Abstract
Reprogramming somatic cells to pluripotency, especially by the induced pluripotent stem cell (iPSC) technology, has become widely used today to generate various types of stem cells for research and for regenerative medicine. However the mechanism(s) of reprogramming still need detailed elucidation, including the roles played by the leukemia inhibitory factor (LIF) signaling pathway. LIF is central in maintaining the ground state pluripotency of mouse embryonic stem cells (ESCs) and iPSCs by activating the Janus kinase-signal transducer and activator of transcription 3 (JAK-STAT3) pathway. Characterizing and understanding this pathway holds the key to generate naïve pluripotent human iPSCs which will facilitate the development of patient-specific stem cell therapy. Here we review the historical and recent developments on how LIF signaling pathway regulates ESC pluripotency maintenance and somatic cell reprogramming, with a focus on JAK-STAT3.
Keywords: JAK, STAT3, LIF, embryonic stem cells, reprogramming, iPSC, epigenetics
Introduction
The importance of leukemia inhibitory factor (LIF) stimulated signaling in mouse embryonic stem cell (ESC) culture in vitro has long been recognized. By activating Janus kinase (JAK) and signal transducer and activator of transcription 3 (STAT3), LIF plays essential roles in the generation and maintenance of mouse pluripotent embryonic stem cells which are capable of infinite self-renewal and differentiation to any cell type of the three-germ layers of an embryo.1,2 Over the past two decades, much information has been obtained regarding the LIF signaling pathway through studying mouse ESCs. However, the exact mechanism by which activated STAT3 controls pluripotency still remains to be elucidated.
The induced pluripotent stem cell (iPSC) technology3-5 provides a powerful tool to reprogram somatic cells such as skin fibroblasts to a pluripotent state, and brings us much closer to the establishment of personalized, cell-based therapy for the treatment of currently incurable diseases such as diabetes and neural degenerative diseases.6-8 In many aspects, somatic cell reprogramming represents a reversed cell differentiation process, by epigenetically resetting the cell nuclei back to a pluripotent state. A the same time reprogrammed cells turn on a cellular signal network capable of both robust cell division and fending off intra- and extra-cellular differentiation stimuli. Recently, the LIF-JAK-STAT3 axis was demonstrated by a number of studies to be essential for the naïve state pluripotency establishment during somatic cell reprogramming.9-11 In addition, these studies provided important insight for further understanding of the mechanism behind LIF regulated ESC pluripotency. In this review, we will summarize the current knowledge on the role of LIF and especially JAK-STAT3 signaling pathway in ESC pluripotency maintenance and in somatic cell reprogramming.
Historical Understanding of LIF
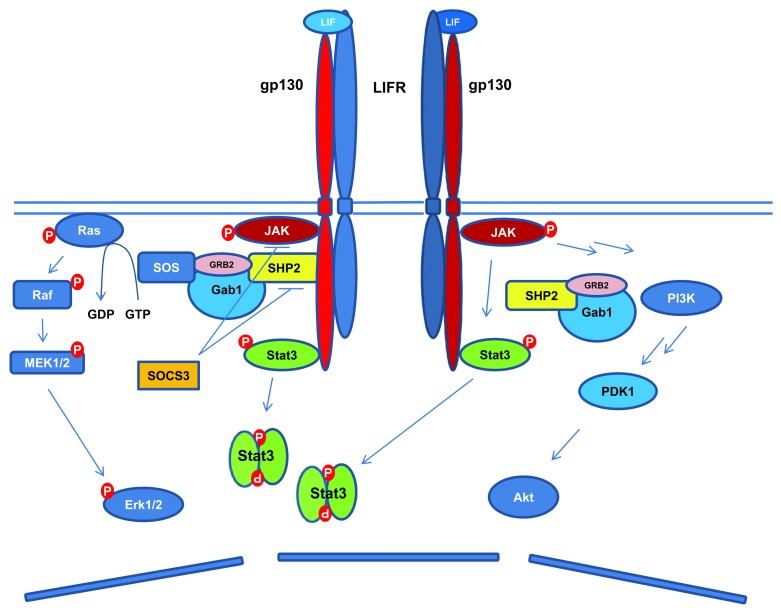
Leukemia inhibitory factor (LIF) is a member of the interleukin (IL)-6-type cytokine family, which includes IL-6, IL-11, IL-27, LIF, ciliary neurotrophic factor (CNTF), cardiotropin-1 (CT-1), oncostatin M (OSM), and cardiotrophin-like cytokine-1/novel neurotrophin-1/B-cell stimulating factor-3 (CLC-1/NNT-1/BSF-3).12,13 LIF was initially discovered to be pleiotropic in different mouse leukemia cell lines, by exerting either an inhibitory or promotional role on cell proliferation.14,15 The same cytokine was also isolated from culture medium conditioned with Buffalo rat liver cells,16 which can promote the self-renewal and sustain the pluripotency of mouse ESCs in the absence of fibroblast feeder cells.16,17 LIF binds to the low-affinity cell surface LIF receptor (LIFR),18 which stimulates the hetero-dimerization of LIFR with the signal transducer glycoprotein 130 (gp130) (Fig. 1).19,20 This triggers the activation of gp130-associated JAK kinases, and the subsequent phosphorylation of tyrosine residues in the gp130 cytoplasmic domain. These phosphor-tyrosines then serve as the docking sites to recruit the Src-homology-2 (SH2) domain containing STAT proteins (STAT1 and 3), which are phosphorylated by activated JAK kinase. Once phosphorylated, the STAT proteins dimerize and enter the cell nucleus to regulate the expression of their target genes.21 Although the JAK kinases including JAK1, JAK2, and Tyk2 can all be phosphorylated following stimulation by LIF as well as other IL-6-type cytokines, JAK1-mediated IL-6/IL-11 signaling cannot be substituted by JAK2 and Tyk2.22,23 Additionally, while RNA inhibition of JAK1 induces mouse ESC differentiation,24 JAK2-deficient ESCs are responsive to LIF stimulation.25 It was also found that in mouse ESCs, the activity of STAT3, but not STAT1, is responsible for pluripotency maintenance,2,26 and artificially activated STAT3 can sustain ESC self-renewal in the absence of LIF.27 These lines of evidence suggest that the prevailing “stemness” signaling is mediated by JAK1 activated STAT3 dimerization in mouse ESCs.
