Abstract
Growing neoplasms employ various mechanisms to evade immunosurveillance. The expression of non-classical MHC class I molecules by both immune and malignant cells in the tumor microenvironment constitute of the strategies used by tumors to circumvent the cytotoxic activity of effector cells of the immune system. The overexpression of HLA-G, -E, and -F is a common finding across a variety of malignancies. However, while the presence of HLA-G and HLA-E has been recently correlated with poor clinical outcome, information on the clinicopathological significance of HLA-F is limited. In the present review, we summarize studies on non-classical MHC class I molecules with special emphasis on their role in the modulation of anticancer immune responses.
Keywords: antigen presentation, cancer, immunomodulation, NK cells, non-classical MHC class I molecules, T cells
Introduction
Paul Erlich was the first to propose that the immune system would play a significant role in protecting the organism from cancer, putting forward the “tumor surveillance” hypothesis as early as in 1909. Unfortunately, the lack of adequate tools and sufficient knowledge did not allow for testing this hypothesis until several decades later. In 1957, Thomas and Burnet reintroduced Erlich’s concept by proposing that specific lymphocyte populations act as sentinels, thus continuously recognizing and eliminating malignant cells before they can cause any harm. This tumor surveillance hypothesis gained support upon the identification of numerous tumor-associated antigens (TAAs).1 Thus, the presentation of TAA-derived antigenic peptides to effector antitumor lymphocytes should, at least theoretically, ensure the clearance of malignant cells. Numerous anticancer vaccines were developed based on the ever increasing number of TAAs. Unfortunately, the overall success of these vaccines was limited by several reasons, including the fact that cancer cells and antigen presenting cells (APCs) downregulate expression of MHC class I molecules, which normally ensure the presentation of TAAs to effector cytotoxic T cells. Recent investigation has unveiled that neoplastic cells are effectively removed by a range of effector immune cells other than CD8+ T lymphocytes, including γδ T cells, natural killer (NK) and NKT cells,2,3 and this irrespective of the limited amounts of MHC class I molecules present on their surface. Interestingly, tumors harness several resources to escape the immune system. As an example, malignant cells often overexpress non-classical MHC I molecules, which have been shown to hamper the cytolytic activity of several effector cells. The precise mechanisms underlying tumor-elicited immunosuppression are not yet precisely understood and are subject of intense investigation.
One of these non-classical MHC I molecules, HLA-G was first described in 1987 by Geraghty and collaborators.4 HLA-E and HLA-F, 2 additional members of this family, were described shortly afterwards.5,6 HLA-G, -E, and -F have been denominated MHC class Ib or non-classical MHC molecules because of multiple features whereby they differ from classical HLA-A, -B and -C molecules. Non-classical HLA molecules, in contrast to classical ones, are characterized by a low genetic diversity as well as by a particular expression pattern, structural organization and functional profile.
In the present review, we will focus on the role of non-classical MHC class I molecules in the immunosuppressive mechanisms whereby malignant cells escape immunosurveillance.
Genetic Organization and Polymorphism of Non-Classical MHC Genes
The genes encoding non-classical HLA molecules are localized on the short arm of chromosome 6.
HLA-G
In contrast to classical HLA molecules, the polymorphic diversity of HLA-G is limited to 46 alleles (including 2 null alleles),7 resulting in only 7 protein isoforms. Four of these isoforms are membrane-bound while 3 of them lack exons 5–7, hence and existing as secreted forms. Of all membrane-bound HLA-G variants, HLA-G1 represents the sole full-length version of the molecule. Conversely, HLA-G2 does not contain exon 3, HLA-G3 is missing exons 3 and 4, and HLA-G4 does not include exon 4. The soluble isoforms of HLA-G (namely, HLA-G5, HLA-G6, and HLA-G7) contain part of intron 4, harboring a stop codon. This results in the expression of truncated proteins lacking exon 5, which encodes the transmembrane domain. HLA-G5, -G6, and -G7 represent the soluble counterparts of HLA-G1, G2, and-G3, respectively.8 Interestingly, there is an additional soluble form of HLA-G resulting from the proteolytic cleavage of HLA-G1.9 Similar to classical MHC class I molecules, HLA-G forms heterodimers with β2 microglobulin. In addition, HLA-G can form functionally active homodimers or tetramers. In fact, dimers and tetramers bind to HLA-G receptors (Table 1) with higher affinity than monomers.10,11
Table 1. Types of HLA-G/E/F receptors and expression on the cells.
| NK cells Macrophages |
monocytes/ T cells |
DC T cells |
CD8+ molecule |
CD4+ | B cells | HLA | |
|---|---|---|---|---|---|---|---|
| ILT2/CD85j/LILRB1 | +/- | + | + | +/- | +/- | + | HLA-G, HLA-F |
| ILT4/CD85d/LILRB2 | + | + | + | - | - | - | HLA-G, HLA-F |
| KIR2DL4/CD158d | + | - | - | +/- | - | - | HLA-G |
| CD8 | +/- | - | - | + | - | - | HLA-G |
| CD160 | + | - | - | +/- | - | - | HLA-G |
| NKG2A/CD159a | + | +/- | - | +/- | +/- | - | HLA-E |
| NKG2C/CD159c | + | + | - | +/- | +/- | - | HLA-E |
| TCR | - | - | - | +/- | - | - | HLA-E |
+/- some
HLA-E
HLA-E consists of 8 exons. Of these, the first encodes the leader peptide sequence, exons 2, 3 and 4 encode the MHC immunoglobulin-like α domains 1, 2, and 3, respectively, exon 5 encodes the transmembrane domain and exons 6 and 7 encode the cytoplasmic tail. Similar to HLA-G, HLA-E forms a complex with β2 microglobulin. To date, only 4 alleles of HLA-E have been described, namely E*01:01, E*0102, E*01:03 and E*01:04. However, the existence of E*0102 and E*01:04 could not be confirmed in recent studies.12 Interestingly, these alleles only differ in a single amino acid that impacts on their peptide-binding affinity.
