Abstract
Dendritic cells (DCs) are essential for the induction of adaptive immune responses against malignant cells by virtue of their capacity to effectively cross-present exogenous antigens to T lymphocytes. Dying cancer cells are indeed a rich source of antigens that may be harnessed for the development of DC-based vaccines. In particular, malignant cells succumbing to apoptosis, rather than necrosis, appear to release antigens in a manner that allows for the elicitation of adaptive immune responses. In this review, we describe the processes that mediate the cross-presentation of antigens released by apoptotic cancer cells to CD8+ T lymphocytes, resulting in the activation of protective tumor-specific immune responses.
Keywords: DAMPs, apoptotic, cross-presentation, dendritic cells, necrotic, storage compartments, type 1 interferon
Introduction
Antigen cross-presentation
The immune system plays an instrumental role in both the development and resolution, be it spontaneous or stimulated by therapy, of malignant lesions.1-3 Within the immune system, dendritic cells (DCs) are pivotal for the activation of immune responses. DCs are generally defined as CD11c+ cells, comprising a collection of distinct cellular subsets that can be discriminated from each other based on additional transmembrane proteins4 and transcription factors.5 DCs are equipped to present fragments of exogenously acquired antigens as peptide/MHC class I complexes to CD8+ T cells, a process referred to as antigen cross-presentation.6,7 Cross-presentation by DCs is essential for developing CD8+ T-cell responses against (cancer) cell-associated antigens, as demonstrated by the lack of cellular immunity against cell-derived antigen in mice depleted of CD11c+ cells.8
Cross-presentation is a specialized process of antigen presentation process for which DCs are particularly well equipped. Other antigen-presenting cells such as B cells, monocytes and macrophages cannot readily stimulate naïve CD8+ T cells after upon encountering exogenous antigens.9 Much effort in the last decade has been dedicated to understanding the mechanisms that underlie optimal cross-presentation. As a result of this effort, specific DC subsets have been shown to possess a superior capacity to cross-present antigens, for instance, CD8+ and CD103+ DCs in mice.6 In 2010, a human counterpart of such a DC subset was found to be characterized by the expression of thrombomodulin (also known as BDCA3 or CD141).10 As these studies unfolded, the idea that specific DC subsets would be superior at cross-presentation was challenged with the postulate that all DCs can cross-present antigens in an optimal fashion when activated by the right conditions.11,12 Inflammatory cytokines, Toll-like receptor ligands and CD40 signaling (as provided by helper CD4+ T cells), can trigger the maturation of DCs and efficiently prime them for antigen cross-presentation.13,14
Uptake of tumor-associated antigens by DCs
The uptake of soluble or complexed antigens by DCs involves various mechanisms including passive pinocytosis, active phagocytosis or translocation via gap junctions.15 The antigen delivery route greatly influences the efficiency of cross-presentation. Hence, antigen cross-presentation can be enhanced by opsonization as well as by specifically targeting antigens to DC surface receptors.16-22 Alternatively, DCs can internalize dying cancer cells, which harbor a wide panel tumor-associated antigens. In this setting, all antigens expressed by the dying cell are theoretically made available for cross-presentation, although only a fraction of antigenic peptides end up being presented as peptide/MHC class I complexes to CD8+ T cells. Researchers have now explored the possibility to use neoplastic cells killed ex vivo as an anticancer vaccine in itself or as a means to load DCs with tumor-associated antigens and thus create DC-based vaccines. Cancer cells can be induced to die ex vivo via different mechanisms, including apoptosis and necrosis. Apoptosis is a form of programmed cell death that can be initiated by multiple signals, eventually resulting in a highly regulated process of intracellular destruction.23,24 This cell death process can take up to 10–20 h. Throughout the execution of apoptosis, one can observe specific alterations of the plasma membrane, but the process also manifests at organized foci within the cytosol and the nucleus of dying cells. Of note, necrosis has long been considered to be a form of incidental cell death that does not follow organized trajectories. Recently, this notion has been challenged by evidence showing that necrosis in vivo is subjected to regulation as well.25 At odds with necrotic cells, apoptotic cells maintain their structural integrity for a prolonged period,26,27 yet eventually will also succumb to the breakdown of the plasma membrane, a setting that is generally referred to as secondary necrosis or late apoptosis.28 From the viewpoint of DCs, (early) apoptotic and necrotic cells presumably constitute very different entities and hence will be handled in a differential manner, with divergent consequences for the elicitation of immune responses.
Approaches to identify different types of cancer cell death
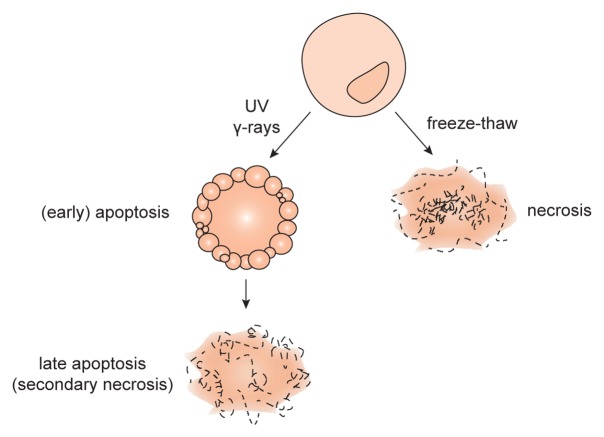
For study purposes, apoptosis is often induced in vitro by introducing DNA damage via irradiation with UV light or γrays (Fig. 1). Apoptotic cells expose phosphatidylserine on the outer leaflet of the plasma membrane,29 rendering this phospholipid available for recognition by Annexin V (AnxV). The integrity of the plasma membrane can be assessed with propidium iodide (PI) or 7-aminoactinomycin D (7-AAD), both of which are not taken up by healthy cells but only by cells with ruptured plasma membranes. Early apoptotic cells can thus be identified by cytofluorometric analyses as AnxV+PI- cells. Experimentally, necrosis is commonly induced by multiple freeze-thaw cycles with liquid nitrogen. Freezing the cells will cause damage to the cell membrane leading to its inevitable rupture. Some investigators induce necrosis by heat or osmotic shock, also resulting in a lethal damage of the plasma membrane. At least theoretically, apoptosis and necrosis are clearly distinguishable from each other. However, inducing cell death in vitro often result in a mixed population of apoptotic and (secondary) necrotic cells. Moreover, some studies refer to late apoptotic cells as to necrotic cells. Such discrepancies can be misleading in crediting specific biological effects to either apoptotic or necrotic cells.