Figure 1. Schematic diagram of LIF signaling pathways. LIF binds to LIFR, which leads to the heterodimerization of LIFR and gp130. This is followed by the activation of JAK-STAT3, PI3K/Akt, and Erk1/2 signaling pathways. The activated STAT3 leads to increased expression of SOCS3, which serves as a negative feedback signal to LIF stimulated activation of STAT3 and Erk1/2.
All IL-6-type cytokines share the common signal transducer gp130. Likewise, LIFR can serve as the receptor to mediate signals of several ligands including LIF, CNTF, CT-1, OSM, and CLC-1 (Table 1).28-30 IL-6-type cytokines induced gp130 homo- or heterodimerization with LIFR also activates the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), and the SH2-domain containing tyrosine phosphatase (SHP2)/extracellular-signal-regulated kinases 1/2 (Erk1/2) pathways.31-35 Activated gp130 recruits SHP2 through its tyrosine residue (Tyr759 in humans, equivalent to Tyr757 in mice), where SHP2 is phosphorylated by JAK kinases and interacts with the growth-factor receptor bound protein 2 (Grb2) and son of sevenless (SOS) complex to activate Erk1/2.31-35 The activation of STAT3 also triggers the expression of the suppressors of cytokine signaling 3 (SOCS3), which competes with SHP2 for the cytoplasmic domain of gp130 phosphorylated at tyrosine residue Tyr759 (human) or Tyr757 (mouse), and inhibits the JAK-STAT3 and Erk1/2 activation (Fig. 1).24,36 The negative regulation of JAK kinases by SOCS3 involves both direct inhibition of the JAK catalytic domain and the promotion of proteasomal degradation of JAK.13,37-39 While constitutively activated Akt was reported to sustain mouse ESC pluripotency independent of STAT3,40 signaling from activated Erk1/2 promotes the neural commitment of ESCs.41,42 As the JAK-STAT3, PI3K/Akt and Erk1/2 pathways are simultaneously activated by LIF, it obviously contributes to the pleiotropic roles that LIF plays in different cell types, and complicates the understanding of LIF signaling regulated pluripotency.
Table 1. Summary of IL-6-type cytokines and their receptor complexes.
| Cytokine | Receptor complex subunits | ||
|---|---|---|---|
| α-receptor | β-receptor | No. of gp130 involved in an induced receptor/ligand complex | |
| IL-6 | IL-6R | - | 2 |
| IL-11 | IL-11R | - | 2 |
| IL-27 | IL-27R | - | 2 |
| LIF | - | LIFR | 1 |
| CNTF | CNTFR | LIFR | 1 |
| CT-1 | ? | LIFR | 1 |
| OSM | - | LIFR or OSMR | 1 |
| CLC-1 | CNTFR | LIFR | 1 |
JAK-STAT3 Signaling and ESC Maintenance
It is well-known that JAK-STAT3 signaling is important in maintaining mouse ESC pluripotency and propagation. Unfortunately, thorough elucidation has not been achieved despite the numerous attempts made to uncover the mechanism behind JAK-STAT3-mediated ESC self-renewal. STAT3 is a transcription factor whose amino acid sequence is highly conserved across species among humans, cattle, pigs, rats, and mice.43-47 Its DNA binding domain recognizes the consensus sequence of TTCC(C/G)GGAA which is present in the regulatory regions of many genes.48,49 The phosphorylation of Tyr705 of STAT3 is important for STAT3 homo-dimerization and nuclear translocation following gp130 activation.45,50-52 By mutating the STAT3 binding tyrosine residues in the cytoplasmic domain of gp130, it was shown that STAT3 activity was necessary for gp130 signaling mediated mouse ESC self-renewal.2 Expressing a mutant form of STAT3 (STAT3F, tyr705 to phenylalanine) leads to ESC differentiation even in the presence of LIF,2,53 while dimerization of STAT3 fused with estrogen receptor (STAT3ER) upon 4-hydroxytamoxifen (4-HT) treatment promoted mouse ESC self-renewal in the absence of LIF.27 Thus it became clear that STAT3 is the key downstream mediator of LIF stimulated pluripotent signal.
STAT3-deficient mouse embryos (Stat3−/−) die on E7.0 upon gastrulation in vivo. These embryos can form embryonic ectoderm and visceral endoderm but not the mesoderm.54 In culture of wild-type ESCs, LIF prevents their differentiation into mesoderm and endoderm but needs either serum supplement or bone morphogenetic proteins (Bmps) to suppress the neuronal differentiation.55 Bmp signaling induces the expression of inhibition of differentiation (Id) proteins, and mouse ESCs transfected with Ids remain undifferentiated in the presence of LIF without serum.55 A number of functional genomic studies have identified many STAT3 target genes.48,49,56-58 Microarray analysis using STAT3ER transfected ESCs identified 58 STAT3 targets, among which 22 are responsible for inhibiting the differentiation of either mesoderm (Ccm4l, Cyr61, Dact1, etc.), endoderm (Krüppel-like factor 4 [Klf4], Gbx2, Pim1, Pim3, Sall4, Smad7, etc.), or both (Klf5).58 Interestingly, 14 of these genes, including Klf4, Klf5, Pim1, Dact1, and Smad7, are co-regulated by Nanog, indicating a partially functional redundancy between STAT3 and Nanog in pluripotency maintenance.58 This is also in line with the finding that overexpression of either Nanog or STAT3 maintains ESC self-renewal in the presence of reduced signaling from one another.58,59
In mouse ESCs, the global DNA binding sites of 13 pluripotency-, cell cycle-, DNA binding-, or reprogramming-related transcription factors were evaluated using chromatin immunoprecipitation coupled with high-throughput sequencing (CHIP-seq). STAT3 occupies an impressive 2546 genomic loci.12,48 Also, a total of 718 genomic loci were identified to be co-bound by STAT3 and at least another 3 of the 13 factors, and STAT3 clusters with Nanog, Oct4 (also known as Pou5f1), or sex determining region Y-box 2 (Sox2) among 56.8% of these loci, clearly demonstrating that STAT3 shares many common targets with the core pluripotent Oct4-Sox2-Nanog circuitry.48,60 Interestingly, STAT3 were found to co-bind with Oct4, Sox2, Nanog, and Smad1 to the regulatory region of key pluripotent genes which were also found to be reprogramming factors such as Nanog, Oct4, Sox2, estrogen-related receptor β (Esrrb), Klf4, c-Myc, T-box transcription factor 3 (Tbx3), Sall4, etc.48
c-Myc had been previously identified as a STAT3 target and overexpression of constitutively active c-Myc mutant maintains mouse ESC pluripotency in the absence of LIF.61 It was postulated that the LIF/STAT3 signaling promotes ESC self-renewal by stimulating c-Myc mRNA expression, and by stabilizing c-Myc protein levels through inhibiting glycogen synthase kinase 3β (GSK3β) mediated threonine 58 (Thr58) phosphorylation.61 However, mouse ESCs with c-Myc disruption were reportedly normal in self-renewal,62 presumably due to the redundant function from N-Myc.63,64 Using CHIP and microarray analysis (CHIP-chip), 948 genes and 1459 genes were found bound by STAT3 and c-Myc in mouse ESCs, respectively, of which STAT3 and c-Myc co-occupy the promoters of 218 genes, including N-Myc, Rest, STAT3, Mbd3, and Jmjd3.49 Interestingly, it was shown that while STAT3 binds to genes that are either transcriptionally activated or repressed, c-Myc mainly occupies actively transcribed genes in ESCs.49 These, together with other studies described above, indicate that while STAT3 functions synergistically with the Oct4-Sox2-Nanog pluripotent circuitry and Myc by co-regulating their common target gene expression, unique function by STAT3 through regulating its specific targets not bound by these factors may exist for the pluripotency maintenance.