HLA-F
HLA-F consists of 8 exons and is organized in a similar way as HLA-E. Thus, the first HLA-F exon encodes the leader peptide, exons 2, 3, and 4 the MHC immunoglobulin-like α domains, exon 5 the transmembrane domain and exons 6 and 7 the cytoplasmic tail. Three splicing variants of HLA-F have been described so far, leading to the synthesis of HLA-F isoforms that differ from each other in the length of the cytoplasmic tail. Similar to other HLA molecules, HLA-F can form a complex with β2 microglobulin.
Role of Non-Classical MHC Class I Molecules in Antigen Presentation
Among the 7 HLA-G isoforms describes so far, only HLA-G1 and HLA-G5 contain a peptide-binding region. Diehl and collaborators demonstrated that HLA-G-associated peptides correspond to classical MHC class I antigenic peptides originating from the proteolysis of intracellular proteins.13
HLA-E binds hydrophobic nanopeptides derived from the N-terminal leader sequences of MHC class I molecules, stress related-factors and pathogen-associated proteins. During transmembrane translocation, the signal peptide of these molecules is cleaved of by the signal peptide peptidase and remains in the endoplasmic reticulum (ER) membrane. The peptide is thus processed by the signal peptide peptidase and its hydrophilic part released back into the cytoplasm. Therein, it undergoes further trimming by the proteasome and can be transported into the ER by the transporter associated with antigen presentation (TAP) system, eventually binding HLA-E.14-17
It has been demonstrated that HLA-E and -G present antigenic peptides to T cells, a process that plays an important role in some lines of antiviral defense.18,19 So far, no report has been published demonstrating that HLA-F molecules also mediate antigen presentation.
Role of Non-Classical MHC Class I molecules Immune Regulation and Cancer
HLA-G
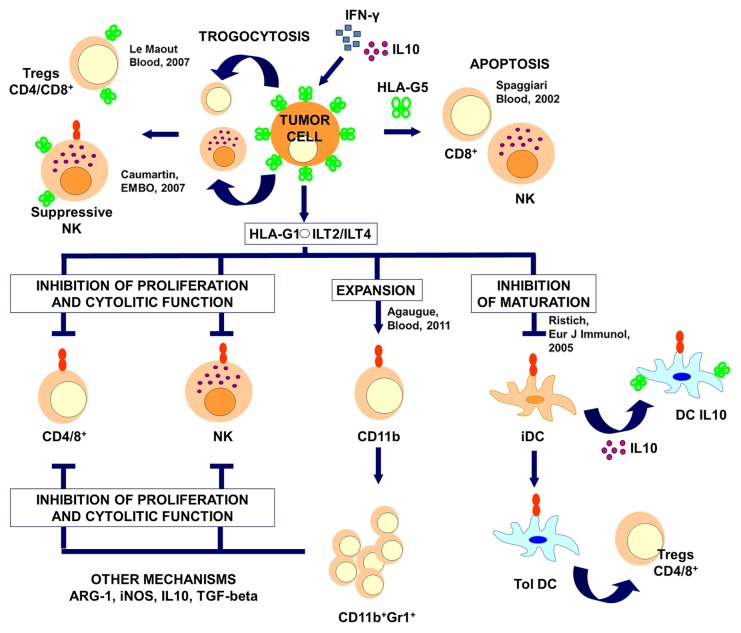
In healthy individuals, the transcription of HLA-G takes place in most cells, but the expression of the corresponding protein is restricted to specific tissues including the trophoblast, thymic epithelium, corneal keratocytes, as well as erythroid and endothelial precursors. In embryonic tissues, HLA-G can be detected from the oocyte to the blastocyst stage, erythroid cells and all organs involved in erythropoiesis.8 HLA-G molecules expressed on trophoblasts effectively suppress local immune responses in the placenta and hence contribute to maternal-fetal tolerance, a mechanism whereby the fetus is not recognized as a non-self tissue.20 In addition, HLA-G is expressed following organ transplantation, viral infection, inflammatory conditions and autoimmune diseases.19,21-25 Interestingly, ectopic HLA-G expression by tumor cells has first been described in 1998. Based on previous data suggesting that HLA-G2 strongly inhibits the lytic activity of NK cells in vitro, Paul and collaborators reported the inhibition of melanoma cell lysis by NK cells. The authors hypothesized that HLA-G plays a role in protecting cancer cells from NK- and cytotoxic T lymphocyte (CTL)-mediated destruction by binding to leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 1 (LILRB1, best known as ILT-2). This observation suggested that cancer cells in general might overexpress HLA-G to evade the host immune system. Since then, HLA-G expression has been detected in various types of cancers including melanomas, carcinomas (breast, renal, ovarian, lung, and colorectal) and leukemia.26,27 Recently, Agaugue and collaborators demonstrated the expression of HLA-G on the surface of malignant cells induces the expansion of myeloid-derived suppressor cells (MDSCs) and shift cytokine production toward a Th2 profile.28 Interestingly, it has also been shown that cancer cells also transfer membrane patches to activated NK cells, a phenomenon that has been named trogocytosis.29-31 As the result of this process, NK cells receive HLA-G1 molecules, stop proliferating and become able to inhibit the cytotoxic effector functions of neighboring NK cells. In addition, high levels of soluble forms of HLA-G (sHLA-G) have been detected in the sera and ascites of melanoma, glioma, ovarian cancer and breast carcinoma patients.32-35 The circulating levels of sHLA-G have been shown to correlate with advanced disease stage and increased tumor load.32 Importantly, sHLA-G molecules are secreted not only by tumor cells but also by tumor-activated monocytes and T cells.36 Tumor-associated myeloid cells such as monocytes and macrophages are usually a bad prognostic feature, as they exert robust immunosuppressive effects.37 In this context, the secretion of sHLA-G contributes to the immunosuppressive functions of tumor-infiltrating myeloid cells not only locally, at the tumor site, but also systemically. Elevated circulating levels of sHLA-G have been observed in the presence of interferon γ (IFNγ)38 and interleukin (IL)-10.39,40 Other cytokine cocktails can stimulate the production of HLA-G (reviewed in ref. 41).