Figure 1. Experimental induction of cell death. Necrosis can be induced by multiple freeze-thaw cycles using liquid nitrogen. In this setting, both the plasma and nuclear membranes are ruptured so that cellular contents leak out and only membrane debris are left. Apoptosis can be induced by radiation with UV light or γ rays. The membrane of apoptotic cells undergo specific alterations, but (at least initially) it remains intact. Eventually, apoptotic cells also lose membrane integrity, a setting that is often referred to as late apoptosis or secondary necrosis.
With these considerations in mind, here we give an overview of studies comparing the ability of apoptotic and necrotic cells to elicit adaptive immune responses against cellular antigens. Herein, we will refer to apoptotic and necrotic cells as they are shown in Figure 1. Moreover, we will discuss how DCs specifically handle dying cells, how this impacts on subsequent immune responses, and which features of dying cells are important for their immunogenicity.
Immune Responses Against Apoptotic vs. Necrotic Cells
In vitro studies of tumor-associated antigen cross-presentation
While immune responses can be raised against antigens harbored by both apoptotic and necrotic cells, the comparison of these 2 cell death modes reveals fundamental differences in their efficiency to elicit adaptive immunity. Given the central position occupied by cross-presentation in the elicitation of adaptive immune responses against cellular antigens,8 this process is often used as a surrogate marker of immunogenicity in vitro. In this setting, DCs are incubated first with either apoptotic or necrotic cells and then with antigen-specific or unfractionated CD8+ T cells, after which cross-presentation is measured in terms of interferon γ (IFNγ) release. Alternatively, the specific lysis of target cells expressing the antigen of choice by activated CD8+ T cells is measured. For several cancer cell lines apoptosis has been shown to be superior to necrosis in facilitating the cross-presentation of tumor-associated antigens to CD8+ T cells by DCs.30,31 These findings were confirmed using primary cancer cells from B-cell lymphoma and melanoma patients.32,33 Of note, when tumor necrosis factor α (TNFα) was supplemented during the incubation of DCs with neoplastic cells, T-cell activation increased and the superior effect of apoptosis over necrosis was lost.33 These studies suggest that an inherent stimulatory effect of apoptotic cells accounts for enhanced CD8+ T-cell activation. Conversely, when non-transformed cells were used as a source of antigens, apoptotic and necrotic cells induced comparable levels of cross-presentation.34,35 In the case of non-transformed cells expressing a specific antigen upon viral infection, apoptosis again turned out to be superior to necrosis at stimulating DC cell cross-presentation.36 Taken together, these data suggest that malignant (or virus-infected) cells, but not their healthy counterparts, are more immunogenic under apoptotic than under necrotic conditions.
The immunogenicity of apoptosis has further been demonstrated in conditions that result in increased numbers of apoptotic cells. Enhanced CD8+ T-cell activation was observed when DCs received cancer cells that were enriched of apoptotic, as opposed to necrotic, cells.37 Interestingly, late apoptotic cells were shown to be even more immunogenic than their early counterparts.38 The same phenomenon was observed by Buttiglieri et al.,39 although the methods for the induction of early and late apoptosis differed in these 2 studies. Thus, the different degree of immunogenicity attained by dying cells in these settings may possibly relate to the specific compounds used to induce cell death instead of the stage of apoptosis.
In vivo studies of tumor-associated antigen cross-presentation
To test the efficacy of dying malignant cells as an anticancer vaccine, they are injected into mice and adaptive immunity against tumor-associated antigens is measured. To this end, CD8+ T cells are isolated from the spleen or lymph nodes and tested for their capacity to lyse tumor cells. Alternatively, mice are re-challenged a week later with living cancer cells of the same type and tumor outgrowth is monitored. Such studies consistently reveal the superior capacity of apoptotic cells for eliciting anti-tumor immune responses.40-44 For example, the differential treatment of established tumors resulting in either the apoptotic or necrotic demise of malignant cells promoted protective or non-protective CD8+ T-cell responses, respectively.41
A different approach to determine the immunogenicity of dying cells in vivo is represented by the injection of DCs that are pulsed with apoptotic or necrotic cells ex vivo. Cancer cell-loaded DCs interact with other immune cells in vivo, thereby eliciting a particular response. Again, DCs loaded with apoptotic cells were superior than DCs loaded with necrotic cells at eliciting efficient CD8+ T-cell responses in both therapeutic and vaccination models.31,45,46 Moreover, the cytolytic activity of splenic T cells was increased when animals received DCs loaded with apoptotic cells as compared with DCs loaded with necrotic cells.45 In contrast, Kotera et al. showed that apoptotic and necrotic cells are equally protective against established tumors as well as against the inoculation of cancer cells in tumor-naïve animals.47
Besides apoptosis, a second routine of programmed cell death exists that involves the breakdown of intracellular material by autophagic vesicles. This type of cell death proved to be favorable from an immunological point of view, inducing antitumor immune responses that were even better than those triggered by apoptotic cells.48 Corroborating these findings, as early as in 2004, Schmitt et al. observed that the inoculation of malignant cells undergoing a caspase-independent cell death mode was more protective than that of cells succumbing to the activation of caspases against a subsequent tumor challenge.49
While in vitro studies remain ambivalent, in vivo studies robustly show that apoptotic cells are the preferential inducers of CD8+ T-cell responses against tumors. However, the experimental design of DC vaccination studies do not always clarify how DCs are specifically handled before injection. For example, in experiments in which dying cells are not removed before the injection of loaded DCs, MHC-deficient cancer cells should be employed to prevent direct presentation. Moreover, when necrotic cells are employed, it is often not stated whether membrane debris or lysates are used. Irrespective of these issues, it is clear that dying cancer cells depend on DCs to trigger antitumor immune responses. This notion has already reached the clinic, since several groups have used DCs loaded with cell lysates as a therapeutic vaccine in patients affected by melanoma and pediatric solid tumors, documenting objective clinical responses in a fraction of these individuals.50-52 It remains a challenge to improve such vaccines by employing optimally immunogenic dying cancer cells instead of necrotic debris.
