Abstract
Introduction
We examined the impact of a 24 hour complete fast (vs. fed state) on two measures of food reward: 1) ‘wanting’, as measured by response to food images and by the relative-reinforcing value of food (RRV), and 2) ‘liking’, as measured by response to food images and the hedonic evaluation of foods consumed.
Methods
Utilizing a randomized crossover design, 15 subjects (9 male; 6 female) aged 28.6±4.5 yrs with body mass index 25.3±1.4 kg/m2 were randomized and counterbalanced to normal feeding (FED) and 24-hour fast (FASTED) conditions. Trait characteristics were measured with the Three Factor Eating Questionnaire. Two computer tasks measured food reward: 1) RRV progressive ratio task, 2) explicit ‘liking’ and ‘wanting’ (Leeds Food Preference Questionnaire, LFPQ). Also measured were ad libitum energy intake (EI; buffet) and food ‘liking’ (visual analogue scale) of personalized stimuli.
Results
There were no significant anthropometric changes between conditions. Appetite scores, hedonic ratings of ‘liking’, and ad libitum EI all significantly increased under the FASTED condition (p<0.05). Under the FASTED condition there were significant increases in the RRV of snack foods; similarly, explicit ‘wanting’ and ‘liking’ significantly increased for all food categories. ‘Liking’ of sweet foods remained high across-meals under FASTED, but savory foods decreased in hedonic saliency.
Conclusion
Relative to a fed state, we observed an increase in hedonic ratings of food, the rewarding value of food, and food intake after a 24 hr fast. Alliesthesia to food and food cues is suggested by heightened hedonic ratings under the FASTED condition relative to FED.
Introduction
In one of the best known and most controlled studies of human feeding it was shown that upon re-feeding after 24 weeks of energy restriction (approximately 1500 kcal/day), subjects who volunteered for the Minnesota Semi-starvation Study demonstrated chronic hyperphagia, obsessive preoccupations with sweet foods and food images, and a host of negative reactions dubbed “semistarvation neurosis” [1]. Similar findings have been reported regarding aberrant feeding with acute periods (1 day) of fasting in clinical populations [2], [3]. Others, however, have found that acute fasting does not necessarily result in excessive energy intake or negative affect upon reinitiating ad libitum feeding in non-clinical subjects [4], [5]. It is still unclear what factors underpin compensatory eating and altered hedonics in the early stages energy deprivation. There are conflicting data, but it appears that the short-term manipulation of satiety, most often achieved with a pre-load paradigm, does not reliably impact palatability [6]–[8]. While the preload paradigm may be sensitive to possible short-term signaling of need (free)-state, a better manipulation has been less well studied and involves increasing the deprivation state through sustained energy deprivation. In support of the external validity of a sustained energy deprivation, it can be pointed out that recent NHANES data show that 63% of respondents were trying to lose weight [9], and older data has shown that approximately 14% of Americans have reported using short-term fasting as a means of losing weight [10]. Furthermore, the relevance of studying subjects under periods of deprivation longer than a few hours is reflected in the observation that day-to-day feeding typically occurs in response to learned cues [11], and not necessarily to a need-state.Thus, although it is clear that under challenges of negative energy balance feeding behavior can be altered with or without measurable changes in body energy reserves, what remains to be better described are the psychological and behavioral factors that drive changes in appetite, feeding, and food reward under such conditions.
Feeding behavior has been shown to be modulated by hunger-state, whereby prolonging inter-meal intervals or inducing weight loss can positively influence perceived taste pleasantness, a concept called alliesthesia [12], [13]. Food reward can be operationalized as stimuli (internal and external) that contribute to the pleasure of and motivation to obtain food. Indeed, rigorous studies involving animal and human models of feeding have led to considerable progress in the understanding of the psychology of food reward [14] and the neural substrates that mediate it (for review see [15], [16]). In order to further examine the potential clinical importance of food reward in body weight regulation, and to better describe the overlapping pathways of food and drug reward [17], there has been a surge in studies examining hunger states (e.g. high hunger vs. sated) with brain imaging [18]–[23]. Concomitantly, there has been an emergence of standardized objective tools to measure psychological constructs of food reward (e.g. hedonics of food and food-related stimuli [24], [25] and food reinforcement [26]). Energy deprivation not only impacts the palatability—or ‘liking’—of a particular food stimulus [27]–[29], but also impacts the desire—or ‘wanting’—to engage a food stimulus [30]. In utilizing a computer task that applies behavioral economic theory to human feeding [31], ongoing research on the reinforcing value of food has demonstrated that ‘wanting’ can change in a state-dependent manner (i.e. hungry vs. satiated) [32]–[34]. Similarly, several groups have demonstrated in human subjects that the hedonic ‘liking’ evaluation of ingested foods can be impacted acutely by hunger-state [35], [36] or chronically by a state of reduced body energy reserves [37], [38]. Taken together, the dual qualities of food reward—‘wanting’ and ‘liking’—can be described as susceptible to changes in the internal milieu, analogous to Michel Cabanac's concept of alliesthesia [13]. It is unclear, however, what impact an acute 24 hour fasting challenge will have on the direction of ‘wanting’ and ‘liking’ for preferred food items or whether a relationship exists with changes in appetite, food reward and ad libitum EI.
There is a growing interest in fasting and alternate day (modified) fasting as methods to achieve weight loss or simply as a way of living (e.g. religious fasting periods). The main objective of this randomized crossover study was to examine the impact of a 24 hour complete fast (vs. fed state) on appetite, food reward, and EI. It was hypothesized that under the fasting condition there would be significantly higher appetite scores and ad libitum EI, and that correlations would exist between these changes. It was further hypothesized that relative to the fed state, snack food would become more rewarding, with increased explicit ‘wanting’ and ‘liking’, and the meal-induced attenuation of explicit ‘liking’ for the fasted condition would be less pronounced.