Current Understanding of LIF/STAT3 Regulated ESC Pluripotency
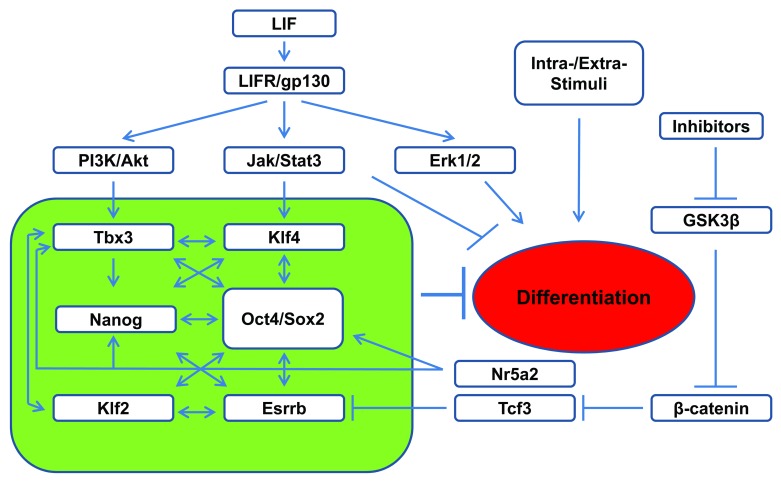
Similar to Nanog, overexpression of Klf4 or Tbx3 gene confers LIF-independent mouse ESC self-renewal.33,59,65 It was elegantly demonstrated that upon LIF stimulation, Klf4 expression in mouse ESCs is preferentially stimulated by JAK-STAT3 activation, whereas Tbx3 expression is stimulated by PI3K/Akt pathway upon the inhibition of Erk activities.33 Based on their finding, Niwa et al.33 proposed a model where LIF regulates pluripotency maintenance by simultaneous stimulation of JAK-STAT3 and PI3K/Akt pathways in ESCs. This leads to the activation of Klf4 and Tbx3 expression, and the subsequent expression of Sox2 and Nanog, respectively. Sox2/Nanog cooperate to maintain Oct4 expression, while Nanog also reinforce the established Oct4-Sox2-Nanog pluripotency network in the absence of Klf4 and Tbx3 (Fig. 2).33
Figure 2. Schematic representation of LIF regulated pluripotency circuit in ESCs. Upon LIF activation, JAK-STAT3 promotes the expression of the core pluripotency circuit (green box) together with PI3K/Akt for pluripotency maintenance. Activated STAT3 also directly suppresses differentiation by inhibiting the expression of lineage commitment genes.
This model of parallel axes by LIF/JAK-STAT3/Sox2 and LIF/PI3K/Nanog to maintain pluripotency are supported by multiple lines of evidences. First, selective activation of Klf4 by STAT3 was reported to synergize with Klf2, activated by Oct4, to ward off the differentiation stimuli to ESCs.66 STAT3 was also reported to directly upregulate Sox2 during neural progenitor cell differentiation from ESCs.67 Second, upregulation of Nanog through PI3K activation or GSK3β inhibition has been reported to contribute to ESC maintenance.68-70 Using PI3K inhibitor LY294002, 646 downstream targets of PI3K pathway were identified in mouse ESCs by microarray analysis.71 These include some key pluripotent genes such as Nanog, Esrrb, Tbx3, and Tcl-1, as well as Zscan4c, one of the Zscan4 family of zinc finger proteins. Interestingly, it was found that inhibition of Zscan4c reduces the proportion of ESCs expressing high levels of Nanog,71 and Zscan4c was found to regulate telomerase elongation and chromosome stability in ESCs.72 In addition, although it is unclear why the constitutively activated Akt confers mouse ESC self-renewal,40 Akt stabilizes Myc by inhibiting GSK3β-mediated phosphorylation and degradation,61,73 and Akt also phosphorylates Sox2, thus enhancing its stability and transcriptional activity in ESCs.74 However, in addition to the PI3K/Nanog axes in ESCs, STAT3 also binds to Nanog promoter with Brachyury which co-activates Nanog expression in early mesoderm progenitor cells derived from ESCs.74
The activation of canonical Wnt pathway leads to phosphorylation and inhibition of GSK3β and the subsequent nuclear accumulation of β-catenin to regulate cell fate and many other biological events. Constitutive activation of the Wnt pathway results in differentiation defects of mouse ESCs.75 It was reported that inhibition of GSK3β using 6-bromoindirubin-3′-oxime promotes mouse ESC self-renewal in the absence of LIF,76 and that activated β-catenin upregulates Nanog expression by interacting with Oct4.77 However, it was also reported that Wnt signaling alone is not sufficient for the maintenance of mouse ESC self-renewal but can cooperate with LIF for this action.78 Part of this synergistic effect of Wnt could be through increasing STAT3 mRNA level in ESCs.79 A more recent study indicates that Wnt regulated expression of orphan nuclear receptor Nr5a2 (also known as LRH-1) is significant in augmenting PI3K/Akt pluripotent signaling through regulating Tbx3, Nanog, and Oct4 expression independent of JAK-STAT3.80 However, unlike Nanog and Tbx3, overexpression of Nr5a2 cannot sustain ESC pluripotency in the absence of LIF.81
The culture of mouse ESCs under the dual inhibition of Erk and GSK3β (2i) ensures ESC self-renewal and pluripotency maintenance without LIF.82 STAT3 null ESCs can be derived under the 2i or triple inhibition of FGF receptor, Erk1/2, and GSK3β culture condition.66,82 The main effect of GSK3β inhibition is the nuclear accumulation of β-catenin. Although β-catenin was found dispensable for ESC self-renewal,83,84 its overexpression enhances pluripotent gene expression and delays the mouse ESC differentiation.85 The pluripotency maintenance effect of GSK3β inhibition was recently found to be through inhibiting transcription factor 3 (Tcf3) protein by activated β-catenin,84 which relieves the suppressive effect of Tcf3, leading to the expression of pluripotent gene Esrrb.86 Smith’s group therefore proposed a model of ESC signal network in which pluripotency is supported by the inhibition of GSK3β and the activation of STAT3 which in turn activate the expression of Tcf3 and Klf4, respectively, and ESC is maintained by an expanded core pluripotent circuitry including Oct4/Sox2, Nanog, Klf4, Tbx2, and Tcf3 (Fig. 2).86 However, Esrrb is also dispensable for ESC maintenance in the presence of LIF,86 which highlights the parallel compensatory capacity by the core pluripotent circuitry for ESC self-renewal. A complementary model was proposed by Savatier’s group which describes the synergistic effect of STAT3 with Nanog in preventing mesoderm and endoderm differentiation, as well as STAT3’s direct effect in preventing endoderm differentiation through regulating its specific target genes in mouse ESCs.58 Together, these studies contribute a comprehensive depiction to our understanding of LIF signal network (Fig. 2).