Impact of Phagosomes Maturation on the Efficiency of Cross-Presentation
Dendritic-cell subsets
Following the discovery that DCs encompass multiple specialized cell types, investigators have demonstrate that murine CD8+ DCs are particularly efficient at cross-presenting cell-associated antigens in lymphoid tissues, while CD103+ DCs are high proficient at doing so in non-lymphoid tissues. This capacity was attributed to the ability of CD8+ DCs to internalize dying cells.53 Indeed, dying cells injected to mice could be traced back to splenic CD8+ DCs.54 Conversely, lung CD103+ (but not CD11b+) DCs were shown to take up apoptotic cells administered intranasally to mice.55 Several subsets of human DCs have also been characterized including BDCA3+ DCs, which are considered as the equivalent of mouse CD8+ DCs, and BDCA1+ DCs. The isolation of circulating BDCA3+ and BDCA1+ DCs allowed for the assessment of their phagocytic capacity in vitro. At odds with their murine counterparts, both BDCA3+ and BDCA1+ human DCs can take up dying cells, yet only the formed was shown to present cell-associated antigens to CD8+ T cells.56 The comparison of lymphoid tissue-derived BDCA3+ and BDCA1+ DCs also revealed an equivalent uptake of dying cells by these 2 DC subsets, but a slightly improved capacity of cross-presentation by BDCA3+ DCs.12
Receptor engagement
Differences in the receptor patterns of DCs that cross-present cell-associated antigens and DCs that do not might account for the distinct functional profile of these DC subsets. C-type lectin domain family 9, member A (CLEC9A, also known as DNGR1), a member of the C-type lectin receptor family, was identified to be selectively expressed to high levels by CD8+, CD103+ and BDCA3+ DCs.57,58 Further investigation showed that CLEC9A can recognize dying cells by binding to F-actin filaments,59 hence promoting the cross-priming of CD8+ T cells.20 Interestingly, CLEC9A- DC subsets can also take up dying cells. Thus, the uptake of dying cells by DCs appears to be independent of CLEC9A. Rather, post-internalization events may be influenced by CLEC9A signaling. Indeed, the activation of CLEC9A did not result in DC activation, as measured by cytokine secretion, but rather caused the co-localization of CLEC9A with markers of early endosomes such as early endosome antigen 1 (EEA1), RAB5A and RAB27A. Likewise, dying cells engulfed by DCs co-localized with RAB5A+ endosomes 60 min upon uptake. Subsequently (240 min after uptake) dying cells were associated with RAB11+ structures, which are considered as recycling endosomes. Of note, such a localization of engulfed dying cells with RAB5A+ and RAB11+ vesicles required the expression of CLEC9A by DCs.60
Receptor engagement can also drive the sorting of endosomal cargo to distinct compartments, such as static and dynamic early endosomes, which mature into recycling or degrading endosomes, respectively.61 Recycling endosomes differ from degrading endosomes in that they maintain a mild acidic lumen whereas degrading endosomes progressively acidify with time. Therefore, the proteolytic activity is lower in recycling endosomes as compared with degrading endosomes. Several studies demonstrate that a reduced proteolytic activity benefits cross-presentation in both mice and human DCs.62-66 As the mannose receptor and CD209 (best-known as DC-SIGN) have been shown to deliver their ligands to slow-maturing endosomes,67 targeting antigens to these receptors can enhance cross-presentation.17,22
Endosomal pH
Data from the last decade suggest that tempering endosomal acidification can benefit antigen cross-presentation. In this regard, it was shown that RAB27A can regulate phagosomal pH by recruiting cytochrome b-245, β polypeptide (CYBB, best known as NOX2) to the phagosomal membrane.63 NOX2 is part of the NADPH oxidase complex that maintains the neutral pH of the endosomal compartment.65 The absence of either RAB27A or NOX2 is detrimental to cross-presentation.63,66 The slow degradation of antigens (as it occurs in a poorly acidic microenvironment) allows for their storage within long-lasting phagosomes. In support of this notion, antigen presentation could be fully recovered when peptides were eluted out of MHC class I molecules on the surface of DCs 16 h after uptake, indicating the antigen was still present within the cell to supplement peptides for MHC class I presentation.68
Therefore, the immunogenicity of as apoptotic cells (upon recognition by CLEC9A) might be the result of their targeting to RAB27A+ storing phagosomes characterized by mild acidification and reduced rates of proteolysis, which constitute optimal compartment for the cross-presentation of cell-associated antigens (Fig. 2).
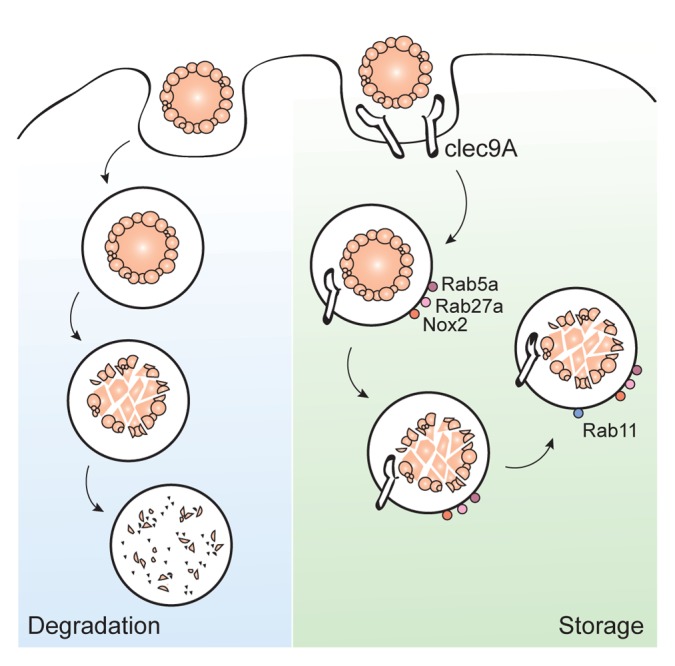
Figure 2. CLEC9A direct apoptotic material toward storage compartments. Apoptotic cells are taken up by dendritic cells (DCs). Upon engagement of C-type lectin domain family 9, member A (CLEC9A) on the DC surface, apoptotic cells are directed to RAB5A+RAB27A+ endosomes. RAB27A rapidly recruits NOX2 to the endosomal membrane, hence preventing an excessive acidification of the maturing endosome and allow for the establishment of a storage compartment. In the absence of CLEC9A the endosomal cargo is quickly dispatcher to lysosomes and fully degraded. CLEC9A thus facilitates the slow degradation and prolonged storage of apoptotic material, a mechanism that may account for the ability of CLEC9A to enhance the cross-presentation of cell-associated antigens.