Methods
Subjects
Fifteen volunteers (9 male; 6 female) aged 28.6±4.5 yrs. with body weight 74.7±4.9 kg and body mass index (BMI) 25.3±1.4 kg/m2 (i.e. normal weight/overweight) participated in this randomized crossover study, once in the fed state (FED) and once after performing a 24-hour complete fast (FASTED). Subjects were free from any illnesses and medication that could have influenced the outcome of the experiment and met the following inclusion criteria: non-diabetic, non-smokers, not pregnant, weight stable for ≥6 months (±2 kg) and aged between 18 and 40 years. Only pre-menopausal women with a regular menstrual cycle (28–35 days) were recruited including those using oral contraceptives. Characteristics of subjects under control (FED) and experimental (FASTED) conditions are presented in Table 1 . This study was conducted according to the guidelines laid down in the Declaration of Helsinki and the University of Ottawa Research Ethics Committee approved all procedures involving human subjects. Written informed consent was obtained from all subjects.
Table 1. Subjects' characteristics under FED (control) and FASTED (experimental) conditions.
| Characteristic | FED | FASTED | %Change | Time | Sex | Time x Sex |
| Anthropometric | ||||||
| Body Weight (kg) | 74.4±4.9 | 74.2±4.9 | 0.3 | 0.12 | 0.10 | 0.60 |
| BMI (kg/m2) | 25.2±1.4 | 25.0±1.4 | 0.8 | 0.09 | 0.56 | 0.69 |
| Appetite (AUC) | ||||||
| Desire to Eat | 555.1±316.9 | 719.2±123.3 | 30.5 | 0.05 | 0.32 | 0.79 |
| Hunger | 415.6±169.5 | 703.5±121.8 | 69.3 | 0.001 | 0.36 | 0.08 |
| Fullness | 560.5±44.4 | 295.6±100.1 | 47.3 | 0.001 | 0.32 | 0.12 |
| PFC | 492.4±43.5 | 741.6±131.5 | 50.6 | 0.001 | 0.14 | 0.75 |
| Food Hedonics | ||||||
| Snack | 120.5±7.6 | 135.1±4.9 | 12.1 | 0.005 | 0.70 | 0.94 |
| Fruit | 112.3±7.1 | 130.9±5.0 | 16.6 | 0.02 | 0.25 | 0.44 |
| Pizza 1 | 107.9±29.9 | 119.6±23.9 | 10.8 | 0.05 | 0.90 | 0.98 |
| Pizza 2 | 95.6±37.2 | 118.6±28.9 | 24.1 | 0.005 | 0.90 | 0.20 |
| Dessert | 121.1±8.6 | 132.2±4.9 | 9.2 | 0.06 | 0.67 | 0.91 |
| AUC | 436.6±79.4 | 502.6±68.8 | 15.1 | 0.001 | 0.58 | 0.26 |
| RRV | ||||||
| Snack Points | 16.9±9.3 | 25.6±12.4 | 51.5 | 0.008 | 0.90 | 0.26 |
| Snack Responses | 381.9±202.2 | 613.5±344.3 | 60.6 | 0.03 | 0.99 | 0.14 |
| Fruit Points | 33.1±9.4 | 23.7±11.9 | 28.4 | 0.03 | 0.98 | 0.37 |
| Fruit Responses | 201.6±52.1 | 145.1±77.3 | 28.0 | 0.008 | 0.95 | 0.09 |
| Ad Libitum EI | ||||||
| Total EI (grams) | 300.0±42.4 | 388.8±55.2 | 29.6 | 0.001 | 0.021 | 0.30 |
| Total EI (kcal) | 491.1±99.6 | 854.9±104.6 | 74.1 | 0.001 | 0.003 | 0.35 |
| Energy Density | 1.65±0.2 | 2.4±0.2 | 45.5 | 0.002 | 0.55 | 0.76 |
| %Sugar | 17.6±1.9 | 29.0±7.5 | 64.8 | 0.06 | 0.19 | 0.71 |
| %Fat | 6.5±1.1 | 12.2±3.7 | 87.7 | 0.21 | 0.42 | 0.35 |
| %Protein | 2.4±0.35 | 4.7±9.0 | 95.8 | 0.05 | 0.76 | 0.26 |
Appetite scores were measured hourly and pre-and post food consumption with 150 mm visual analogue scales (VAS). Similarly, the change in food hedonics was measured by VAS ratings for palatability immediately following the ingestion of preferred foods. Food reinforcement was measured with a behavioral choice computer task.
Area under the curve (AUC) was calculated with the trapezoid method and included all variables described in the Food Hedonics category. Note that BMI is body mass index; PFC is prospective food consumption; EI is energy intake in kilocalories (kcal); Energy Density is kcal/gram of food consumed; and RRV is relative-reinforcing value. Values are means ± SD.
Design and procedure
Screening visit
The study began during the initial (screening) visit to the laboratory, where written informed consent was obtained and then height and body weight were measured. Each subject was then asked to complete a questionnaire to indicate his or her favorite snack food and favorite fruit/or vegetable. For this decision, they were asked to circle five choices each from a comprehensive list of common food products (chips, candies, cakes, and chocolate bars) and common fruits/vegetables, or if the choice was not on the list, they were asked to indicate what their favorite food items were. In this manner each subject would have his single favorite snack food and fruit/or vegetable as reinforcers for the RRV paradigm. Note that no subjects chose a vegetable as their preferred food item, so all non-snack foods were fruits. Also, subjects were required to complete the Three Factor Eating Questionnaire (TFEQ). In order to minimize the hormonal effects on main outcomes, measurements for women were scheduled between days 1–5 of the menstrual cycle, where ovarian hormones are at their lowest levels [39]. As such, women had at least a 1-month period, and men at least a 2-week period between the FED and FASTED sessions.