HLA-G limit the activity of effector cells by binding to inhibitory receptors ILT-2, LILRB2 (best known as ILT-4) and killer cell immunoglobulin-like receptor, 2 domains, long cytoplasmic tail, 4 (KIR2DL4) on NK cells, T and B lymphocytes as well as APCs. sHLA-G binds to the CD8+ co-receptor (as other sHLA molecules do) and induces apoptosis.42 Moreover, Fond and collaborators have demonstrated the ability of sHLA-G to bind CD160, hence stimulating angiogenesis.43,44 Le Maoult and coworkers demonstrated that HLA-G promotes the expression of increased levels of inhibitory receptors.45 The binding partners of HLA-G are summarized in Table 1.
As demonstrated by several authors, the role of HLA-G in oncogenesis and tumor progression does not stem from its contribution to antigen presentation, and therefore deviates from that of classical MHC molecules. HLA-G triggers inhibitory receptors instead, hence switching off the effector function of multiple antitumor immune effectors (Fig. 1). The concerted efforts of several groups over the recent years have unveiled the molecular mechanisms underlying such an activity of HLA-G. In particular, it has been shown that renal carcinoma and melanoma cells expressing HLA-G are protected from the cytotoxic activity of NK cells and antigen-specific CD8+ T cells. This protective effect can be abolished by the administration of HLA-G-specific antibodies.46,47 Ristich and collaborators demonstrated HLA-G inhibits the maturation and activation of myeloid DCs at fetal-maternal interface, hence promoting the development of tolerogenic DCs that suppress CD4+ and CD8+ T cells.48 The same group described the involvement of IL-6 signaling and signal transducer and activator of transcription 3 (STAT3) in this process.48,49 It is possible, yet remains to be formally demonstrated, that cancer cells tolerize DCs in a similar fashion.
Figure 1. Immunological mechanisms of HLA-G-mediated immunosuppression by cancer cells. Cancer cells expose HLA-G molecules (in green) on their surface or secrete it (HLA-G5), inducing the apoptotic demise of effector cells. The transfer of HLA-G-containing membranes to T and natural killer (NK) cells renders them immunosuppressive. The binding of HLA-G1 to inhibitory receptors such as ILT-2 and ILT-4 mediated immunosuppressive mechanisms in a variety of immune cells. ARG-1, arginase 1; iDC, immature dendritic cell; IFNγ, interferon γ; IL-10, interleukin-10; iNOS, inducible nitric oxide synthase; TGFβ, transforming growth factor β; Tol DC, tolerogenic dendritic cell.
HLA-E
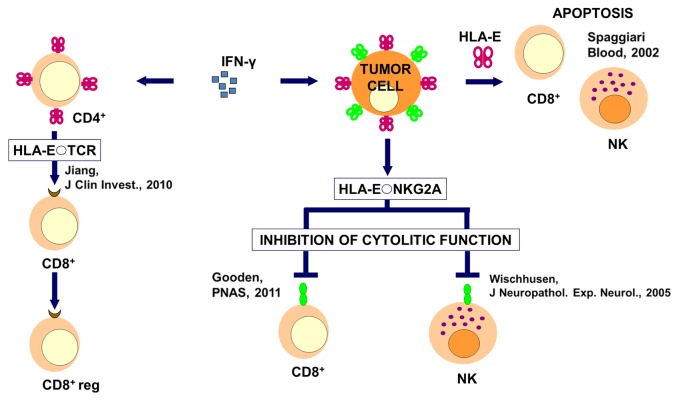
Similar to HLA-G, HLA-E is expressed on the surface of the trophoblastic cells (during pregnancy) as well as following organ transplantation, viral infection, inflammatory conditions, and autoimmune diseases (reviewed in ref. 50). The transcription of HLA-E has been detected in almost all cell types, although the expression of the corresponding protein on the cell surface is mainly restricted to endothelial cells, T and B lymphocytes, monocytes, and macrophages. Interestingly, while cells of the immune system express HLA-E at high levels, endothelial cells exhibit reduced levels of HLA-E on their surface, much lower than those of most MHC class Ia genes. Only when endothelial cells are exposed to pro-inflammatory cytokines such as tumor necrosis factor α (TNFα) IL-1β and IFNγ, they produce and display on the surface increased levels of HLA-E. Such an upregulation also results in HLA-E secretion.51 The precise mechanism underlying sHLA-E production remains to be determined. Secreted forms of HLA-E may indeed originate from translation of an alternatively spliced transcript or, as suggested by several authors, result from metalloprotease-dependent shedding of membrane-bound HLA-E molecules. These possibilities are not mutually exclusive.51,52 The upregulation of HLA-E on the surface of endothelial cells correlates with an increased protection against the effector functions of NK cells, hence constituting a potential means of controlling excessive cytotoxic responses. Increased levels of surface-exposed and soluble HLA-E were observed in cultured melanoma cells.53 In this setting, NK cytotoxicity was increased upon blocking HLA-E or HLA-E-dependent ligand-receptor interactions with specific antibodies. Some evidence indicates that HLA-G could act in concert with HLA-E in immune regulation. Thus, it has been proposed that some HLA-G isoforms stimulate the expression of HLA-E on the cell surface.54 Braud and collaborators have shown that this process (the upregulation of HLA-E on the cell surface) is TAP- and tapasin-dependent.17 Some authors propose that low levels of surface-exposed HLA-E result from low-affinity interactions with β2 microglobulin and inefficient peptide loading in the ER.55,56 HLA-E binds killer cell lectin-like receptor subfamily C, member 1 (KLRC1, an activatory receptor best known as NKG2A) and KLRC2 (an inhibitory receptor best known as NKG2C) on NK cells, T lymphocytes and macrophages. The binding of HLA-E to NKG2A is much stronger than that to NKG2C, suggesting a subordinate role of this latter interaction in normal conditions.57 Michaëlsson and collaborators have demonstrated that, upon binding to heat-shock protein-derived peptides, HLA-E loses its affinity for NKG2A, pointing to the existence of a peptide-dependent mechanism that regulates HLA-E functions.58 Interestingly, several groups have observed an interaction between peptide-loaded HLA-E molecules and the T-cell receptor (TCR) of CD8+ T cells,59 and CD160 (in thus far resembling HLA-G).60 The interaction of HLA-E with the TCR have been ascribed an important role in antiviral responses.18 However, the binding of HLA-E to the TCR might also stimulate the generation of CD8+ regulatory T cells (Tregs).61 Although it might be possible that the peptide presented by HLA-E would play a critical role in this settings, such a dual functionality has not yet been elucidated in detail.