Apoptotic cells induce prolonged immune responses
The superior cross-priming capacities of apoptotic cells may at least in part originate from the prolonged storage of their antigens within DCs, although the biological bearing for this process are not yet fully understood. The comparison of CD8+ and CD11b+ DCs showed that the cross-presentation of apoptotic material not only was more pronounced in the former, but also lasted for longer periods.69 A third subset of DCs known as merocytic DCs (mcDCs) has been shown to have a cross-presentation activity that nearly exceed that of CD8+ DCs.42 This effect could be blocked by the administration of diphenylene iodonium (DPI), a compound that accelerates endosomal acidification,65,66 suggesting that aberrant antigen processing and storage negatively affect cross-presentation. Indeed, the phagosomal pH remains high in CD8+ and mcDCs but decreases in CD11b+ DCs after antigen uptake. In vivo CD8+ T-cell responses to vaccination with loaded DCs were also stronger and more prolonged when CD8+ or mcDCs were used as compared with when CD11b+ DCs were employed.69 Similar observations were made upon the direct injection of apoptotic or necrotic cancer cells in mice.40 In particular, CD8+ T cells developed a cytolytic activity that lasted for up to 9 d upon the injection of apoptotic cells. Conversely, necrotic cells only induced CD8+ T-cell responses that lasted for up to 4 d, with initial responses also being inferior to those induced by apoptotic cells.40 The histological study of vaccination sites revealed a rapid recruitment of T cells, B cells, macrophages and DCs by both apoptotic and necrotic cells.44 However, T cells and DCs were still present 10 d after vaccination only in case of apoptotic cell-based vaccines.44 The importance of antigen persistence was also established by the use of MHC class I-deficient cells. Such cells were rapidly removed by natural killer (NK) cells and hence were unable to elicit CD8+ T cell responses. However, when NK cells were depleted, MHC class I-deficient cells persisted for sufficient time to induce the cross-priming of antigen-specific CD8+ T cells.70
Taken together, these studies point to a dual role for apoptotic cells in antigen persistence. First, by interfering with phagosomal pH, antigens from apoptotic cells persist longer than antigens from necrotic cells within DCs, resulting in prolonged cross-presentation. Second, beyond stimulating the recruitment of immune cells (as necrotic cells do), apoptotic cells promote their persistence or survival.
A Role Role for Type I Interferons in Antigen Persistence
Upon exposure to apoptotic cells, mcDCs secreted Type I interferons (IFNs) whereas CD8+ DCs or CD11b+ DCs did not.69 Bone marrow-derived DCs stimulated with the FLT3 ligand also respond to apoptotic, but not necrotic cells, by producing Type I interferons.42 In humans, to date only plasmacytoid DCs (pDCs) were found to secrete IFNα upon stimulation with apoptotic cells.71,72
Interestingly, Type I interferons are of great importance for the elicitation of tumor-rejecting immune response in vivo.73 Indeed, blocking the IFN (α and β) receptor 1 (IFNAR1) with specific antibodies significantly increase tumor outgrowth. Similarly, Ifnar1−/− mice exhibit accelerated rates of oncogenesis and tumor progression. The importance of Type I interferons was also shown in a model based on the induction of cancer cell death in vivo. Indeed, the cross-presentation of tumor-associated antigens was diminished in IFNα/β-deficient mice receiving wild-type antigen-specific CD8+ T cells, suggesting that the elicitation of adaptive antitumor immune responses requires IFNα/β sensitivity on immune cells other than CD8+ T cells.74 Indeed, Diamond et al. demonstrated that the induction of antitumor CD8+ T-cell responses relies on the IFNα/β sensitivity of CD8+ DCs.73 In line with this notion, the pre-treatment of CD8+ DCs with IFNα increased the cross-presentation of cell-associated antigens.75 Apoptotic cells were shown to persist for longer periods in IFN-treated DCs as compared with their untreated counterparts. As this phenomenon was sensitive to DPI, Type I interferons may somehow modulate the phagosomal pH of DCs. Human DCs exposed to apoptotic cells also manifested a delayed endosomal acidification as well as the storage of cell-associated antigens in RAB5+ and RAB11+ compartments.76 Additionally, MHC class I molecules were shown to localized to DC antigen storage compartments upon IFNα treatment. Remarkably, IFNα does not only promote the persistence of antigens within DCs, but also the survival of DCs themselves. At least in part, this stems from the fact that CD8+ DCs that internalize apoptotic cells express increased levels of anti-apoptotic proteins such as BCL-2 and BCL-XL.75
In pDCs, IFNα secretion has been associated with the appearance of LC3-coated phagosomes that internalize DNA-immune complexes.77 In macrophages, apoptotic cells were shown to be taken up into LC3-coated phagosomes.78 Moreover, the production of reactive oxygen species (ROS) by NOX2 specifically recruited LC3 toward the endosomal membrane.79 Since apoptotic cells taken up by DCs were shown to indirectly attract NOX2 to phagosomal membranes, it is tempting to speculate that they are also engulfed in LC3-coated structures. However, whether the recruitment of LC3 to phagosomes is required for IFNα secretion by DCs remains elusive.
While it is clear that DCs benefit from IFNα signaling for the cross-presentation of cell-associated antigens, the very same DCs do not per se produce Type I interferons upon exposure to apoptotic cells. Rather, neighboring immune cells have been shown to produce these cytokines when they encounter apoptotic cells. Accordingly, pDCs can help CD11c+ DCs to cross-prime tumor-specific CD8+ T cells in an IFNα-dependent manner.80 Also in a vaccination setting based on CD8+ and mcDCs, the pre-incubation of mcDCs with dying cancer cells in the presence of pDCs increased their survival rates, suggesting a synergism between pDCs and mcDCs in the elicitation of antitumor immune responses.74 Thus, an optimal handling of the apoptotic cells may be achieved by DCs in response to the secretion of IFNα by surrounding cells, including pDCs.