Experimental manipulation of FED state
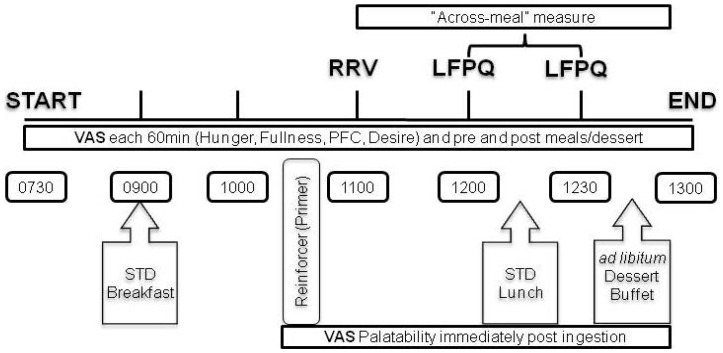
Subjects arrived at 0800 fasted from 1900 the prior evening for the FED session and body weight and height were recorded. Subjects were then presented with a calorically-clamped breakfast which had to be consumed in 15 minutes at approximately 0900. Appetite measures (VAS) were taken first while fasted, and then every hour after completing the breakfast meal. Similarly, food ‘liking’ was measured immediately after the standardized breakfast, after the RRV primers, after lunch, and after the ad libitum dessert. The RRV computer task was administered at 1100 after consuming a 50 kcal primer each of the personalized snack and of the personalized fruit (see Figure 1 ). The second computer task—the LFPQ—measured explicit ‘liking’ and ‘wanting’ for food and was employed once at ∼1145, prior to eating lunch, and then once again immediately following lunch. Because there was a lunch meal in between the two measures of LFPQ, the term “across-meal” measure was used to describe this comparison of ‘wanting’ and ‘liking’. A standardized (calorically-clamped) lunch was served at ∼1200. At approximately 1230, after consuming lunch and after performing the post lunch LFPQ computer task, subjects were offered an ad libitum dessert buffet with a 30-minute time limit to eat as much or as little as desired. The session ended immediately after completing the buffet and VAS measures.
Figure 1. Protocol for the two testing sessions.
Note that in order to perform the 24; the fasting period was slightly less than 24 hours due to the standardized consumption (100 kcal: 50 kcal snack and 50 kcal fruit) of the primers used in the RRV task.
Experimental manipulation of FASTED state
Subjects arrived at 0800 fasted from 1200 the prior day for the FASTED session body weight and height were recorded. Subjects were only allowed water during the 24 hours that defined the FASTED interval, which started outside of the lab at 1200 the day prior to testing lasting until 1100 on the day of testing in the lab. A self-reported checklist was used to verify compliance to the fast and indirect calorimetry was used as a complement to corroborate that the respiratory quotient was indicative of a fasted state (i.e., RQ<0.84). Appetite measures (VAS) were taken every hour. Food ‘liking’ was measured immediately after the food reinforcers for the RRV task (∼1100), and immediately following the lunch (∼1200) and ad libitum dessert (∼1230). The experimental design was a repeated measures and the only difference between the FASTED and FED sessions in the lab was that during the FASTED session subjects did not consume a breakfast on the day of testing.
Measurements
Anthropometric measures
Height (HR-100 Height Rod; Tanita Corporation of America Inc. Arlington Heights, IL) and body weight (HR-100; BWB-800AS, Tanita Corporation, Arlington Heights, IL., USA) were measured after a 12 hour overnight fast, after voiding, while wearing a standard hospital gown.
Questionnaires: TFEQ
The TFEQ was administered during the initial screening process to determine the subject's individual attitude towards eating at a specific moment in time. It is understood that this questionnaire measures “trait” characteristics which are relatively stable, but are subject to change under periods of weight-loss, for example [40]. This 51 item questionnaire is an instrument that assesses three important attitudes to food: 1) chronic dietary restraint, which describes strategic dieting behavior, attitude to self-regulation, etc.; 2) disinhibition, which describes the vulnerability to lose control and over-consume and the responsiveness to the sight and smell of food; and 3) susceptibility to hunger, which describes internal and external loci of hunger [41].
Appetite and hedonic ‘liking’: visual analogue scales (VAS)
Appetite ratings were measured using a pen and paper on a 150-mm visual analogue scale (VAS) adapted from Hill and Blundell [42]. Desire to eat, hunger, fullness and prospective food consumption (PFC) were rated using the following questions: 1) “How strong is your desire to eat?” (Very weak- Very strong); 2) “How hungry do you feel?” (Not hungry at all- As hungry as I have ever felt); 3) “How full do you feel?” (Not full at all- Very full), and 4) “How much food do you think you could eat?” (Nothing at all- A large amount). Hedonic measures of ‘liking’ were similarly measured immediately following the ingestion of the personalized snack and fruit reinforcers (∼1045), and also immediately following each slice of pizza (∼1200), and finally after the ad libitum dessert (∼1300) with the following question: “How palatable was the meal?” (Not at all-Extremely). The trapezoid method was employed to measure the area under the curve (AUC) for all appetite-related variables, as previously described [43].
Standardized meals and personalized buffet
During the FED condition subjects were presented with a standardized breakfast, which had to be consumed within 15 minutes. The meal consisted of 2 pieces of whole wheat toast (D'Italiano®, 147 kcal), 17 grams of peanut butter (Kraft Smooth Peanut Butter®, 101 kcal), 15 grams of raspberry jam (Smuckers®, 50 kcal), and 250 ml of water for a total of 298 kcal. At 1200 in the FED and FASTED sessions, subjects were presented with a standardized lunch, which had to be consumed in 30 min. The meal consisted of 2 slices of cheese pizza (Michelina's Zap ‘Ems Gourmet®, 781 kcal). The ad libitum buffet consisted of 5 snack food items and 4 fruit/or vegetable items that were individualized—that is, the buffet items were previously indicated as being favorite food items on the food questionnaire from the RRV task. All items were presented on white foam plates and in standardized quantities (e.g. 70 g potato chip serving, 100 g apple serving, 100 g candy bar serving, etc.). Subjects were allotted 30 minutes to eat as much or as little of the buffet as they wanted and were told they could request more servings of any item. All food items were weighed to the nearest 0.1 gram (Scout Pro SP2001; Ohaus Corporation) and the difference calculated and subsequently analyzed with Food Processor SQL software (version 9.6.2.; ESHA Research).