A few studies also investigated the STAT3 regulated ESC pluripotency and gene expression at the epigenetic level. One study discovered that in mouse ESCs, STAT3, and Oct4 co-bind to the promoter region of embryonic ectoderm development (Eed) gene and promote its expression.87 As Eed is a core component of Polycomb repressive complex 2 (PRC2), this stabilizes the histone 3 lysine 27 tri-methylation (H3K27me3) at the promoter regions of several lineage commitment genes, therefore suppresses their expression.87 The pluripotent cell-specific ATP-dependent SWI/SNF chromatin remodeling helicase complex (esBAF) is essential for ESC pluripotency and self-renewal, with Brg1 (also known as Smarca4) as a key esBAF component.88 In mouse ESCs, Brg1 was shown to be required to prepare the chromatin access for STAT3 to activate many of its target genes, whose promoters are otherwise occupied by PRC2 complex.89 Other studies also demonstrated that Brg1 binding to STAT3 is necessary to allow STAT3 to access the promoter region of INF regulatory factor 1 to control its expression, as well as the promoter of the p21waf1 gene followed by STAT3-cdk9 interaction promoted transcriptional elongation.90,91
JAK-STAT3 in Somatic Cell Reprogramming
iPSCs are ESC-like cells reprogrammed from somatic cells by overexpression a set of reprogramming factors including Oct4, Klf4, Sox2, and c-Myc (OKSM) in the mice and humans, or Oct4, Sox2, Nanog, and Lin28 in humans.3,5,92 This technology represents a powerful way for regenerative medicine in terms of massively producing patient-specific pluripotent cells within a short time. However, many technical issues still remain unsolved, including the efficiency of reprogramming, the development of integration-free reprogramming method, the quality and safety of induced iPSCs for clinical application, and lastly but probably most importantly, the understanding of the molecular mechanisms of reprogramming. Such information is required to ensure a proper translation of the iPSC technology to human medicine.
Many major differences exist between the mouse ESCs/iPSCs and human ESCs/iPSCs. For example, human ESCs/iPSCs are flat in colony morphology; depend on FGF/Activin signaling for their pluripotency maintenance; and have completed X chromosome inactivation. They divide slowly (36 h doubling time), and survive poorly after single cell dissociation―a property essential for gene targeting.93-96 On the contrary, mouse ESCs/iPSCs are dome-shaped, depend on LIF signaling for pluripotency, divide rapidly (16 h doubling time), and are amenable for single-cell colonization and gene targeting.2,16 The human ESCs/iPSCs are in fact similar to mouse epiblast stem cells (mEpiSCs) derived from pre- or post-implantation mouse embryos which have limited in vivo differentiation capacity,97-99 and these cells are collectively referred to as being in the “primed” pluripotent state, while the mouse ESCs/iPSCs from pre-implantation embryos are referred as in the “naïve” state.100
So far numerous studies have focused on the role of JAK-STAT3 in the maintenance of the steady-state pluripotency of ESCs; however, until recently little had been known whether LIF signaling is essential for iPSC induction, despite that LIF was preferably included in the induction medium. Interestingly, withdrawing LIF and supplementing the culture medium with FGF/Activin A results in the transition of naïve mouse ESCs to primed EpiSC state.101 At the same time, EpiSCs can be reprogrammed into naïve mouse iPSCs by overexpression of Klf4, Klf2, or Nanog.66,101-103 Using this EpiSC-ESC reprogramming system, Yang et al. in Smith’s group9 studied the effect of LIF signaling in the conversion of primed to naïve state pluripotency. They first found that LIF is necessary for EpiSC-ESCs transition induced by Klf4 or Nanog overexpression under the 2i condition.9 Previously a chimeric receptor was described (GY118F),2 in which the ligand binding domain of the granulocyte colony stimulating factor (GCSF) receptor was fused to the trans-membrane and cytoplasmic domains of gp130 modified at residue Tyr118 (corresponding to the tyr757 of the wild-type mouse gp130) to phenylalanine. This ensures constitutive activation of STAT3 upon GCSF stimulation.2 By expressing the GY118F receptor in EpiSCs with GCSF stimulation, Yang et al. discovered that activation of STAT3 can convert EpiSCs directly to naïve iPSCs in 2i medium. This was also confirmed with overexpression of STAT3ER activated by 4-HT.9 They further demonstrated that STAT3 activation in EpiSCs does not stimulate Klf4 or Nanog expression. Rather, STAT3 acts synergistically with either of them to dramatically improve the reprogramming efficiency. Finally, they demonstrated that activated STAT3 also improves the reprogramming of mouse neural stem cells and partially reprogrammed cells (pre-iPSCs) to naïve state iPSCs induced by Oct4, Klf4, c-Myc (OKM), or OKSM, respectively.9
The study by Smith’s group for the first time demonstrated that STAT3 activation is necessary for the reprogramming between mouse EpiSCs to iPSCs. A later study also demonstrated that enhanced STAT3 activation can reprogram mouse EpiSCs to naïve pluripotent cells even in the presence of FGF/Activin differentiation signals.10 Thus the STAT3 activity becomes essential for converting the primed state to naïve state pluripotency in the mouse. However, it remained unclear whether the LIF signaling is necessary for reprogramming of terminally differentiated mouse somatic cells, which bypasses the EpiSC stage during reprogramming with LIF containing medium. Furthermore, the molecular mechanism of STAT3 induced reprogramming remains elusive, albeit much information has been obtained by studying the steady-state pluripotency maintenance.