Interactions Between Apoptotic Cells and Dendritic Cells
Recently, the concept of immunogenic cell death has emerges, proposing that cells can die in either a silent or immunogenic way, depending on the lethal stimulus that they received.81-84 Immunogenic cell death appears to be accompanied by the release of so-called “danger-associated molecular patterns” (DAMPs), including high mobility group box 1 HMGB1, heat-shock proteins (HSPs) and ATP. DAMPs are secreted or exposed on the plasma membrane in response to stress or death, hence acquiring the ability to stimulate immune responses.
An apparent paradox emerges when one considers that apoptotic cells display a superior immunogenicity than necrotic cells, while the latter release more DAMPs than the former.48,85,86 Indeed, necrotic cells have been shown to efficiently stimulate the maturation of DCs in vitro,87 whereas apoptotic cells were not as effective at doing so. Such observations would suggest that DAMPs do not endorse the immunogenicity of dying cells. However, several studies show a critical role for DAMPs in the elicitation of antitumor immune responses.
The supernatants of cells succumbing to necrosis was shown to operate as an adjuvant to antigen vaccination,88 an effect that was significantly inhibited when HMGB1-depleted cells were employed. In line with this notion, adding recombinant HMGB1 to cellular antigens enhanced the elicitation of antitumor immunity and the degree of protection conferred to mice against a tumor challenge. These results indicate that HMGB1 is a potent stimulator of adaptive immune responses in the presence of cellular antigen, but not the exclusive one, as its knockdown did not result in a complete rescue phenotype. The importance of HMGB1 was also established by means of blocking antibodies and RNA interference in setting of cross-presentation of cell-associated antigens in vivo and induction of antitumor immunity.89 In particular, HMGB1 was shown to bind Toll-like receptor 4 (TLR4), and Tlr4−/− mice to exhibit a decreased capacity to clear malignant cells.
HSPs have also been shown to operate as DAMPs and accumulating evidence supports their importance in antitumor immune responses.90-92 The cross-presentation of soluble and cell-associated antigens was increased when these were complexed with HSPs.91,93-95 Human recombinant HSP70 did not induce the maturation of DCs, as measured by the expression of co-stimulatory molecules on their surface or cytokine secretion.93 Thus, increased immune signaling is probably not responsible for the enhanced cross-presentation by HSPs. An increased antigen uptake could account for this effect, as the intracellular concentrations of specific peptides were higher when antigens were delivered in complex with HSP70. Similar results were obtained with the ER-resident protein calreticulin. Calreticulin can translocate to the plasma membrane during apoptosis, a condition in which it might function as a DAMP and facilitate the uptake of dying cells by DCs.96 The supplementation of recombinant calreticulin to soluble antigens, however, did not enhance cross-presentation,97 indicating that also calreticulin does not engage in immune signaling with DCs.
Finally, there is evidence for an involvement of the NLR family, pyrin domain containing 3 (NLRP3) inflammasome in DC-mediated adaptive immune responses against tumors. Thus, the exposure of DCs to dying cancer cells activated the NLRP3 inflammasome within DCs, a process that was dependent on the expression of purinergic receptors.98 Purinergic receptors can bind ATP, which secreted in large amounts by dying cells. The NLRP3 inflammasome can mediate the activation of caspase-1, thereby facilitating the secretion of bioactive interleukin-1β (IL-1β). Indeed, mice deficient for caspase-1 or the IL-1β receptor revealed the requirement for an intact IL-1β system for efficient cross-priming. The release of ATP by dying cells is therefore of great importance for antitumor immune responses. This said, TLR ligands have also been observed to stimulate the NLRP3-dependent production of IL-1β by DCs.99 Thus, DAMPs other than ATP may contribute to the activation of the NLRP3 inflammasome in DCs.
In summary, the cross-presentation of cell-associated antigens involves many factors that are either intrinsically expressed by DCs or present in the microenvironment (Table 1). Both apoptosis and the release of DAMPs by necrotic cells are important for the induction of tumor-specific adaptive immune responses. At first glance, these observations do not seem to reconcile but such an interpretation might actually be oversimplistic. As mentioned above, apoptotic cells also go through a necrotic state when the integrity of their plasma membranes is eventually lost. Current definitions point to apoptotic and necrotic cells as to static conformations, but these may actually be considered as the same entity parted by time. As additional insights into this subject are obtained, this apparent paradox will likely be resolved. The regulated changes that are associated with apoptosis are required to make dying cells visible to DCs. Apoptotic cells are thus recognized and internalized, a phenomenon that is presumably stimulated by DAMPs such as calreticulin and HSPs, leading to degradation of the engulfed material. Only when DCs express CLEC9A is the apoptotic material redirected to storage compartments. In the course of recognition and internalization, apoptotic cells are expected to progress along the cascade of events that delineates this cell death modality. One possibility is that apoptotic cells may undergo secondary necrosis within the phagosomal compartment of DCs. In this setting, DAMPs would be released locally and exert immunogenic effects. Because CLEC9A direct the apoptotic material toward long-lived recycling compartments, chances are that recycled TLRs will encounter DAMPs released by dying cells. Alternatively, DAMPs might be released by surrounding dying cells that are not (yet) internalized. In both these scenarios, TLR signaling will activate DCs and cause their maturation, supporting the cross-priming of antigen-specific CD8+ T cells. In contrast, the dispatch of apoptotic material toward degrading lysosomes is expected to result in its complete and rapid degradation, hence delineating an immunologically silent cell death.