Food reward: RRV and LFPQ computer tasks
The reinforcing value of a stimulus can be objectively observed as the increased (or decreased) willingness—a quantitative measure—to work (button presses) at obtaining food points via a progressive ratio computer task to obtain a desirable food stimulus (vs. some alternative). Specifically, for this study, computer software was used to set up a split screen that alternated between two different choices (a healthy food and palatable snack food) and was navigated with a mouse/pad (see [26]). Food points were earned by selectively working for the food item of choice. To obtain one fruit/vegetable point the reinforcement schedule was set at a progressive linear ratio with response requirements across five trials as follows: 4, 6, 8, 10, and 12. Thus, for example, at VR8 (3rd of 5 trials) the subject performed 8 button presses. For the snack foods the schedule was also set at a progressive ratio that doubled at each session as follows: 4, 8, 16, 32, and 64. The schedules of reinforcement were set to change differently between the two food stimuli in an effort to covertly disguise the discrepancy between the higher responding for the snack food. Note that 1 point was equivalent to 1 gram of the snack or fruit. Prior to performing the RRV task subjects were required to ingest a “primer” of the foods they picked as their favorite—approximately a 50 kcal portion of the snack and fruit reinforcers, respectively. Each subject was then shown what each food portion would look like if they worked entirely for the snack food or entirely for the fruit; this was done to help control for reward expectation [44] and previous research has shown that a primer can increase the ability to show differences in food reinforcement [45].
The LFPQ is designed to allow the separate and concurrent assessments of explicit ‘liking’ and ‘wanting’ for the same array of foods. This stimuli used in the task were photographic images of food varying in fat content (high or low) and taste (savory or sweet). These dimensions can be separated into four categories: high fat savory (HFSA), low fat savory (LFSA), high fat sweet (HFSW), and low fat sweet (LFSW). Five food items for a total of twenty different food stimuli represent each of the four categories. For the explicit measures of ‘liking’ and ‘wanting’, the 20 foods were rated according to “How pleasant would you find the taste of this food right now?“ and “How much do want some of this food right now?”.
Alliesthesia: ‘liking’ evaluation of food and food cues (images)
The phenomenon of alimentary alliesthesia was defined by Cabanac [46] as a change in the hedonic ‘liking’ of a stimulus that is modulated by the current bodily state—influenced by interoceptive signals and energetic needs. Briefly, alliesthesia was measured by Cabanac by the change in subjective pleasantness ratings for multiple ingestions of sweet tasting 25% solutions of aqueous glucose [13]. It is in this light that we measure the phenomenon of alliesthesia in the current study by evaluating the post-ingestive VAS ‘liking’ response to preferred food items (e.g. primers from the RRV task, pizza from the standardized lunch, and items from the dessert buffet) while under FED and FASTED conditions. Similarly, alliesthesia to food cues was measured by analogue scales in the LFPQ task for ‘liking’ of individual food images.
Statistical methods
To test for differences in anthropometric variables, appetite, ad libitum EI, ‘liking’, RRV, and LFPQ across FED and FASTED sessions, repeated measures Analysis of Variance (ANOVAs) were used where Condition (FED vs FASTED) represented the within-subjects effects and sex represented the between subjects effects. Bivariate correlations were used to determine the strength of the relationship between the changes in food reward and the change in EI, and the relationship between TFEQ and food reward and EI; partial correlations controlling for age and sex were then used to follow up significant relationships. Statistical analyses were performed using SPSS version 17 (Chicago, SPSS Inc.). Results are presented as means ± SD and effects were considered significant at p<0.05.
Results
As expected there were no significant changes in body weight or BMI between FED and FASTED conditions (see Table 1 ). Mean scores (maximum and minimum in parentheses) for Three Factor Eating Questionnaire (TFEQ) scores were 9 (±3.9) (4–17), 7.5 (±3.6) (2–13), and 7.6 (±3.0 (3–12), for dietary restraint, dietary disinhibition, and susceptibility to hunger, respectively.
There were significant differences (Condition effects) under the FASTED condition for all four measures of AUC (mm) for appetite (see Table 1 ). Relative to FED, ad libitum EI and all hedonic measures of ‘liking’ significantly increased under the FASTED condition. There were no significant sex or Condition x sex effects for variables of appetite or food hedonics (see Table 1 ).
As indicated by higher mean snack points (p<0.001) and higher mean snack responses (p<0.05) for the RRV task, significant Condition effects were noted for the RRV measure of food reward where preferred snack food was more reinforcing under the FASTED condition compared to FED (see Table 1 ). Relatedly, mean fruit points (p<0.05) and mean fruit responses (p<0.01) significantly decreased from FED to FASTED. There were no significant sex or time x sex effects for any RRV measure.
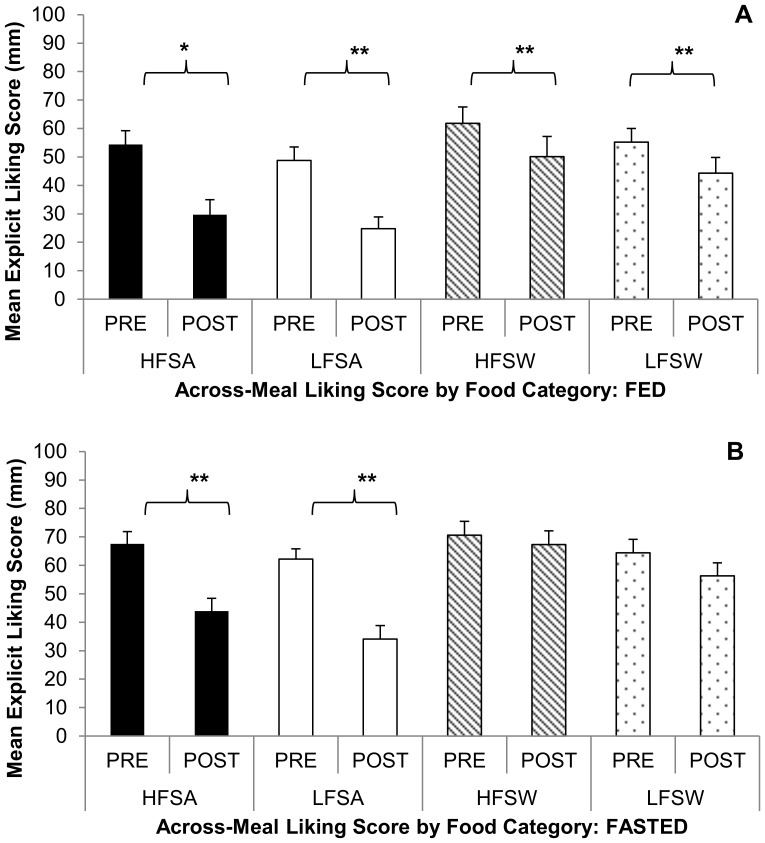