We addressed these questions by studying the role of STAT3 during the reprogramming of terminally differentiated mouse embryonic fibroblasts (MEFs). Using STAT3C, a constitutively activated STAT3 mutant with two residues in its SH2 domain mutated to cysteine (A662C and N664C),104 we discovered that enhanced STAT3 activation significantly promotes the reprogramming from MEF cells to iPSCs by Oct4, Klf4, Sox2 (OKS), or OKSM overexpression.11 This result was doubly confirmed by expressing a mutant gp130 with Tyr757 converted to phenylalanine, as well as by deprivation of LIF during reprogramming.11 We further found that the complete MEF reprogramming, as indicated by expression of GFP transgene driven by the Oct4 distal enhancer,105 was completely blocked in the presence of JAK inhibitor 1 (JAKi) but not the PI3K inhibitor, LY294002. However, the total number of induced colonies was not affected by the JAKi treatment. These observations demonstrated that STAT3 activation plays a vital role in the late-stage somatic cell reprogramming, i.e., the activation of the endogenous Oct4 gene.11
We also studied the role of STAT3 activation in the complete reprogramming of pre-iPSCs induced by OKM. These cells form colonies but do not express endogenous Oct4, and can be completely reprogrammed by the expression of Sox2 or the addition of RepSox, an inhibitor of transforming growth factor β receptor (Tgfbr).106 Again our results show that STAT3 activation is necessary for Sox2 or Tgfβ-signaling pathway inhibitor promoted reprogramming of pre-iPSCs.11
Examining the promoter DNA methylation status revealed that JAK-STAT3 inhibition prevents the demethylation of endogenous Oct4 and Nanog during reprogramming. This is associated with increased expression of DNA methyltransferase 1 (Dnmt1), class I histone deacetylases (HDACs), and other chromatin-repressive genes.11 Inhibition of Dnmt1 and HDACs has been shown to significantly improve somatic cell reprogramming.107-110 In order to test whether the STAT3 induced inhibition of Dnmt1 and HDAC expression is important for complete reprogramming, we applied inhibitors for Dnmt1 and HDACs107-110 together with the inhibition of STAT3 during reprogramming. Indeed we found that inhibiting either Dnmt1 or HDACs but not the overexpression of Nanog can overcome the halted reprogramming by STAT3 inactivation.11
During this study, we also observed that STAT3 inhibition resulted in continued expression of the retroviral transgenes which are otherwise gradually silenced at late-stage reprogramming. This lack of external gene silencing is tightly associated with the diminished expression of de novo DNA methyltransferase Dnmt3l.11 It has previously been demonstrated that expression of Dnmt3l in mouse ESCs is crucial to the activation of de novo DNA methylation which silences the viral transgenes.111 Our finding therefore reveals a new role of STAT3 in regulating de novo DNA methylation for epigenetic gene silencing during reprogramming. Taken together, our work demonstrated that (1) JAK-STAT3 activation is essential for the reprogramming of murine somatic cells, (2) STAT3 activity is crucial for the pluripotent gene promoter demethylation, and (3) STAT3 is important for retroviral transgene silencing during the reprogramming.
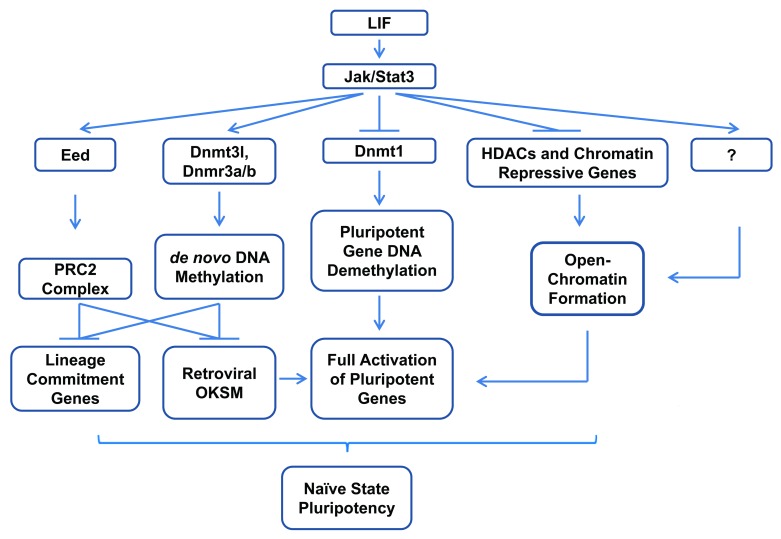
The discovery that STAT3 activity is essential for the reprogramming from mouse EpiSCs and somatic cells to naïve iPSCs, as demonstrated by others and us, respectively,9-11 is significant for the understanding of reprogramming and pluripotency. The continued high-level expression of ectopic transgenes in pre-iPSCs and in reprogrammed cells caused by STAT3 inhibition9,11 strongly indicates the existence of a reprogramming barrier which cannot be simply overcome by a greater dosage of reprogramming factors in the absence of STAT3 activity, even with the overexpression of Klf4 and Nanog which can sustain ESC self-renewal in the absence of LIF.33,59,65 A passive model of genomic DNA demethylation as a result of inhibited methylation maintenance has been proposed to explain the somatic cell reprogramming.112,113 It was also found that transient STAT3 activation poises the EpiSCs for complete reprogramming by Klf4 or Nanog.9 These are in accordance with our finding that JAK-STAT3 functions epigenetically to inhibit the expression of Dnmt1 and other heterochromatin-promoting genes, which in turn promotes the demethylation of pluripotent loci during reprogramming. Together, our results strongly suggest a central role of STAT3 during reprogramming, by orchestrating the establishment of open-chromatin to completely activate the pluripotent genes, and by promoting the de novo DNA methylation activity to silence the expression of viral transgenes and possibly the lineage commitment genes, in addition to the STAT3 promoted suppression through PRC2 (Fig. 3).87
Figure 3. Schematic representation of STAT3 regulated somatic cell reprogramming via epigenetic mechanisms. During late-stage reprogramming, STAT3 orchestrates the epigenetic changes leading to complete pluripotency, by ensuring full activation of core pluripotency genes through promoting DNA demethylation and open chromatin formation, while suppressing the viral transgenes and lineage commitment genes by promoting de novo DNA methylation and probably PRC2 mediated histone modifications.