Table 1. Factors that contribute to the cross-presentation of tumor-associated antigens by dendritic cells.
| Intrinsic | Ref. | Extrinsic | Ref. |
|---|---|---|---|
| Membrane receptors | Inflammatory cytokines | ||
| CLEC9A | 20 | Type I IFNs | 73–76 |
| Purinergic receptors | 98 | TNFα | 33 |
| CD40 | 14 | GM-CSF | 13 |
| TLR4 | 89 | ||
| HSP receptor | 19 | ||
| Fcγ receptor* | 18, 21 | ||
| CD209 (DC-SIGN)* | 22 | ||
| LY75 (DEC-205)* | 16 | ||
| Cytosolic proteins | DAMPs | ||
|---|---|---|---|
| RAB27A | 63 | HMGB1 | 88, 89 |
| RAC2 | 66 | HSPs | 91, 93–95 |
| NOX2 | 66 | ATP | 98 |
Abbreviations: CLEC9A, C-type lectin domain family 9, member A; GM-CSF, granulocyte macrophage colony-stimulating factor; HMGB1, high mobility group box 1; HSP, heat-shock protein; IFN, interferon; LY75, lymphocyte antigen 75; RAB27A, RAB27A, member RAS oncogene family; RAC2, ras-related C3 botulinum toxin substrate 2; TLR4, Toll-like receptor 4; TNFα, tumor necrosis factor α. *To date not linked to dying cells
As such, apoptotic cells and DAMP release appear to cooperate to elicit optimal CD8+ T-cell responses against (cancer) cell-associated antigens. In particular, apoptotic cells facilitate the efficient uptake and persistence of the antigen, whereas the DAMPs promote the maturation and activation of DCs (Fig. 3).
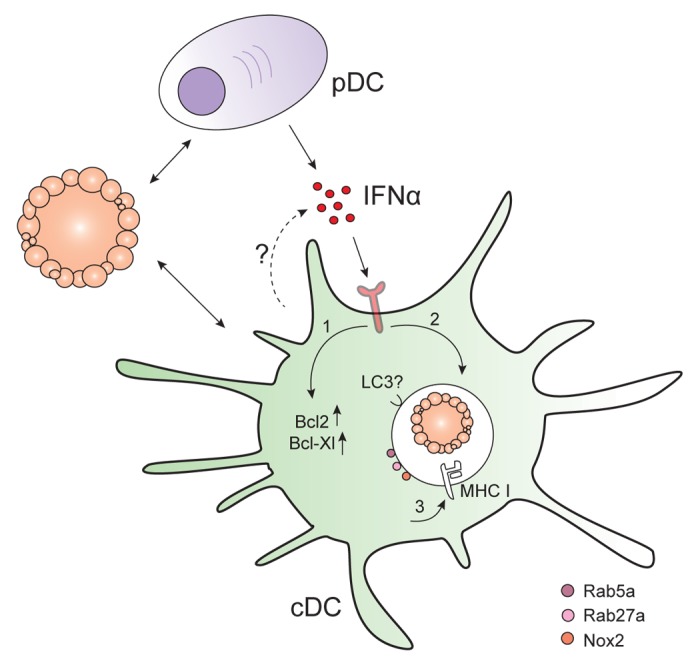
Figure 3. Type I interferon signaling influences antigen persistence within dendritic cells. Plasmacytoid DCs (pDC) and perhaps also conventional DCs (cDC) produce interferon α (IFNα) upon the recognition of apoptotic cells. The exposure of cDCs to IFNα results in1 the upregulation of pro-survival factors such as BCL-2 and BCL-XL;2 the prolonged storage of apoptotic material in intracellular compartments; and3 the localization of MHC class I molecules to storage compartments.
Concluding Remarks
As we learn more about this process, it has become increasing clearer that the cross-presentation of cell-associated antigens by DCs is complex, involving several factors that will eventually determine the outcome of tumor-specific immune responses. An interesting and hitherto unresolved question is whether some DC subsets have a preference for cross-presenting antigens associated with specific forms of cell death. Moreover, it remains to be determined whether a specific combination of cell death-associated parameters and DAMPs truly defines the immunogenicity of the cell demise. Much interest currently goes to understanding the mechanisms of antigen loading and handling by DCs that lead to optimal cross-presentation and cross-priming of CD8+ T cells. Irrespective of whether DAMPs specifically contribute to antigen processing or not, we believe that it is important to understand how adaptive immune responses against tumor cells are raised, especially in the context of DC-vaccines, which have become increasingly appealing as a treatment of cancer patients. DC vaccines have achieved significant results, but there is a clear need (and room) for improvements. By elucidating the processes that are critical for the induction of robust and protracted antitumor immune responses by DCs, we can continue to improve not only DC-based vaccines and but also anticancer immunotherapy in general.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge the Villa Joep Foundation, Ammodo, KiKa, Vrienden Wilhelmina Kinderziekenhuis en NWO/ZonMW for financial support (to S.N. and J.B.).