There were significant Condition effects as indicated by pre-meal increases in the LFPQ explicit ‘liking’ scores from FED to FASTED in the following: HFSA (54.4±18.6 vs. 67.5±16.4, p<0.05) and LFSW (55.2±17.3 vs. 64.4±18.2, p<0.05). Similarly, there were significant Condition effects for post-meal scores as indicated by increases in the explicit ‘liking’ scores from FED to FASTED for the following: HFSA (29.7±20.6 vs. 43.9±17.6, p<0.05), HFSW (50.6±27.6 vs. 67.3±18.8, p<0.01), and LFSW (44.3±21.4 vs. 56.3±17.9, p<0.05). There were significant Condition effects as indicated by pre-meal increases in all the LFPQ explicit ‘wanting’ scores for each specific food category from FED to FASTED: HFSA (49.8±18.8 vs. 68.9±18.2, p<0.05), LFSA (45.2±19.1 vs. 64.2±13.9, p<0.001), HFSW (50.3±22.2 vs. 69.1±20.4, p<0.005), and LFSW (47.6±15.1 vs. 59.9±17.9, p<0.05). Similarly, significant Condition effects were noted for the post-meal explicit ‘wanting’ rating from FED to FASTED, showing increases for each category: HFSA (21.8±20.5 vs. 40.2±22.1, p<0.01), LFSA (18.7±16.9 vs. 31.6±20.3, p<0.05), HFSW (38.3±23.4 vs. 62.8±22.0, p<0.005), and LFSW (34.7±18.9 vs. 52.9±18.9, p<0.01). In the FED condition there were significant across-meal decreases in explicit ‘liking’ for all four food categories: HFSA (p<0.05), LFSA (p<0.001), HFSW (p<0.001), and LFSW (p<0.001) (see Figure 2 ). In the FASTED condition, there were only across-meal decreases in explicit ‘liking’ for HFSA (p<0.001) and LFSA (p<0.001), while no significant decreases were noted for the two sweet categories (see Figure 2 ). The only noted sex effect for the LFPQ variables was for post-meal ‘wanting’ of HFSA, where men wanted HFSA foods more than women post-meal; there were no time x sex interactions for any LFPQ variables.
Figure 2. Results from the Leeds Food Preference Questionnaire for ‘liking’ of food images under FASTED and FED conditions.
Showing the change in across-meal mean ‘liking’ score (± SD) for each of the four food categories: high fat savory (HFSA), low fat savory (LFSA), high fat sweet (HFSW) and low fat sweet (LFSW). In the FED condition (panel A) across-meal ‘liking’ for all four categories decreased. Panel B demonstrates the contrast of hedonic ‘liking’ ratings in the experimental condition and suggests the absence of negative alliesthesia to food images of the sweet food category after completing a 24 hour fast. *p<0.05, **p<0.001.
Bivariate correlations revealed that the noted increase in ad libitum EI under the FASTED condition was positively correlated with the change in the AUC appetite score for desire to eat (r = 0.55, p = 0.04). Partial correlations controlling for sex revealed: that TFEQ disinhibition scores were positively correlated with snack points earned under the FED condition (r = 0.56, p<0.05); and that under the FASTED condition, disinhibition was positively correlated with snack points (r = 0.55, p<0.05) and snack responses (r = 0.52, p<0.05). There were no other significant correlations between variables.
Discussion
This study demonstrated evidence of acute changes in the rewarding qualities of preferred foods after a 24-hour fast. Ad libitum EI from the buffet increased 74% from FED to FASTED and food was not only subjectively rated with higher post-ingestive ‘liking’ scores (VAS) in the FASTED condition, but food images were also perceived as more liked both pre and post lunch, suggestive of alliesthesia for orosensory and visual stimuli. Across-meal measures of the LFPQ for ‘liking’ in the FASTED condition demonstrated an attenuated score for savory foods, but sweet foods maintained a strong hedonic saliency by failing to show the decrease in ‘liking’ scores noted in the FED condition from pre- to post-meal.
Intuitively, one would anticipate that energy deprivation results in increased hunger ratings. But evidence suggests that as the fasting period is carried over from hours to days the appetite response can normalize to baseline levels previously noted in the fed state after approximately 4-5 days of fasting [5], [47]. Similar seemingly discrepant results have been found with less dramatic methods of energy deprivation, namely very low calorie diets, where on average subjects reported decreased appetite during the intervention—described as “less food less hunger” [48]–[50]. In a study with a fasting period (19 hours) comparable to our design, subjects without clinical eating pathology did not eat significantly more when allowed access to ad libitum feeding [2]. Compared to FED, the appetite response to the current 24-hour fast was significantly impacted for all four AUC appetite measures. Most affected was hunger, which increased by 69%, followed by 50% and 30% increases in prospective food consumption and desire to eat, respectively, along with a 47% decrease in fullness. After 24 hours of fasting we demonstrate a significant 74% increase in mean ad libitum EI (kcal), which was positively correlated with increased AUC desire to eat scores. A similar study found significantly increased appetite scores following a 36-hour fast but they noted that when ad libitum feeding was reinstated there was only a 20% increase in EI, and this was unrelated to the changes in appetite [4].
What is interesting is that our data is suggestive of alliesthesia for preferred food stimuli; that is, subjects rated ‘liking’ of the ad libitum dessert significantly higher under the FASTED condition even after eating 74% more energy (see Table 1 ). Corroborating these results are findings from deprivation periods of much shorter duration. By manipulating the period of energy deprivation with two separate test days, one day with a 3.5 hour period of deprivation and another with an overnight fast of approximately 12–15 hours, Speigel et al. [51] found that the deprivation period enhanced palatability, again consistent with the concept of alliesthesia. A strength of our findings lies in the fact that although neither the macronutrient nor the item content of the dessert buffet were controlled between subjects, there were no significant differences in percent fat or carbohydrate intake between the ad libitum EI in the FED and FASTED conditions (see Table 1 ). This observation is somewhat analogous to controlling for macronutrient intake and allows for better comparisons in overall changes in ‘liking’, either measured by VAS or by the LFPQ, thereby permitting a better analogue when comparing our results with previous work on alliesthesia (e.g. [46], [52]). To be sure, our ‘liking’ measures obtained immediately post-consumption of the preferred food items do not mimic the pure measures of ‘liking’ obtained by Cabanac with a 25% aqueous solution of glucose [13], but our findings are indeed consistent with the concept of alliesthesia.