Alike HLA-G, HLA-E is expressed to high levels by different types of cancer, including lymphomas,62 melanomas,53 gliomas,63 and carcinomas.64,65 In some of these scenarios, HLA-E expression levels have been correlated with its tolerogenic activity. The effects of HLA-E on immune effectors are summarized in Figure 2.
Figure 2. Immunological mechanisms of HLA-E-mediated immunosuppression by cancer cells. In Cancer cells expose HLA-G (in green) and HLA-E (in red) molecules of their surface. The secretion of HLA-E induces apoptosis in immune effectors. Moreover, in the presence of interferon γ (IFNγ), HLA-G and HLA-E molecules are expressed at increased levels on the surface of both cancer cells and CD4+ T lymphocytes. The binding of HLA-E expressed on CD4+ T cells to the T-cell receptor (TCR) of CD4+ T lymphocytes can induce the differentiation of the latter into regulatory CD8+ T cells (CD8+ reg). The binding of HLA-E to NKG2A inhibits the cytotoxic functions of CD8+ T lymphocytes and NK cells.
HLA-F
Although HLA-F was first described together with other MHC class Ib molecules, it represents the most enigmatic member of this protein family. HLA-F is expressed by peripheral blood B cells, B-cell lines as well as by all tissues containing B cells (adult tonsils, thymus, bladder, skin, and the fetal liver, major sites of B-cell development).66 HLA-F has also been detected in embryonic tissues, including the extravillous trophoblasts invading maternal deciduas, and in spermatozoids.67-69 The expression of HLA-F by trophoblasts correlates with the protection of the fetus from destruction by the maternal immune system, an observation that also applies to HLA-G and -E.
The majority of HLA-F is localize within the cell. Recently, HLA-F was detected on the surface of activated B-, T-, and NK cells.70 HLA-F molecules exposed form a complex with β2 microglobulin and can bind ILT-2 and ILT-4.71 Boyle and coworkers investigated the mechanisms whereby HLA-F is transported to the cell surface and proved that this process relies on the cytoplasmic tail of HLA-F, something that differentiates HLA-F from other MHC class I molecules.72 Different authors disagree on the fact that the exposure of HLA-F on the cell surface might depend or not on tapasin and TAP.73 The presence of HLA-F molecules on activated lymphocytes but not on Tregs suggests that HLA-F might be involved in providing signals that invoke the tolerogenic function of Tregs upon the secretion of anti-inflammatory cytokines.70 Thus, HLA-F may mediate important immunosuppressive functions that regulate the maternal tolerance to the fetus.
Gobin and coworkers performed a study on non-classical HLA promoter regions and found that HLA-F transcription can be induced by IFNγ.74 HLA-F-coding transcripts have been detected in various cancer tissues. In addition, HLA-F-specific antibodies are present at increased levels in the serum of patients affected by multiple types of cancer.75 The analysis of HLA-F expression in a cohort of esophageal squamous cell carcinoma patients revealed that high HLA-F levels correlate with poor disease outcome.76 Similar results were obtained in patients with non-small lung carcinoma.77
Conclusions
HLA-G, -E, and -F are important regulators of the immune system and their role in tumor immunology is attracting a steadily growing interest. The upregulation of HLA-G, -E, and -F following IFNγ stimulation suggests that non-classical MHC class I molecules may be involved in negative feedback responses to potentially harmful pro-inflammatory responses. Inflammation is also one of the hallmarks of cancer. While inflammatory responses are required to eliminate cancer cells, they also trigger strong immunoregulatory mechanisms that limit the recognition of malignant cells by the immune system, hence favoring tumor progression. Thus, tumor-caused results in the recruitment strongly immunosuppressive cells such as Tregs, MDSCs and tumor-infiltrating macrophages. Non-classical MHC class I molecules constitute another means whereby malignant cells escape immunosurveillance. Indeed, these molecules inhibit the activity of the immune system by binding to inhibitory receptors expressed by effector cells, hence suppressing their functions or inducing their apoptotic demise.42,78 Unfortunately, data concerning HLA-F are scarce. Alike other non-classical MHC class I molecules, HLA-F molecules contribute to the maintenance of tolerance to semi-allogenic fetal allografts. Perhaps, HLA-F might exert similar immunosuppressive effects in cancer.
Based on the observation presented above, several groups have proposed the use of HLA-G and HLA-E as cancer biomarkers upon detection with ELISA or in situ immunostaining. Some authors have suggested that the presence of non-classical MHC class I molecules can be a predictor of poor prognosis.79-81 Even though some studies classify HLA-E as rather neutral,82 or even favorable,83,84 for disease outcome, accumulating evidence indicated that HLA-E overexpression is associated with poor prognosis.
In summary, a growing amount of data highlights an important role of non-classical MHC class I molecules in immune regulation and cancer. Thus, the molecules might become an important target for anticancer immunotherapy. Interfering with the functions of non-classical MHC class I molecules might robustly boost the antineoplastic potential of cytotoxic effectors while antagonizing the activity of intrinsically immunosuppressive cells. The specific mechanisms whereby these molecules control excessive inflammation are currently under investigation for therapeutic purposes, not only in the context of anticancer immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Karine Breckpot is a post-doctoral fellow funded by FWO-Vlaanderen and research of her group on immunospuppressive mechanisms exerted by tumor and its environment is also funded by the same organization (iMECHs grant, G.0234.11), David Escors has been funded by an Arthritis Research UK Career Development Fellowship (18433) and currently by a Miguel Servet Fellowship from the Instituto de Salud Carlos III, Spain.