Citation: Spel L, Boelens J, Nierkens S, Boes M. Anti-tumor immune reactivity mediated by dendritic cells: How signals derived from dying tumor cells drive antigen cross-presentation. OncoImmunology 2013; 2:e26403; 10.4161/onci.26403
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26403
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Progress in the development of immunotherapy for the treatment of patients with cancer. J Intern Med. 2001;250:462–75. doi: 10.1046/j.1365-2796.2001.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collin M, Bigley V, Haniffa M, Hambleton S. Human dendritic cell deficiency: the missing ID? Nat Rev Immunol. 2011;11:575–83. doi: 10.1038/nri3046. [DOI] [PubMed] [Google Scholar]
- 5.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, et al. Immunological Genome Consortium Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–99. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol. 1999;60:562–7. doi: 10.1016/S0198-8859(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 8.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/S1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 10.Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1131–4. doi: 10.1084/jem.20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nierkens S, Tel J, Janssen E, Adema GJ. Antigen cross-presentation by dendritic cell subsets: one general or all sergeants? Trends Immunol. 2013;34:361–70. doi: 10.1016/j.it.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med. 2013;210:1035–47. doi: 10.1084/jem.20121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dresch C, Leverrier Y, Marvel J, Shortman K. Development of antigen cross-presentation capacity in dendritic cells. Trends Immunol. 2012;33:381–8. doi: 10.1016/j.it.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Toes RE, Schoenberger SP, van der Voort EI, Offringa R, Melief CJ. CD40-CD40Ligand interactions and their role in cytotoxic T lymphocyte priming and anti-tumor immunity. Semin Immunol. 1998;10:443–8. doi: 10.1006/smim.1998.0147. [DOI] [PubMed] [Google Scholar]
- 15.Pang B, Neijssen J, Qiao X, Janssen L, Janssen H, Lippuner C, Neefjes J. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J Immunol. 2009;183:1083–90. doi: 10.4049/jimmunol.0900861. [DOI] [PubMed] [Google Scholar]
- 16.Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, Nussenzweig MC, Piperno AG, Steinman RM. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–94. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 18.Flinsenberg TW, Compeer EB, Koning D, Klein M, Amelung FJ, van Baarle D, Boelens JJ, Boes M. Fcγ receptor antigen targeting potentiates cross-presentation by human blood and lymphoid tissue BDCA-3+ dendritic cells. Blood. 2012;120:5163–72. doi: 10.1182/blood-2012-06-434498. [DOI] [PubMed] [Google Scholar]
- 19.Murshid A, Gong J, Calderwood SK. The role of heat shock proteins in antigen cross presentation. Front Immunol. 2012;3:63. doi: 10.3389/fimmu.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, van Schip JJ, Sedlik C, Melief CJ, Verbeek JS, Ossendorp F. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J Immunol. 2002;168:2240–6. doi: 10.4049/jimmunol.168.5.2240. [DOI] [PubMed] [Google Scholar]
- 22.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, et al. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–85. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 23.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/S0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 25.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 26.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–9. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Kanduc D, Mittelman A, Serpico R, Sinigaglia E, Sinha AA, Natale C, Santacroce R, Di Corcia MG, Lucchese A, Dini L, et al. Cell death: apoptosis versus necrosis (review) Int J Oncol. 2002;21:165–70. [PubMed] [Google Scholar]
- 28.Silva MT. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett. 2010;584:4491–9. doi: 10.1016/j.febslet.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Fadok VA, Daleke DL, Henson PM, Bratton DL, de Cathelineau A Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–7. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 30.Schnurr M, Scholz C, Rothenfusser S, Galambos P, Dauer M, Röbe J, Endres S, Eigler A. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells. Cancer Res. 2002;62:2347–52. [PubMed] [Google Scholar]
- 31.Strome SE, Voss S, Wilcox R, Wakefield TL, Tamada K, Flies D, Chapoval A, Lu J, Kasperbauer JL, Padley D, et al. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res. 2002;62:1884–9. [PubMed] [Google Scholar]
- 32.Manches O, Lui G, Molens JP, Sotto JJ, Chaperot L, Plumas J. Whole lymphoma B cells allow efficient cross-presentation of antigens by dendritic cells. Cytotherapy. 2008;10:642–9. doi: 10.1080/14653240802317647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pietra G, Mortarini R, Parmiani G, Anichini A. Phases of apoptosis of melanoma cells, but not of normal melanocytes, differently affect maturation of myeloid dendritic cells. Cancer Res. 2001;61:8218–26. [PubMed] [Google Scholar]
- 34.Ferlazzo G, Semino C, Spaggiari GM, Meta M, Mingari MC, Melioli G. Dendritic cells efficiently cross-prime HLA class I-restricted cytolytic T lymphocytes when pulsed with both apoptotic and necrotic cells but not with soluble cell-derived lysates. Int Immunol. 2000;12:1741–7. doi: 10.1093/intimm/12.12.1741. [DOI] [PubMed] [Google Scholar]
- 35.Fonteneau JF, Kavanagh DG, Lirvall M, Sanders C, Cover TL, Bhardwaj N, Larsson M. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood. 2003;102:4448–55. doi: 10.1182/blood-2003-06-1801. [DOI] [PubMed] [Google Scholar]
- 36.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 37.Galetto A, Buttiglieri S, Forno S, Moro F, Mussa A, Matera L. Drug- and cell-mediated antitumor cytotoxicities modulate cross-presentation of tumor antigens by myeloid dendritic cells. Anticancer Drugs. 2003;14:833–43. doi: 10.1097/00001813-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Brusa D, Garetto S, Chiorino G, Scatolini M, Migliore E, Camussi G, Matera L. Post-apoptotic tumors are more palatable to dendritic cells and enhance their antigen cross-presentation activity. Vaccine. 2008;26:6422–32. doi: 10.1016/j.vaccine.2008.08.063. [DOI] [PubMed] [Google Scholar]
- 39.Buttiglieri S, Galetto A, Forno S, De Andrea M, Matera L. Influence of drug-induced apoptotic death on processing and presentation of tumor antigens by dendritic cells. Int J Cancer. 2003;106:516–20. doi: 10.1002/ijc.11243. [DOI] [PubMed] [Google Scholar]
- 40.Buckwalter MR, Srivastava PK. Mechanism of dichotomy between CD8+ responses elicited by apoptotic and necrotic cells. Cancer Immun. 2013;13:2. [PMC free article] [PubMed] [Google Scholar]
- 41.Gamrekelashvili J, Ormandy LA, Heimesaat MM, Kirschning CJ, Manns MP, Korangy F, Greten TF. Primary sterile necrotic cells fail to cross-prime CD8(+) T cells. Oncoimmunology. 2012;1:1017–26. doi: 10.4161/onci.21098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssen E, Tabeta K, Barnes MJ, Rutschmann S, McBride S, Bahjat KS, Schoenberger SP, Theofilopoulos AN, Beutler B, Hoebe K. Efficient T cell activation via a Toll-Interleukin 1 Receptor-independent pathway. Immunity. 2006;24:787–99. doi: 10.1016/j.immuni.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Ronchetti A, Rovere P, Iezzi G, Galati G, Heltai S, Protti MP, Garancini MP, Manfredi AA, Rugarli C, Bellone M. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J Immunol. 1999;163:130–6. [PubMed] [Google Scholar]