Another interesting question that arises is whether there are differences in food reward or underlying trait differences (e.g. TFEQ) that can be attributed to the energy deprivation or to the noted increase in EI and palatability. The RRV task employed in the current study demonstrated that relative to FED, there were FASTED increases in the reinforcing value of palatable food as evidenced by significantly higher points earned and responses made for the snack item versus the preferred fruit (see Table 1 ). To our knowledge there is only one other study using the RRV task under comparable levels of energy deprivation; after ∼13 hours of fasting food also became more reinforcing for normal weight non-dietary restrained females [34]. What is interesting is that there is some evidence that this increase in the reinforcing value of food can predict ad libitum EI, independently of rated ‘liking’, or hedonic ratings of foods [53], [54]. Our data, however, did not demonstrate a relationship between RRV and EI. That is, although snack foods were significantly more reinforcing under the FASTED condition, we found no indication that this increased desire or motivation to obtain more energy dense foods was related to the increased ad libitum EI noted under the FASTED condition. Regarding eating behavior traits, we have replicated recent findings that TFEQ scores for dietary disinhibition are related to food reinforcement [26]. Specifically, our data show a positive association between disinhibition scores and the ‘wanting’ for palatable energy dense snack food in the RRV task. Although the relationship between RRV, disinhibition and reward responsiveness remains to be better defined, there seems to be more consistent data confirming a positive relationship between BMI, or weight gain, and disinhibition scores [55]. This would suggest that individuals who score high in disinhibited eating may be more vulnerable to increases in food reward and food palatability ratings following a period of food deprivation, which may put them at greater risk of subsequently overconsuming food under such circumstances.
The LFPQ is different from the RRV task in that it allows the separate and concurrent assessments of explicit ‘liking’ and ‘wanting’ for the same target stimuli [56]. In our sample, explicit ‘wanting’ and ‘liking’ of food images increased under the FASTED relative to FED condition: except for the lack of change in ‘liking’ for LFSW foods, both pre-meal scores and post-meal scores significantly increased for all four food categories. In previous work with the LFPQ measuring ‘wanting’ and ‘liking’, once in a hungry state (3–4 hours post-prandial) and then after eating a lunch meal on the same test day [57], it was shown that ‘liking’ for sweet foods did not decrease as much as that for fat foods. Here we show similar results in the FED condition, but under the FASTED condition only images of savory foods decreased in ‘liking’, while images of sweet foods maintained significantly heightened across-meal ‘liking’ (see Figure 2 ). Our results are in agreement with recent imaging studies showing a robust activation of brain reward areas to sucrose taste [58] and to images of high calorie foods [18], particularly after a period of fasting. Similar results were found in a study investigating alliesthesia to food images and found that foods rated with the highest ‘liking’ scores (i.e. desserts) were less affected by energy deprivation than the savory foods [22], suggesting that the hedonic value of a food stimulus may predict the magnitude of alliesthesia. Limited data on alliesthesia to food images exists: no effect of deprivation on ratings of ‘liking’ has been reported [59], while others have reported higher ‘liking’ ratings under hungry versus sated subjects [22], [23], [60], [61]. It should be noted that the explicit ‘liking’ responses from the LFPQ as they relate to alliesthesia are distinct from the ‘liking’ measures we obtained from the VAS palatability measure immediately post-ingestion. That is, the ‘liking’ of food images is a composite of learned hedonic features of the food (e.g. previous exposure of taste, texture, post-ingestive consequences, etc.), whereas, the VAS ‘liking’ is a momentary evaluation of sensory qualia of the food.
Limitations for the current study are the fact that the fasting period was out of the laboratory and therefore there was the possibility that contrary to their self-reported fast, some subjects did not precisely follow the protocol. The relatively small number of moderately overweight but otherwise healthy subjects limits the scope of the current findings to similar populations. Also, because pizza was served as the standardized lunch, there could have existed a sensory specific satiety to savory food cues, which may partially explain the decreased post-prandial ‘liking’ of savory food images. It is worthwhile to restate here that ‘wanting’ of savory foods after the lunch was higher in males, thus we cannot discount sex-related differences in food evaluation [62], [63]. Some of the strengths are in the randomized crossover design, that women were tested in the same phase of the menstrual cycle, and that the food items were personalized. Finally, to confirm our findings, more research is needed to clarify the role that sex has in responding to various degrees of negative energy balance, and in the subsequent response to food or food-related cues.
As a result of a 24-hour complete fast, preferred foods were both wanted and liked significantly more than in the fed state. Scores for appetite were dramatically affected, pushing up hunger, desire to eat and PFC, while simultaneously attenuating fullness. Higher disinhibition scores correlated with responding for palatable snack food stimuli in the RRV task, further indicating that RRV has strong ties with impulsivity and food sensitivity. Considering that there was a 74% increase in EI under the FASTED condition, our data suggest that alliesthesia was demonstrated by heightened hedonic ratings of ‘liking’ following this much larger eating episode compared to the FED condition. The results from the LFPQ were in agreement with the abovementioned finding, whereby there was an increase in explicit ‘wanting’ and ‘liking’ to food images after 24 hours of fasting. The current study is also timely due to the recent, but still nascent findings, that alternating days of 24 hours of fasting may confer protection against coronary artery disease [64] and improve insulin sensitivity [65] in humans. More research is needed to better describe the degree that these measures of food reward are conceptually unique or structurally overlapping; furthermore, given the surge in brain imaging research and recent controversy regarding food addiction [66], [67], it becomes clear that validated computer tasks like those discussed here will compliment a more sophisticated biological and behavioral understanding of the human response to energy deprivation.