Glossary
Abbreviations:
- APC
antigen-presenting cell
- ARG
arginase CTL, cytotoxic T lymphocyte
- DC
dendritic cell
- ER
endoplasmic reticulum
- IFNγ
interferon γ
- IL
interleukin
- ILT
immunoglobulin-like transcript
- iNOS
inducible nitric oxide synthase
- MDSC
myeloid-derived suppressor cell
- NK
natural killer
- sHLA
soluble human leukocyte antigen
- STAT3
signal transducer and activator of transcription 3
- TAA
tumor-associated antigen
- TAP
transporter associated with antigen presentation
- TNF
tumor necrosis factor
- Treg
regulatory T cell
Citation: Escors D, Breckpot K, Guerrero-Setas D, Kochan G. Role of non-classical MHC class I molecules in cancer immunosuppression. OncoImmunology 2013; 2:e26491; 10.4161/onci.26491
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26491
References
- 1.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–37. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 3.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geraghty DE, Koller BH, Orr HT. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci U S A. 1987;84:9145–9. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koller BH, Geraghty DE, Shimizu Y, DeMars R, Orr HT. HLA-E. A novel HLA class I gene expressed in resting T lymphocytes. J Immunol. 1988;141:897–904. [PubMed] [Google Scholar]
- 6.Geraghty DE, Wei XH, Orr HT, Koller BH. Human leukocyte antigen F (HLA-F). An expressed HLA gene composed of a class I coding sequence linked to a novel transcribed repetitive element. J Exp Med. 1990;171:1–18. doi: 10.1084/jem.171.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castelli EC, Mendes-Junior CT, Veiga-Castelli LC, Roger M, Moreau P, Donadi EA. A comprehensive study of polymorphic sites along the HLA-G gene: implication for gene regulation and evolution. Mol Biol Evol. 2011;28:3069–86. doi: 10.1093/molbev/msr138. [DOI] [PubMed] [Google Scholar]
- 8.Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N. HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol. 2003;81:199–252. doi: 10.1016/S0065-2776(03)81006-4. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo R, Trentini A, Bortolotti D, Manfrinato MC, Rotola A, Castellazzi M, Melchiorri L, Di Luca D, Dallocchio F, Fainardi E, et al. Matrix metalloproteinase-2 (MMP-2) generates soluble HLA-G1 by cell surface proteolytic shedding. Mol Cell Biochem. 2013;381:243–55. doi: 10.1007/s11010-013-1708-5. [DOI] [PubMed] [Google Scholar]
- 10.Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008;111:4862–70. doi: 10.1182/blood-2007-12-127662. [DOI] [PubMed] [Google Scholar]
- 11.Shiroishi M, Kuroki K, Ose T, Rasubala L, Shiratori I, Arase H, Tsumoto K, Kumagai I, Kohda D, Maenaka K. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J Biol Chem. 2006;281:10439–47. doi: 10.1074/jbc.M512305200. [DOI] [PubMed] [Google Scholar]
- 12.Grimsley C, Kawasaki A, Gassner C, Sageshima N, Nose Y, Hatake K, Geraghty DE, Ishitani A. Definitive high resolution typing of HLA-E allelic polymorphisms: Identifying potential errors in existing allele data. Tissue Antigens. 2002;60:206–12. doi: 10.1034/j.1399-0039.2002.600302.x. [DOI] [PubMed] [Google Scholar]
- 13.Diehl M, Münz C, Keilholz W, Stevanović S, Holmes N, Loke YW, Rammensee HG. Nonclassical HLA-G molecules are classical peptide presenters. Curr Biol. 1996;6:305–14. doi: 10.1016/S0960-9822(02)00481-5. [DOI] [PubMed] [Google Scholar]
- 14.LeMaoult J, Rouas-Freiss N, Carosella ED. Immuno-tolerogenic functions of HLA-G: relevance in transplantation and oncology. Autoimmun Rev. 2005;4:503–9. doi: 10.1016/j.autrev.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Bland FA, Lemberg MK, McMichael AJ, Martoglio B, Braud VM. Requirement of the proteasome for the trimming of signal peptide-derived epitopes presented by the nonclassical major histocompatibility complex class I molecule HLA-E. J Biol Chem. 2003;278:33747–52. doi: 10.1074/jbc.M305593200. [DOI] [PubMed] [Google Scholar]
- 16.Braud V, Jones EY, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol. 1997;27:1164–9. doi: 10.1002/eji.1830270517. [DOI] [PubMed] [Google Scholar]
- 17.Braud VM, Allan DS, Wilson D, McMichael AJ. TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide. Curr Biol. 1998;8:1–10. doi: 10.1016/S0960-9822(98)70014-4. [DOI] [PubMed] [Google Scholar]
- 18.Mazzarino P, Pietra G, Vacca P, Falco M, Colau D, Coulie P, Moretta L, Mingari MC. Identification of effector-memory CMV-specific T lymphocytes that kill CMV-infected target cells in an HLA-E-restricted fashion. Eur J Immunol. 2005;35:3240–7. doi: 10.1002/eji.200535343. [DOI] [PubMed] [Google Scholar]
- 19.Yan WH, Lin A, Chen BG, Chen SY. Induction of both membrane-bound and soluble HLA-G expression in active human cytomegalovirus infection. J Infect Dis. 2009;200:820–6. doi: 10.1086/604733. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo R, Melchiorri L, Stignani M, Baricordi OR. HLA-G expression is a fundamental prerequisite to pregnancy. Hum Immunol. 2007;68:244–50. doi: 10.1016/j.humimm.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Zarkhin V, Talisetti A, Li L, Wozniak LJ, McDiarmid SV, Cox K, Esquivel C, Sarwal MM. Expression of soluble HLA-G identifies favorable outcomes in liver transplant recipients. Transplantation. 2010;90:1000–5. doi: 10.1097/TP.0b013e3181f546af. [DOI] [PubMed] [Google Scholar]
