] [PubMed] [Google Scholar]
- 93. Goetz C, Gromeier M (2010) Preparing an oncolytic poliovirus recombinant for clinical application against glioblastoma multiforme. Cytokine Growth Factor Rev 21: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fitzgerald KD, Semler BL (2011) Re-localization of cellular protein SRp20 during poliovirus infection: bridging a viral IRES to the host cell translation apparatus. PLoS Pathog 7: e1002127 doi:10.1371/journal.ppat.1002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kastan MB, Bartek J (2004) Cell-cycle checkpoints and cancer. Nature 432: 316–323. [DOI] [PubMed] [Google Scholar]
- 96. Ben-Israel H, Kleinberger T (2002) Adenovirus and cell cycle control. Front Biosci 7: d1369–1395. [DOI] [PubMed] [Google Scholar]
- 97. Crescenzi M, Soddu S, Tato F (1995) Mitotic cycle reactivation in terminally differentiated cells by adenovirus infection. J Cell Physiol 162: 26–35. [DOI] [PubMed] [Google Scholar]
- 98. Poggioli GJ, DeBiasi RL, Bickel R, Jotte R, Spalding A, et al. (2002) Reovirus-induced alterations in gene expression related to cell cycle regulation. J Virol 76: 2585–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yoo NK, Pyo CW, Kim Y, Ahn BY, Choi SY (2008) Vaccinia virus-mediated cell cycle alteration involves inactivation of tumour suppressors associated with Brf1 and TBP. Cell Microbiol 10: 583–592. [DOI] [PubMed] [Google Scholar]
- 100. Bandara LR, La Thangue NB (1991) Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature 351: 494–497. [DOI] [PubMed] [Google Scholar]
- 101. Zamanian M, La Thangue NB (1992) Adenovirus E1a prevents the retinoblastoma gene product from repressing the activity of a cellular transcription factor. EMBO J 11: 2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, et al. (2000) A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 19: 2–12. [DOI] [PubMed] [Google Scholar]
- 103. Kim M, Williamson CT, Prudhomme J, Bebb DG, Riabowol K, et al. (2010) The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status. Oncogene 29: 3990–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Martin S, Harris DT, Shisler J (2012) The C11R gene, which encodes the vaccinia virus growth factor, is partially responsible for MVA-induced NF-kappaB and ERK2 activation. J Virol 86: 9629–9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tao Y, Song X, Deng X, Xie D, Lee LM, et al. (2005) Nuclear accumulation of epidermal growth factor receptor and acceleration of G1/S stage by Epstein-Barr-encoded oncoprotein latent membrane protein 1. Exp Cell Res 303: 240–251. [DOI] [PubMed] [Google Scholar]
- 106. Twardzik DR, Brown JP, Ranchalis JE, Todaro GJ, Moss B (1985) Vaccinia virus-infected cells release a novel polypeptide functionally related to transforming and epidermal growth factors. Proc Natl Acad Sci U S A 82: 5300–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Holbro T, Civenni G, Hynes NE (2003) The ErbB receptors and their role in cancer progression. Exp Cell Res 284: 99–110. [DOI] [PubMed] [Google Scholar]
- 108. Esfandiarei M, Luo H, Yanagawa B, Suarez A, Dabiri D, et al. (2004) Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J Virol 78: 4289–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hsu MJ, Wu CY, Chiang HH, Lai YL, Hung SL (2010) PI3K/Akt signaling mediated apoptosis blockage and viral gene expression in oral epithelial cells during herpes simplex virus infection. Virus Res 153: 36–43. [DOI] [PubMed] [Google Scholar]
- 110. Soares JA, Leite FG, Andrade LG, Torres AA, De Sousa LP, et al. (2009) Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infections is required for both host survival and viral replication. J Virol 83: 6883–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Werden SJ, Lanchbury J, Shattuck D, Neff C, Dufford M, et al. (2009) The myxoma virus m-t5 ankyrin repeat host range protein is a novel adaptor that coordinately links the cellular signaling pathways mediated by Akt and Skp1 in virus-infected cells. J Virol 83: 12068–12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sypula J, Wang F, Ma Y, Bell J, McFadden G (2004) Myxoma virus tropism in human tumor cells. Gene Ther Mol Biol 8: 103–114. [Google Scholar]