Funding Statement
GSG is supported by an Endowed Scholar Award from the Children's Hospital of 429 Eastern Ontario Volunteer Association. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Franklin JC, Schiele BC, Brozek J, Keys A (1948) Observations on human behavior in experimental semistarvation and rehabilitation. J Clin Psychol 4: 28–45. [DOI] [PubMed] [Google Scholar]
- 2. Hetherington MM, Stoner SA, Andersen AE, Rolls BJ (2000) Effects of acute food deprivation on eating behavior in eating disorders. Int J Eat Disord 28: 272–283. [DOI] [PubMed] [Google Scholar]
- 3. Telch CF, Agras WS (1996) The effects of short-term food deprivation on caloric intake in eating-disordered subjects. Appetite 26: 221–233. [DOI] [PubMed] [Google Scholar]
- 4. Johnstone AM, Faber P, Gibney ER, Elia M, Horgan G, et al. (2002) Effect of an acute fast on energy compensation and feeding behaviour in lean men and women. Int J Obes Relat Metab Disord 26: 1623–1628. [DOI] [PubMed] [Google Scholar]
- 5. Oh SY, Kim BS, Choue R (2002) Appetite sensations and eating behaviors to complete fasting in obese and non-obese individuals. Eur J Clin Nutr 56: 86–89. [DOI] [PubMed] [Google Scholar]
- 6. Booth DA, Mather P, Fuller J (1982) Starch content of ordinary foods associatively conditions human appetite and satiation, indexed by intake and eating pleasantness of starch-paired flavours. Appetite 3: 163–184. [DOI] [PubMed] [Google Scholar]
- 7. Johnson J, Vickers Z (1993) Effects of flavor and macronutrient composition of food servings on liking, hunger and subsequent intake. Appetite 21: 25–39. [DOI] [PubMed] [Google Scholar]
- 8. Yeomans MR, Gray RW, Conyers TH (1998) Maltodextrin preloads reduce food intake without altering the appetiser effect. Physiol Behav 64: 501–506. [DOI] [PubMed] [Google Scholar]
- 9. Nicklas JM, Huskey KW, Davis RB, Wee CC (2012) Successful weight loss among obese U.S. adults. Am J Prev Med 42: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. French SA, Jeffery RW, Forster JL, McGovern PG, Kelder SH, et al. (1994) Predictors of weight change over two years among a population of working adults: the Healthy Worker Project. Int J Obes Relat Metab Disord 18: 145–154. [PubMed] [Google Scholar]
- 11. De Castro JM (1996) How can eating behavior be regulated in the complex environments of free-living humans? Neurosci Biobehav Rev 20: 119–131. [DOI] [PubMed] [Google Scholar]
- 12. Cabanac M (1971) Physiological role of pleasure. Science 173: 1103–1107. [DOI] [PubMed] [Google Scholar]
- 13. Cabanac M, Duclaux R (1973) Olfactory-gustatory alliesthesia and food intake in humans. J Physiol (Paris) 66: 113–135. [PubMed] [Google Scholar]
- 14.Blundell JE (1992) Psychobiology of the appetite. Journ Annu Diabetol Hotel Dieu: 239–253. [PubMed]
- 15. Berridge KC, Robinson TE, Aldridge JW (2009) Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 9: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berthoud HR, Munzberg H, Richards BK, Morrison CD (2012) Neural and metabolic regulation of macronutrient intake and selection. Proc Nutr Soc 71: 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang DW, Fellows LK, Small DM, Dagher A (2012) Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav 106: 317–324. [DOI] [PubMed] [Google Scholar]
- 18. Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, et al. (2009) Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci 30: 1625–1635. [DOI] [PubMed] [Google Scholar]
- 19. LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, et al. (2001) Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci 115: 493–500. [DOI] [PubMed] [Google Scholar]
- 20. Piech RM, Lewis J, Parkinson CH, Owen AM, Roberts AC, et al. (2009) Neural correlates of appetite and hunger-related evaluative judgments. PLoS One 4: e6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stice E, Burger K, Yokum S (2013) Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage 67: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stoeckel LE, Cox JE, Cook EW 3rd, Weller RE (2007) Motivational state modulates the hedonic value of food images differently in men and women. Appetite 48: 139–144. [DOI] [PubMed] [Google Scholar]
- 23. Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC (2006) Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res 169: 111–119. [DOI] [PubMed] [Google Scholar]
- 24. Finlayson G, King N, Blundell JE (2007) Liking vs. wanting food: importance for human appetite control and weight regulation. Neurosci Biobehav Rev 31: 987–1002. [DOI] [PubMed] [Google Scholar]
- 25. Lemmens SG, Schoffelen PF, Wouters L, Born JM, Martens MJ, et al. (2009) Eating what you like induces a stronger decrease of ‘wanting’ to eat. Physiol Behav 98: 318–325. [DOI] [PubMed] [Google Scholar]
- 26. Epstein LH, Lin H, Carr KA, Fletcher KD (2012) Food reinforcement and obesity. Psychological moderators. Appetite 58: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berridge KC (1991) Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite 16: 103–120. [DOI] [PubMed] [Google Scholar]
- 28. Sorensen LB, Moller P, Flint A, Martens M, Raben A (2003) Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int J Obes Relat Metab Disord 27: 1152–1166. [DOI] [PubMed] [Google Scholar]
- 29. Yeomans MR (1996) Palatability and the micro-structure of feeding in humans: the appetizer effect. Appetite 27: 119–133. [DOI] [PubMed] [Google Scholar]
- 30. Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28: 309–369. [DOI] [PubMed] [Google Scholar]
- 31. Lappalainen R, Epstein LH (1990) A behavioral economics analysis of food choice in humans. Appetite 14: 81–93. [DOI] [PubMed] [Google Scholar]
- 32. Bulik CM, Brinded EC (1994) The effect of food deprivation on the reinforcing value of food and smoking in bulimic and control women. Physiol Behav 55: 665–672. [DOI] [PubMed] [Google Scholar]
- 33. Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA (2003) Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav 78: 221–227. [DOI] [PubMed] [Google Scholar]
- 34. Raynor HA, Epstein LH (2003) The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite 40: 15–24. [DOI] [PubMed] [Google Scholar]