Perspective
The JAK-STAT3 pathway has been identified as the core signaling pathway for pluripotency maintenance, and was also demonstrated recently to be essential for the complete reprogramming of mouse somatic cells. Although a number of STAT3 downstream target genes had been identified, a thorough understanding of the STAT3 mediated reprogramming still remains unachieved. An emerging area of study on STAT3 mechanism is to investigate the epigenetic roles of STAT3 in the maintenance of steady-state pluripotency and during the dynamic changes of somatic cell reprogramming. Special emphasis may be placed on the STAT3 induced formation of euchromatin for the pluripotent gene activation, and formation of heterochromatin for lineage commitment gene silencing. Interestingly, recently several groups reported the generation of naïve state human iPSCs, which also rely on LIF signaling for their self-renewal.114-116 Together, these series of studies have brought JAK-STAT3 to the central stage of iPSCs research.
Acknowledgments
This project was supported by USDA grant (1265-31000-091-02S).
Glossary
Abbreviations:
- BMP
bone morphogenetic protein
- CHIP
chromatin immunoprecipitation
- CLC-1/NNT-1/BSF-3
cardiotrophin-like cytokine-1/novel neurotrophin-1/B-cell stimulating factor-3
- CNTF
ciliary neurotrophic factor
- CT-1
cardiotropin-1
- Dnmt
DNA methyltransferase
- EpiSC
epiblast stem cell
- Esrrb
estrogen-related receptor beta
- ESC
embryonic stem cell
- Erk
extracellular-signal-regulated kinases
- FGF
fibroblast growth factor
- GCSF
granulocyte colony stimulating factor
- gp130
glycoprotein 130
- GSK3
Glycogen synthase kinase 3
- HDAC
histone deacetylase
- iPSC
induced pluripotent stem cell
- IL
interleukin
- JAK
Janus kinase
- Klf4
Krüppel-like factor 4
- LIF
leukemia inhibitory factor
- LIFR
LIF receptor
- Nr5a2
nuclear receptor subfamily 5, group A, member 2
- Oct4
Octamer-binding transcription factor 4
- OSM
oncostatin M
- PI3K
phosphatidylinositol 3-kinase
- PKB/Akt
protein kinase B
- PRC2
Polycomb repressive complex 2
- SH2
sarcoma (Src)-homology-2
- SHP2
SH2-domain containing tyrosine phosphatase
- SOCS3
suppressors of cytokine signaling 3
- Sox2
sex determining region Y-box 2
- STAT3
signal transducer and activator of transcription 3
- STAT3ER
a fusion protein of STAT3 with estrogen receptor
- Tbx3
T-box transcription factor 3
- Tcf3
transcription factor 3
- Tgfbr
transforming growth factor beta receptor
- Tyk2
tyrosine kinase 2
- Tyr
tyrosine
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/24935
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–60. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 6.Aasen T, Izpisúa Belmonte JC. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat Protoc. 2010;5:371–82. doi: 10.1038/nprot.2009.241. [DOI] [PubMed] [Google Scholar]
- 7.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–84. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 8.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, van Oosten AL, Theunissen TW, Guo G, Silva JC, Smith A. Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell. 2010;7:319–28. doi: 10.1016/j.stem.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Oosten AL, Costa Y, Smith A, Silva JC. JAK/STAT3 signalling is sufficient and dominant over antagonistic cues for the establishment of naive pluripotency. Nat Commun. 2012;3:817. doi: 10.1038/ncomms1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y, Luo Y, Jiang Z, Ma Y, Lin CJ, Kim C, et al. Jak/Stat3 signaling promotes somatic cell reprogramming by epigenetic regulation. Stem Cells. 2012;30:2645–56. doi: 10.1002/stem.1225. [DOI] [PubMed] [Google Scholar]
- 12.Hirai H, Karian P, Kikyo N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem J. 2011;438:11–23. doi: 10.1042/BJ20102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreau JF, Donaldson DD, Bennett F, Witek-Giannotti J, Clark SC, Wong GG. Leukaemia inhibitory factor is identical to the myeloid growth factor human interleukin for DA cells. Nature. 1988;336:690–2. doi: 10.1038/336690a0. [DOI] [PubMed] [Google Scholar]
- 15.Tomida M, Yamamoto-Yamaguchi Y, Hozumi M. Purification of a factor inducing differentiation of mouse myeloid leukemic M1 cells from conditioned medium of mouse fibroblast L929 cells. J Biol Chem. 1984;259:10978–82. [PubMed] [Google Scholar]
- 16.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–90. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 17.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–7. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 18.Gearing DP, Thut CJ, VandeBos T, Gimpel SD, Delaney PB, King J, et al. Leukemia inhibitory factor receptor is structurally related to the IL-6 signal transducer, gp130. EMBO J. 1991;10:2839–48. doi: 10.1002/j.1460-2075.1991.tb07833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gearing DP, Comeau MR, Friend DJ, Gimpel SD, Thut CJ, McGourty J, et al. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992;255:1434–7. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- 20.Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, et al. LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993;260:1805–8. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- 21.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 22.Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995;14:1421–9. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahmen H, Horsten U, Küster A, Jacques Y, Minvielle S, Kerr IM, et al. Activation of the signal transducer gp130 by interleukin-11 and interleukin-6 is mediated by similar molecular interactions. Biochem J. 1998;331:695–702. doi: 10.1042/bj3310695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst M, Oates A, Dunn AR. Gp130-mediated signal transduction in embryonic stem cells involves activation of Jak and Ras/mitogen-activated protein kinase pathways. J Biol Chem. 1996;271:30136–43. doi: 10.1074/jbc.271.47.30136. [DOI] [PubMed] [Google Scholar]
- 25.Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/S0092-8674(00)81168-X. [DOI] [PubMed] [Google Scholar]
- 26.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–50. doi: 10.1016/S0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–9. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–31. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 29.Robledo O, Fourcin M, Chevalier S, Guillet C, Auguste P, Pouplard-Barthelaix A, et al. Signaling of the cardiotrophin-1 receptor. Evidence for a third receptor component. J Biol Chem. 1997;272:4855–63. doi: 10.1074/jbc.272.8.4855. [DOI] [PubMed] [Google Scholar]
- 30.Senaldi G, Varnum BC, Sarmiento U, Starnes C, Lile J, Scully S, et al. Novel neurotrophin-1/B cell-stimulating factor-3: a cytokine of the IL-6 family. Proc Natl Acad Sci U S A. 1999;96:11458–63. doi: 10.1073/pnas.96.20.11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulton TG, Stahl N, Yancopoulos GD. Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J Biol Chem. 1994;269:11648–55. [PubMed] [Google Scholar]