- 22.Verbruggen LA, Rebmann V, Demanet C, De Cock S, Grosse-Wilde H. Soluble HLA-G in rheumatoid arthritis. Hum Immunol. 2006;67:561–7. doi: 10.1016/j.humimm.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 23.LeMaoult J, Le Discorde M, Rouas-Freiss N, Moreau P, Menier C, McCluskey J, Carosella ED. Biology and functions of human leukocyte antigen-G in health and sickness. Tissue Antigens. 2003;62:273–84. doi: 10.1034/j.1399-0039.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 24.Crispim JC, Duarte RA, Soares CP, Costa R, Silva JS, Mendes-Júnior CT, Wastowski IJ, Faggioni LP, Saber LT, Donadi EA. Human leukocyte antigen-G expression after kidney transplantation is associated with a reduced incidence of rejection. Transpl Immunol. 2008;18:361–7. doi: 10.1016/j.trim.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Lafon M, Prehaud C, Megret F, Lafage M, Mouillot G, Roa M, Moreau P, Rouas-Freiss N, Carosella ED. Modulation of HLA-G expression in human neural cells after neurotropic viral infections. J Virol. 2005;79:15226–37. doi: 10.1128/JVI.79.24.15226-15237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul P, Rouas-Freiss N, Moreau P, Cabestre FA, Menier C, Khalil-Daher I, Pangault C, Onno M, Fauchet R, Martinez-Laso J, et al. HLA-G, -E, -F preworkshop: tools and protocols for analysis of non-classical class I genes transcription and protein expression. Hum Immunol. 2000;61:1177–95. doi: 10.1016/S0198-8859(00)00154-3. [DOI] [PubMed] [Google Scholar]
- 27.Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res. 2005;65:10139–44. doi: 10.1158/0008-5472.CAN-05-0097. [DOI] [PubMed] [Google Scholar]
- 28.Agaugué S, Carosella ED, Rouas-Freiss N. Role of HLA-G in tumor escape through expansion of myeloid-derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood. 2011;117:7021–31. doi: 10.1182/blood-2010-07-294389. [DOI] [PubMed] [Google Scholar]
- 29.Caumartin J, Lemaoult J, Carosella ED. Intercellular exchanges of membrane patches (trogocytosis) highlight the next level of immune plasticity. Transpl Immunol. 2006;17:20–2. doi: 10.1016/j.trim.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26:1423–33. doi: 10.1038/sj.emboj.7601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, Carosella ED. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–8. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 32.Ugurel S, Rebmann V, Ferrone S, Tilgen W, Grosse-Wilde H, Reinhold U. Soluble human leukocyte antigen--G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer. 2001;92:369–76. doi: 10.1002/1097-0142(20010715)92:2<369::AID-CNCR1332>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.Rebmann V, Ugurel S, Tilgen W, Reinhold U, Grosse-Wilde H. Soluble HLA-DR is a potent predictive indicator of disease progression in serum from early-stage melanoma patients. Int J Cancer. 2002;100:580–5. doi: 10.1002/ijc.10524. [DOI] [PubMed] [Google Scholar]
- 34.Singer G, Rebmann V, Chen YC, Liu HT, Ali SZ, Reinsberg J, McMaster MT, Pfeiffer K, Chan DW, Wardelmann E, et al. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9:4460–4. [PubMed] [Google Scholar]
- 35.Rebmann V, Regel J, Stolke D, Grosse-Wilde H. Secretion of sHLA-G molecules in malignancies. Semin Cancer Biol. 2003;13:371–7. doi: 10.1016/S1044-579X(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 36.Lila N, Rouas-Freiss N, Dausset J, Carpentier A, Carosella ED. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: a CD4+ T cell regulatory mechanism. Proc Natl Acad Sci U S A. 2001;98:12150–5. doi: 10.1073/pnas.201407398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emeagi PU, Goyvaerts C, Maenhout S, Pen J, Thielemans K, Breckpot K. Lentiviral vectors: a versatile tool to fight cancer. Curr Mol Med. 2013;13:602–25. doi: 10.2174/1566524011313040011. [DOI] [PubMed] [Google Scholar]
- 38.Gobin SJ, van Zutphen M, Woltman AM, van den Elsen PJ. Transactivation of classical and nonclassical HLA class I genes through the IFN-stimulated response element. J Immunol. 1999;163:1428–34. [PubMed] [Google Scholar]
- 39.Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, Carosella ED, Paul P. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol. 1999;11:803–11. doi: 10.1093/intimm/11.5.803. [DOI] [PubMed] [Google Scholar]
- 40.Urosevic M, Kurrer MO, Kamarashev J, Mueller B, Weder W, Burg G, Stahel RA, Dummer R, Trojan A. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am J Pathol. 2001;159:817–24. doi: 10.1016/S0002-9440(10)61756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.González A, Rebmann V, LeMaoult J, Horn PA, Carosella ED, Alegre E. The immunosuppressive molecule HLA-G and its clinical implications. Crit Rev Clin Lab Sci. 2012;49:63–84. doi: 10.3109/10408363.2012.677947. [DOI] [PubMed] [Google Scholar]
- 42.Contini P, Ghio M, Poggi A, Filaci G, Indiveri F, Ferrone S, Puppo F. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol. 2003;33:125–34. doi: 10.1002/immu.200390015. [DOI] [PubMed] [Google Scholar]