- 113. Fu X, Tao L, Cai R, Prigge J, Zhang X (2006) A mutant type 2 herpes simplex virus deleted for the protein kinase domain of the ICP10 gene is a potent oncolytic virus. Mol Ther 13: 882–890. [DOI] [PubMed] [Google Scholar]
- 114. Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW (2007) Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther 15: 1522–1530. [DOI] [PubMed] [Google Scholar]
- 115. Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW (1998) The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J 17: 3351–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Endo T, Toda M, Watanabe M, Iizuka Y, Kubota T, et al. (2002) In situ cancer vaccination with a replication-conditional HSV for the treatment of liver metastasis of colon cancer. Cancer Gene Ther 9: 142–148. [DOI] [PubMed] [Google Scholar]
- 117. Melcher A, Parato K, Rooney CM, Bell JC (2009) Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther 19: 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, et al. (2009) Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res 15: 4374–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vigil A, Martinez O, Chua MA, Garcia-Sastre A (2008) Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther 16: 1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Guo ZS, Naik A, O'Malley ME, Popovic P, Demarco R, et al. (2005) The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res 65: 9991–9998. [DOI] [PubMed] [Google Scholar]
- 121. Vijayalingam S, Subramanian T, Ryerse J, Varvares M, Chinnadurai G (2009) Down-regulation of multiple cell survival proteins in head and neck cancer cells by an apoptogenic mutant of adenovirus type 5. Virology 392: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jin J, Liu H, Yang C, Li G, Liu X, et al. (2009) Effective gene-viral therapy of leukemia by a new fiber chimeric oncolytic adenovirus expressing TRAIL: in vitro and in vivo evaluation. Mol Cancer Ther 8: 1387–1397. [DOI] [PubMed] [Google Scholar]
- 123. Sathaiah M, Thirunavukkarasu P, O'Malley ME, Kavanagh MA, Ravindranathan R, et al. (2012) Oncolytic poxvirus armed with Fas ligand leads to induction of cellular Fas receptor and selective viral replication in FasR-negative cancer. Cancer Gene Ther 19: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chalikonda S, Kivlen MH, O'Malley ME, Eric Dong XD, McCart JA, et al. (2008) Oncolytic virotherapy for ovarian carcinomatosis using a replication-selective vaccinia virus armed with a yeast cytosine deaminase gene. Cancer Gene Ther 15: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zheng FQ, Xu Y, Yang RJ, Wu B, Tan XH, et al. (2009) Combination effect of oncolytic adenovirus therapy and herpes simplex virus thymidine kinase/ganciclovir in hepatic carcinoma animal models. Acta Pharmacol Sin 30: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ma S, Qu W, Mao L, Zhu Z, Jia L, et al. (2012) Antitumor effects of oncolytic adenovirus armed with Drosophila melanogaster deoxyribonucleoside kinase in colorectal cancer. Oncol Rep 27: 1443–1450. [DOI] [PubMed] [Google Scholar]
- 127. Sunamura M, Oonuma M, Motoi F, Abe H, Saitoh Y, et al. (2002) Gene therapy for pancreatic cancer targeting the genomic alterations of tumor suppressor genes using replication-selective oncolytic adenovirus. Hum Cell 15: 138–150. [DOI] [PubMed] [Google Scholar]
- 128. Schepelmann S, Ogilvie LM, Hedley D, Friedlos F, Martin J, et al. (2007) Suicide gene therapy of human colon carcinoma xenografts using an armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer Res 67: 4949–4955. [DOI] [PubMed] [Google Scholar]
- 129. Stubdal H, Perin N, Lemmon M, Holman P, Bauzon M, et al. (2003) A prodrug strategy using ONYX-015-based replicating adenoviruses to deliver rabbit carboxylesterase to tumor cells for conversion of CPT-11 to SN-38. Cancer Res 63: 6900–6908. [PubMed] [Google Scholar]
- 130. Mahoney DJ, Lefebvre C, Allan K, Brun J, Sanaei CA, et al. (2011) Virus-tumor interactome screen reveals ER stress response can reprogram resistant cancers for oncolytic virus-triggered caspase-2 cell death. Cancer Cell 20: 443–456. [DOI] [PubMed] [Google Scholar]
- 131. Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]