- 35. Blundell JE, Hill AJ (1988) On the mechanism of action of dexfenfluramine: effect on alliesthesia and appetite motivation in lean and obese subjects. Clin Neuropharmacol 11 Suppl 1S121–134. [PubMed] [Google Scholar]
- 36. Laeng B, Berridge KC, Butter CM (1993) Pleasantness of a sweet taste during hunger and satiety: effects of gender and “sweet tooth”. Appetite 21: 247–254. [DOI] [PubMed] [Google Scholar]
- 37. Cameron JD, Goldfield GS, Cyr MJ, Doucet E (2008) The effects of prolonged caloric restriction leading to weight-loss on food hedonics and reinforcement. Physiol Behav 94: 474–480. [DOI] [PubMed] [Google Scholar]
- 38. Frankham P, Gosselin C, Cabanac M (2005) Diet induced weight loss accelerates onset of negative alliesthesia in obese women. BMC Public Health 5: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McNeil J, Cameron JD, Finlayson G, Blundell JE, Doucet E (2013) Greater overall olfactory performance, explicit wanting for high fat foods and lipid intake during the mid-luteal phase of the menstrual cycle. Physiol Behav 112–113C: 84–89. [DOI] [PubMed] [Google Scholar]
- 40. Bryant EJ, Caudwell P, Hopkins ME, King NA, Blundell JE (2012) Psycho-markers of weight loss. The roles of TFEQ Disinhibition and Restraint in exercise-induced weight management. Appetite 58: 234–241. [DOI] [PubMed] [Google Scholar]
- 41. Stunkard AJ, Messick S (1985) The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 29: 71–83. [DOI] [PubMed] [Google Scholar]
- 42. Hill AJ, Magson LD, Blundell JE (1984) Hunger and palatability: tracking ratings of subjective experience before, during and after the consumption of preferred and less preferred food. Appetite 5: 361–371. [DOI] [PubMed] [Google Scholar]
- 43. Drapeau V, King N, Hetherington M, Doucet E, Blundell J, et al. (2007) Appetite sensations and satiety quotient: predictors of energy intake and weight loss. Appetite 48: 159–166. [DOI] [PubMed] [Google Scholar]
- 44. Robbins TW, Everitt BJ (1996) Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6: 228–236. [DOI] [PubMed] [Google Scholar]
- 45. Reiss S, Havercamp S (1996) The sensitivity theory of motivation: implications for psychopathology. Behav Res Ther 34: 621–632. [DOI] [PubMed] [Google Scholar]
- 46. Cabanac M, Pruvost M (1972) Gustatory allachesthesia for sugar: complementary study on the nature of the internal signal. J Physiol (Paris) 65 Suppl 3370A. [PubMed] [Google Scholar]
- 47. Lappalainen R, Sjoden PO, Hursti T, Vesa V (1990) Hunger/craving responses and reactivity to food stimuli during fasting and dieting. Int J Obes 14: 679–688. [PubMed] [Google Scholar]
- 48. Foster GD, Wadden TA, Swain RM, Stunkard AJ, Platte P, et al. (1998) The Eating Inventory in obese women: clinical correlates and relationship to weight loss. Int J Obes Relat Metab Disord 22: 778–785. [DOI] [PubMed] [Google Scholar]
- 49. Pekkarinen T, Takala I, Mustajoki P (1996) Two year maintenance of weight loss after a VLCD and behavioural therapy for obesity: correlation to the scores of questionnaires measuring eating behaviour. Int J Obes Relat Metab Disord 20: 332–337. [PubMed] [Google Scholar]
- 50. Wadden TA, Stunkard AJ, Day SC, Gould RA, Rubin CJ (1987) Less food, less hunger: reports of appetite and symptoms in a controlled study of a protein-sparing modified fast. Int J Obes 11: 239–249. [PubMed] [Google Scholar]
- 51. Spiegel TA, Shrager EE, Stellar E (1989) Responses of lean and obese subjects to preloads, deprivation, and palatability. Appetite 13: 45–69. [DOI] [PubMed] [Google Scholar]
- 52. Cabanac M, Lafrance L (1990) Postingestive alliesthesia: the rat tells the same story. Physiol Behav 47: 539–543. [DOI] [PubMed] [Google Scholar]
- 53. Epstein LH, Carr KA, Lin H, Fletcher KD (2011) Food reinforcement, energy intake, and macronutrient choice. Am J Clin Nutr 94: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, et al. (2007) Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci 121: 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. French SA, Epstein LH, Jeffery RW, Blundell JE, Wardle J (2012) Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite 59: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Finlayson G, King N, Blundell JE (2007) Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiol Behav 90: 36–42. [DOI] [PubMed] [Google Scholar]
- 57. Finlayson G, King N, Blundell J (2008) The role of implicit wanting in relation to explicit liking and wanting for food: Implications for appetite control. Appetite 50: 120–127. [DOI] [PubMed] [Google Scholar]
- 58. Haase L, Cerf-Ducastel B, Murphy C (2009) Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage 44: 1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Drobes DJ, Miller EJ, Hillman CH, Bradley MM, Cuthbert BN, et al. (2001) Food deprivation and emotional reactions to food cues: implications for eating disorders. Biol Psychol 57: 153–177. [DOI] [PubMed] [Google Scholar]
- 60. Jiang T, Soussignan R, Rigaud D, Martin S, Royet JP, et al. (2008) Alliesthesia to food cues: heterogeneity across stimuli and sensory modalities. Physiol Behav 95: 464–470. [DOI] [PubMed] [Google Scholar]
- 61. Rolls ET, Rolls BJ, Rowe EA (1983) Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol Behav 30: 185–192. [DOI] [PubMed] [Google Scholar]
- 62. Lovejoy JC, Sainsbury A (2009) Sex differences in obesity and the regulation of energy homeostasis. Obes Rev 10: 154–167. [DOI] [PubMed] [Google Scholar]
- 63. Macdiarmid JI, Vail A, Cade JE, Blundell JE (1998) The sugar-fat relationship revisited: differences in consumption between men and women of varying BMI. Int J Obes Relat Metab Disord 22: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 64. Varady KA, Hellerstein MK (2007) Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr 86: 7–13. [DOI] [PubMed] [Google Scholar]
- 65. Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, et al. (2005) Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res 13: 574–581. [DOI] [PubMed] [Google Scholar]
- 66. Volkow ND, Wang GJ, Tomasi D, Baler RD (2013) Obesity and addiction: neurobiological overlaps. Obes Rev 14: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ziauddeen H, Fletcher PC (2013) Is food addiction a valid and useful concept? Obes Rev 14: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]