- 43.Fons P, Chabot S, Cartwright JE, Lenfant F, L’Faqihi F, Giustiniani J, Herault JP, Gueguen G, Bono F, Savi P, et al. Soluble HLA-G1 inhibits angiogenesis through an apoptotic pathway and by direct binding to CD160 receptor expressed by endothelial cells. Blood. 2006;108:2608–15. doi: 10.1182/blood-2005-12-019919. [DOI] [PubMed] [Google Scholar]
- 44.Le Bouteiller P, Fons P, Herault JP, Bono F, Chabot S, Cartwright JE, Bensussan A. Soluble HLA-G and control of angiogenesis. J Reprod Immunol. 2007;76:17–22. doi: 10.1016/j.jri.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 45.LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 2005;19:662–4. doi: 10.1096/fj.04-1617fje. [DOI] [PubMed] [Google Scholar]
- 46.Bukur J, Malenica B, Huber C, Seliger B. Altered expression of nonclassical HLA class Ib antigens in human renal cell carcinoma and its association with impaired immune response. Hum Immunol. 2003;64:1081–92. doi: 10.1016/j.humimm.2003.08.350. [DOI] [PubMed] [Google Scholar]
- 47.Paul P, Rouas-Freiss N, Khalil-Daher I, Moreau P, Riteau B, Le Gal FA, Avril MF, Dausset J, Guillet JG, Carosella ED. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci U S A. 1998;95:4510–5. doi: 10.1073/pnas.95.8.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35:1133–42. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 49.Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6--STAT3 signaling pathway. Proc Natl Acad Sci U S A. 2008;105:8357–62. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwaszko M, Bogunia-Kubik K. Clinical significance of the HLA-E and CD94/NKG2 interaction. Arch Immunol Ther Exp (Warsz) 2011;59:353–67. doi: 10.1007/s00005-011-0137-y. [DOI] [PubMed] [Google Scholar]
- 51.Coupel S, Moreau A, Hamidou M, Horejsi V, Soulillou JP, Charreau B. Expression and release of soluble HLA-E is an immunoregulatory feature of endothelial cell activation. Blood. 2007;109:2806–14. doi: 10.1182/blood-2006-06-030213. [DOI] [PubMed] [Google Scholar]
- 52.Dong Y, Lieskovska J, Kedrin D, Porcelli S, Mandelboim O, Bushkin Y. Soluble nonclassical HLA generated by the metalloproteinase pathway. Hum Immunol. 2003;64:802–10. doi: 10.1016/S0198-8859(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 53.Derré L, Corvaisier M, Charreau B, Moreau A, Godefroy E, Moreau-Aubry A, Jotereau F, Gervois N. Expression and release of HLA-E by melanoma cells and melanocytes: potential impact on the response of cytotoxic effector cells. J Immunol. 2006;177:3100–7. doi: 10.4049/jimmunol.177.5.3100. [DOI] [PubMed] [Google Scholar]
- 54.Teklemariam T, Zhao L, Hantash BM. Full-length HLA-G1 and truncated HLA-G3 differentially increase HLA-E surface localization. Hum Immunol. 2012;73:898–905. doi: 10.1016/j.humimm.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Ulbrecht M, Couturier A, Martinozzi S, Pla M, Srivastava R, Peterson PA, Weiss EH. Cell surface expression of HLA-E: interaction with human beta2-microglobulin and allelic differences. Eur J Immunol. 1999;29:537–47. doi: 10.1002/(SICI)1521-4141(199902)29:02<537::AID-IMMU537>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Ulbrecht M, Kellermann J, Johnson JP, Weiss EH. Impaired intracellular transport and cell surface expression of nonpolymorphic HLA-E: evidence for inefficient peptide binding. J Exp Med. 1992;176:1083–90. doi: 10.1084/jem.176.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wada H, Matsumoto N, Maenaka K, Suzuki K, Yamamoto K. The inhibitory NK cell receptor CD94/NKG2A and the activating receptor CD94/NKG2C bind the top of HLA-E through mostly shared but partly distinct sets of HLA-E residues. Eur J Immunol. 2004;34:81–90. doi: 10.1002/eji.200324432. [DOI] [PubMed] [Google Scholar]
- 58.Michaëlsson J, Teixeira de Matos C, Achour A, Lanier LL, Kärre K, Söderström K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002;196:1403–14. doi: 10.1084/jem.20020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Goldstein I, Glickman-Nir E, Jiang H, Chess L. Induction of TCR Vbeta-specific CD8+ CTLs by TCR Vbeta-derived peptides bound to HLA-E. J Immunol. 2001;167:3800–8. doi: 10.4049/jimmunol.167.7.3800. [DOI] [PubMed] [Google Scholar]
- 60.Rabot M, Tabiasco J, Polgar B, Aguerre-Girr M, Berrebi A, Bensussan A, Strbo N, Rukavina D, Le Bouteiller P. HLA class I/NK cell receptor interaction in early human decidua basalis: possible functional consequences. Chem Immunol Allergy. 2005;89:72–83. doi: 10.1159/000087914. [DOI] [PubMed] [Google Scholar]
- 61.Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, Chess L. HLA-E-restricted regulatory CD8(+) T cells are involved in development and control of human autoimmune type 1 diabetes. J Clin Invest. 2010;120:3641–50. doi: 10.1172/JCI43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marín R, Ruiz-Cabello F, Pedrinaci S, Méndez R, Jiménez P, Geraghty DE, Garrido F. Analysis of HLA-E expression in human tumors. Immunogenetics. 2003;54:767–75. doi: 10.1007/s00251-002-0526-9. [DOI] [PubMed] [Google Scholar]
- 63.Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Weller M. HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: implications for immune escape in vivo. J Neuropathol Exp Neurol. 2005;64:523–8. doi: 10.1093/jnen/64.6.523. [DOI] [PubMed] [Google Scholar]