- 32.Yin T, Yang YC. Mitogen-activated protein kinases and ribosomal S6 protein kinases are involved in signaling pathways shared by interleukin-11, interleukin-6, leukemia inhibitory factor, and oncostatin M in mouse 3T3-L1 cells. J Biol Chem. 1994;269:3731–8. [PubMed] [Google Scholar]
- 33.Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–22. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 34.Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–70. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 35.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–8. doi: 10.1016/S0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz J, Weissenbach M, Haan S, Heinrich PC, Schaper F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J Biol Chem. 2000;275:12848–56. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- 37.Boyle K, Zhang JG, Nicholson SE, Trounson E, Babon JJ, McManus EJ, et al. Deletion of the SOCS box of suppressor of cytokine signaling 3 (SOCS3) in embryonic stem cells reveals SOCS box-dependent regulation of JAK but not STAT phosphorylation. Cell Signal. 2009;21:394–404. doi: 10.1016/j.cellsig.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babon JJ, Kershaw NJ, Murphy JM, Varghese LN, Laktyushin A, Young SN, et al. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity. 2012;36:239–50. doi: 10.1016/j.immuni.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babon JJ, Nicola NA. The biology and mechanism of action of suppressor of cytokine signaling 3. Growth Factors. 2012;30:207–19. doi: 10.3109/08977194.2012.687375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- 41.Stavridis MP, Lunn JS, Collins BJ, Storey KG. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 2007;134:2889–94. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- 42.Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 43.Zhong Z, Wen Z, Darnell JE., Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 44.Ripperger JA, Fritz S, Richter K, Hocke GM, Lottspeich F, Fey GH. Transcription factors Stat3 and Stat5b are present in rat liver nuclei late in an acute phase response and bind interleukin-6 response elements. J Biol Chem. 1995;270:29998–30006. doi: 10.1074/jbc.270.50.29998. [DOI] [PubMed] [Google Scholar]
- 45.Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, et al. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 46.Wen L, Craig J, Dyce PW, Li J. Cloning of porcine signal transducer and activator of transcription 3 cDNA and its expression in reproductive tissues. Reproduction. 2006;132:511–8. doi: 10.1530/rep.1.01055. [DOI] [PubMed] [Google Scholar]
- 47.Zimin AV, Delcher AL, Florea L, Kelley DR, Schatz MC, Puiu D, et al. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009;10:R42. doi: 10.1186/gb-2009-10-4-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–17. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 49.Kidder BL, Yang J, Palmer S. Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PLoS One. 2008;3:e3932. doi: 10.1371/journal.pone.0003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minami M, Inoue M, Wei S, Takeda K, Matsumoto M, Kishimoto T, et al. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc Natl Acad Sci U S A. 1996;93:3963–6. doi: 10.1073/pnas.93.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, et al. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–60. doi: 10.1016/S1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, et al. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15:3651–8. [PMC free article] [PubMed] [Google Scholar]
- 53.Boeuf H, Hauss C, Graeve FD, Baran N, Kedinger C. Leukemia inhibitory factor-dependent transcriptional activation in embryonic stem cells. J Cell Biol. 1997;138:1207–17. doi: 10.1083/jcb.138.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci U S A. 1997;94:3801–4. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–92. doi: 10.1016/S0092-8674(03)00847-X. [DOI] [PubMed] [Google Scholar]
- 56.Snyder M, Huang XY, Zhang JJ. Identification of novel direct Stat3 target genes for control of growth and differentiation. J Biol Chem. 2008;283:3791–8. doi: 10.1074/jbc.M706976200. [DOI] [PubMed] [Google Scholar]
- 57.Sekkaï D, Gruel G, Herry M, Moucadel V, Constantinescu SN, Albagli O, et al. Microarray analysis of LIF/Stat3 transcriptional targets in embryonic stem cells. Stem Cells. 2005;23:1634–42. doi: 10.1634/stemcells.2005-0182. [DOI] [PubMed] [Google Scholar]
- 58.Bourillot PY, Aksoy I, Schreiber V, Wianny F, Schulz H, Hummel O, et al. Novel STAT3 target genes exert distinct roles in the inhibition of mesoderm and endoderm differentiation in cooperation with Nanog. Stem Cells. 2009;27:1760–71. doi: 10.1002/stem.110. [DOI] [PubMed] [Google Scholar]
- 59.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55. doi: 10.1016/S0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 60.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–53. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 61.Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–96. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 62.Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993;7:671–82. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- 63.Malynn BA, de Alboran IM, O’Hagan RC, Bronson R, Davidson L, DePinho RA, et al. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 2000;14:1390–9. [PMC free article] [PubMed] [Google Scholar]
- 64.Varlakhanova NV, Cotterman RF, deVries WN, Morgan J, Donahue LR, Murray S, et al. myc maintains embryonic stem cell pluripotency and self-renewal. Differentiation. 2010;80:9–19. doi: 10.1016/j.diff.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–42. doi: 10.1016/S0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 66.Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, et al. Oct4 and LIF/Stat3 additively induce Krüppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell. 2009;5:597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Foshay KM, Gallicano GI. Regulation of Sox2 by STAT3 initiates commitment to the neural precursor cell fate. Stem Cells Dev. 2008;17:269–78. doi: 10.1089/scd.2007.0098. [DOI] [PubMed] [Google Scholar]
- 68.Kingham E, Welham M. Distinct roles for isoforms of the catalytic subunit of class-IA PI3K in the regulation of behaviour of murine embryonic stem cells. J Cell Sci. 2009;122:2311–21. doi: 10.1242/jcs.046557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Storm MP, Bone HK, Beck CG, Bourillot PY, Schreiber V, Damiano T, et al. Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem. 2007;282:6265–73. doi: 10.1074/jbc.M610906200. [DOI] [PubMed] [Google Scholar]