- 64.Levy EM, Bianchini M, Von Euw EM, Barrio MM, Bravo AI, Furman D, Domenichini E, Macagno C, Pinsky V, Zucchini C, et al. Human leukocyte antigen-E protein is overexpressed in primary human colorectal cancer. Int J Oncol. 2008;32:633–41. [PubMed] [Google Scholar]
- 65.Gooden M, Lampen M, Jordanova ES, Leffers N, Trimbos JB, van der Burg SH, Nijman H, van Hall T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8⁺ T lymphocytes. Proc Natl Acad Sci U S A. 2011;108:10656–61. doi: 10.1073/pnas.1100354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei XH, Orr HT. Differential expression of HLA-E, HLA-F, and HLA-G transcripts in human tissue. Hum Immunol. 1990;29:131–42. doi: 10.1016/0198-8859(90)90076-2. [DOI] [PubMed] [Google Scholar]
- 67.Le Bouteiller P, Lenfant F. Antigen-presenting function(s) of the non-classical HLA-E, -F and -G class I molecules: the beginning of a story. Res Immunol. 1996;147:301–13. doi: 10.1016/0923-2494(96)89643-X. [DOI] [PubMed] [Google Scholar]
- 68.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol. 2003;171:1376–84. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 69.Fiszer D, Ulbrecht M, Fernandez N, Johnson JP, Weiss EH, Kurpisz M. Analysis of HLA class Ib gene expression in male gametogenic cells. Eur J Immunol. 1997;27:1691–5. doi: 10.1002/eji.1830270715. [DOI] [PubMed] [Google Scholar]
- 70.Lee N, Ishitani A, Geraghty DE. HLA-F is a surface marker on activated lymphocytes. Eur J Immunol. 2010;40:2308–18. doi: 10.1002/eji.201040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lepin EJ, Bastin JM, Allan DS, Roncador G, Braud VM, Mason DY, van der Merwe PA, McMichael AJ, Bell JI, Powis SH, et al. Functional characterization of HLA-F and binding of HLA-F tetramers to ILT2 and ILT4 receptors. Eur J Immunol. 2000;30:3552–61. doi: 10.1002/1521-4141(200012)30:12<3552::AID-IMMU3552>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 72.Boyle LH, Gillingham AK, Munro S, Trowsdale J. Selective export of HLA-F by its cytoplasmic tail. J Immunol. 2006;176:6464–72. doi: 10.4049/jimmunol.176.11.6464. [DOI] [PubMed] [Google Scholar]
- 73.Lee N, Geraghty DE. HLA-F surface expression on B cell and monocyte cell lines is partially independent from tapasin and completely independent from TAP. J Immunol. 2003;171:5264–71. doi: 10.4049/jimmunol.171.10.5264. [DOI] [PubMed] [Google Scholar]
- 74.Gobin SJ, van den Elsen PJ. Transcriptional regulation of the MHC class Ib genes HLA-E, HLA-F, and HLA-G. Hum Immunol. 2000;61:1102–7. doi: 10.1016/S0198-8859(00)00198-1. [DOI] [PubMed] [Google Scholar]
- 75.Noguchi K, Isogai M, Kuwada E, Noguchi A, Goto S, Egawa K. Detection of anti-HLA-F antibodies in sera from cancer patients. Anticancer Res. 2004;24(5C):3387–92. [PubMed] [Google Scholar]
- 76.Zhang X, Lin A, Zhang JG, Bao WG, Xu DP, Ruan YY, Yan WH. Alteration of HLA-F and HLA I antigen expression in the tumor is associated with survival in patients with esophageal squamous cell carcinoma. Int J Cancer. 2013;132:82–9. doi: 10.1002/ijc.27621. [DOI] [PubMed] [Google Scholar]
- 77.Lin A, Zhang X, Ruan YY, Wang Q, Zhou WJ, Yan WH. HLA-F expression is a prognostic factor in patients with non-small-cell lung cancer. Lung Cancer. 2011;74:504–9. doi: 10.1016/j.lungcan.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 78.Contini P, Ghio M, Merlo A, Brenci S, Filaci G, Indiveri F, Puppo F. Soluble HLA class I/CD8 ligation triggers apoptosis in EBV-specific CD8+ cytotoxic T lymphocytes by Fas/Fas-ligand interaction. Hum Immunol. 2000;61:1347–51. doi: 10.1016/S0198-8859(00)00212-3. [DOI] [PubMed] [Google Scholar]
- 79.Bossard C, Bézieau S, Matysiak-Budnik T, Volteau C, Laboisse CL, Jotereau F, Mosnier JF. HLA-E/β2 microglobulin overexpression in colorectal cancer is associated with recruitment of inhibitory immune cells and tumor progression. Int J Cancer. 2012;131:855–63. doi: 10.1002/ijc.26453. [DOI] [PubMed] [Google Scholar]
- 80.Wolpert F, Roth P, Lamszus K, Tabatabai G, Weller M, Eisele G. HLA-E contributes to an immune-inhibitory phenotype of glioblastoma stem-like cells. J Neuroimmunol. 2012;250:27–34. doi: 10.1016/j.jneuroim.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 81.Allard M, Oger R, Vignard V, Percier JM, Fregni G, Périer A, Caignard A, Charreau B, Bernardeau K, Khammari A, et al. Serum soluble HLA-E in melanoma: a new potential immune-related marker in cancer. PLoS One. 2011;6:e21118. doi: 10.1371/journal.pone.0021118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kren L, Fabian P, Slaby O, Janikova A, Soucek O, Sterba J, Krenova Z, Michalek J, Kral Z. Multifunctional immune-modulatory protein HLA-E identified in classical Hodgkin lymphoma: possible implications. Pathol Res Pract. 2012;208:45–9. doi: 10.1016/j.prp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Benevolo M, Mottolese M, Tremante E, Rollo F, Diodoro MG, Ercolani C, Sperduti I, Lo Monaco E, Cosimelli M, Giacomini P. High expression of HLA-E in colorectal carcinoma is associated with a favorable prognosis. J Transl Med. 2011;9:184. doi: 10.1186/1479-5876-9-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spaans VM, Peters AA, Fleuren GJ, Jordanova ES. HLA-E expression in cervical adenocarcinomas: association with improved long-term survival. J Transl Med. 2012;10:184. doi: 10.1186/1479-5876-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]