- 70.Kim JS, Kim BS, Kim J, Park CS, Chung IY. The phosphoinositide-3-kinase/Akt pathway mediates the transient increase in Nanog expression during differentiation of F9 cells. Arch Pharm Res. 2010;33:1117–25. doi: 10.1007/s12272-010-0719-y. [DOI] [PubMed] [Google Scholar]
- 71.Storm MP, Kumpfmueller B, Thompson B, Kolde R, Vilo J, Hummel O, et al. Characterization of the phosphoinositide 3-kinase-dependent transcriptome in murine embryonic stem cells: identification of novel regulators of pluripotency. Stem Cells. 2009;27:764–75. doi: 10.1002/stem.3. [DOI] [PubMed] [Google Scholar]
- 72.Zalzman M, Falco G, Sharova LV, Nishiyama A, Thomas M, Lee SL, et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature. 2010;464:858–63. doi: 10.1038/nature08882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bechard M, Dalton S. Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Mol Cell Biol. 2009;29:2092–104. doi: 10.1128/MCB.01405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeong CH, Cho YY, Kim MO, Kim SH, Cho EJ, Lee SY, et al. Phosphorylation of Sox2 cooperates in reprogramming to pluripotent stem cells. Stem Cells. 2010;28:2141–50. doi: 10.1002/stem.540. [DOI] [PubMed] [Google Scholar]
- 75.Kielman MF, Rindapää M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet. 2002;32:594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- 76.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 77.Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 78.Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–66. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- 79.Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 80.Wagner RT, Xu X, Yi F, Merrill BJ, Cooney AJ. Canonical Wnt/β-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells. 2010;28:1794–804. doi: 10.1002/stem.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo G, Smith A. A genome-wide screen in EpiSCs identifies Nr5a nuclear receptors as potent inducers of ground state pluripotency. Development. 2010;137:3185–92. doi: 10.1242/dev.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–23. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol. 2011;13:753–61. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, et al. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol. 2011;13:838–45. doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW. β-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214–27. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martello G, Sugimoto T, Diamanti E, Joshi A, Hannah R, Ohtsuka S, et al. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell. 2012;11:491–504. doi: 10.1016/j.stem.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ura H, Usuda M, Kinoshita K, Sun C, Mori K, Akagi T, et al. STAT3 and Oct-3/4 control histone modification through induction of Eed in embryonic stem cells. J Biol Chem. 2008;283:9713–23. doi: 10.1074/jbc.M707275200. [DOI] [PubMed] [Google Scholar]
- 88.Ho L, Ronan JL, Wu J, Staahl BT, Chen L, Kuo A, et al. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc Natl Acad Sci U S A. 2009;106:5181–6. doi: 10.1073/pnas.0812889106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho L, Miller EL, Ronan JL, Ho WQ, Jothi R, Crabtree GR. esBAF facilitates pluripotency by conditioning the genome for LIF/STAT3 signalling and by regulating polycomb function. Nat Cell Biol. 2011;13:903–13. doi: 10.1038/ncb2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giraud S, Hurlstone A, Avril S, Coqueret O. Implication of BRG1 and cdk9 in the STAT3-mediated activation of the p21waf1 gene. Oncogene. 2004;23:7391–8. doi: 10.1038/sj.onc.1207972. [DOI] [PubMed] [Google Scholar]
- 91.Ni Z, Bremner R. Brahma-related gene 1-dependent STAT3 recruitment at IL-6-inducible genes. J Immunol. 2007;178:345–51. doi: 10.4049/jimmunol.178.1.345. [DOI] [PubMed] [Google Scholar]
- 92.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 93.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 94.Camus A, Perea-Gomez A, Moreau A, Collignon J. Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol. 2006;295:743–55. doi: 10.1016/j.ydbio.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 95.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–82. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 96.Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 97.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–5. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 98.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–9. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 99.Najm FJ, Chenoweth JG, Anderson PD, Nadeau JH, Redline RW, McKay RD, et al. Isolation of epiblast stem cells from preimplantation mouse embryos. Cell Stem Cell. 2011;8:318–25. doi: 10.1016/j.stem.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–92. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 101.Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–9. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–37. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hanna J, Markoulaki S, Mitalipova M, Cheng AW, Cassady JP, Staerk J, et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell. 2009;4:513–24. doi: 10.1016/j.stem.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 105.Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122:881–94. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- 106.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, et al. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi Y, Do JT, Desponts C, Hahm HS, Schöler HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–8. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 110.Mali P, Chou BK, Yen J, Ye Z, Zou J, Dowey S, et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010;28:713–20. doi: 10.1002/stem.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ooi SK, Wolf D, Hartung O, Agarwal S, Daley GQ, Goff SP, et al. Dynamic instability of genomic methylation patterns in pluripotent stem cells. Epigenetics Chromatin. 2010;3:17. doi: 10.1186/1756-8935-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–25. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang W, Yang J, Liu H, Lu D, Chen X, Zenonos Z, et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc Natl Acad Sci U S A. 2011;108:18283–8. doi: 10.1073/pnas.1100893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, et al. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A. 2010;107:9222–7. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buecker C, Chen HH, Polo JM, Daheron L, Bu L, Barakat TS, et al. A murine ESC-like state facilitates transgenesis and homologous recombination in human pluripotent stem cells. Cell Stem Cell. 2010;6:535–46. doi: 10.1016/j.stem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]