- 44.Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, Manns MP, Greten TF. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer. 2003;103:205–11. doi: 10.1002/ijc.10777. [DOI] [PubMed] [Google Scholar]
- 45.Henry F, Boisteau O, Bretaudeau L, Lieubeau B, Meflah K, Grégoire M. Antigen-presenting cells that phagocytose apoptotic tumor-derived cells are potent tumor vaccines. Cancer Res. 1999;59:3329–32. [PubMed] [Google Scholar]
- 46.Hoffmann TK, Meidenbauer N, Dworacki G, Kanaya H, Whiteside TL. Generation of tumor-specific T-lymphocytes by cross-priming with human dendritic cells ingesting apoptotic tumor cells. Cancer Res. 2000;60:3542–9. [PubMed] [Google Scholar]
- 47.Kotera Y, Shimizu K, Mulé JJ. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001;61:8105–9. [PubMed] [Google Scholar]
- 48.Uhl M, Kepp O, Jusforgues-Saklani H, Vicencio JM, Kroemer G, Albert ML. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ. 2009;16:991–1005. doi: 10.1038/cdd.2009.8. [DOI] [PubMed] [Google Scholar]
- 49.Schmitt E, Parcellier A, Ghiringhelli F, Casares N, Gurbuxani S, Droin N, Hamai A, Pequignot M, Hammann A, Moutet M, et al. Increased immunogenicity of colon cancer cells by selective depletion of cytochrome C. Cancer Res. 2004;64:2705–11. doi: 10.1158/0008-5472.CAN-03-2475. [DOI] [PubMed] [Google Scholar]
- 50.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mulé JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–9. [PubMed] [Google Scholar]
- 51.Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–57. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 52.Salcedo M, Bercovici N, Taylor R, Vereecken P, Massicard S, Duriau D, Vernel-Pauillac F, Boyer A, Baron-Bodo V, Mallard E, et al. Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother. 2006;55:819–29. doi: 10.1007/s00262-005-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulz O, Reis e Sousa C. Cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–9. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, Jakubzick CV. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 2011;208:1789–97. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–60. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. 2008;283:16693–701. doi: 10.1074/jbc.M709923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sancho D, Mourão-Sá D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang JG, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S, Tullett KM, Robin AY, Brammananth R, van Delft MF, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity. 2012;36:646–57. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Zelenay S, Keller AM, Whitney PG, Schraml BU, Deddouche S, Rogers NC, Schulz O, Sancho D, Reis e Sousa C. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J Clin Invest. 2012;122:1615–27. doi: 10.1172/JCI60644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–4. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 63.Jancic C, Savina A, Wasmeier C, Tolmachova T, El-Benna J, Dang PM, Pascolo S, Gougerot-Pocidalo MA, Raposo G, Seabra MC, et al. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9:367–78. doi: 10.1038/ncb1552. [DOI] [PubMed] [Google Scholar]
- 64.Mantegazza AR, Savina A, Vermeulen M, Pérez L, Geffner J, Hermine O, Rosenzweig SD, Faure F, Amigorena S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–22. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Duménil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–18. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 66.Savina A, Peres A, Cebrian I, Carmo N, Moita C, Hacohen N, Moita LF, Amigorena S. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 2009;30:544–55. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–6. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 68.van Montfoort N, Camps MG, Khan S, Filippov DV, Weterings JJ, Griffith JM, Geuze HJ, van Hall T, Verbeek JS, Melief CJ, et al. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc Natl Acad Sci U S A. 2009;106:6730–5. doi: 10.1073/pnas.0900969106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reboulet RA, Hennies CM, Garcia Z, Nierkens S, Janssen EM. Prolonged antigen storage endows merocytic dendritic cells with enhanced capacity to prime anti-tumor responses in tumor-bearing mice. J Immunol. 2010;185:3337–47. doi: 10.4049/jimmunol.1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jusforgues-Saklani H, Uhl M, Blachère N, Lemaître F, Lantz O, Bousso P, Braun D, Moon JJ, Albert ML. Antigen persistence is required for dendritic cell licensing and CD8+ T cell cross-priming. J Immunol. 2008;181:3067–76. doi: 10.4049/jimmunol.181.5.3067. [DOI] [PubMed] [Google Scholar]
- 71.Heyder P, Bekeredjian-Ding I, Parcina M, Blank N, Ho AD, Herrmann M, Lorenz HM, Heeg K, Schiller M. Purified apoptotic bodies stimulate plasmacytoid dendritic cells to produce IFN-alpha. Autoimmunity. 2007;40:331–2. doi: 10.1080/08916930701356515. [DOI] [PubMed] [Google Scholar]
- 72.Schiller M, Parcina M, Heyder P, Foermer S, Ostrop J, Leo A, Heeg K, Herrmann M, Lorenz HM, Bekeredjian-Ding I. Induction of type I IFN is a physiological immune reaction to apoptotic cell-derived membrane microparticles. J Immunol. 2012;189:1747–56. doi: 10.4049/jimmunol.1100631. [DOI] [PubMed] [Google Scholar]
- 73.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nierkens S, den Brok MH, Garcia Z, Togher S, Wagenaars J, Wassink M, Boon L, Ruers TJ, Figdor CG, Schoenberger SP, et al. Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res. 2011;71:6428–37. doi: 10.1158/0008-5472.CAN-11-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lorenzi S, Mattei F, Sistigu A, Bracci L, Spadaro F, Sanchez M, Spada M, Belardelli F, Gabriele L, Schiavoni G. Type I IFNs control antigen retention and survival of CD8α(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J Immunol. 2011;186:5142–50. doi: 10.4049/jimmunol.1004163. [DOI] [PubMed] [Google Scholar]
- 76.Spadaro F, Lapenta C, Donati S, Abalsamo L, Barnaba V, Belardelli F, Santini SM, Ferrantini M. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119:1407–17. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 77.Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, et al. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–97. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci U S A. 2011;108:17396–401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang J, Brumell JH. NADPH oxidases contribute to autophagy regulation. Autophagy. 2009;5:887–9. doi: 10.4161/auto.9125. [DOI] [PubMed] [Google Scholar]
- 80.Shimizu K, Asakura M, Shinga J, Sato Y, Kitahara S, Hoshino K, Kaisho T, Schoenberger SP, Ezaki T, Fujii S. Invariant NKT cells induce plasmacytoid dendritic cell (DC) cross-talk with conventional DCs for efficient memory CD8+ T cell induction. J Immunol. 2013;190:5609–19. doi: 10.4049/jimmunol.1300033. [DOI] [PubMed] [Google Scholar]
- 81.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoves S, Sutton VR, Haynes NM, Hawkins ED, Fernández Ruiz D, Baschuk N, Sedelies KA, Schnurr M, Stagg J, Andrews DM, et al. A critical role for granzymes in antigen cross-presentation through regulating phagocytosis of killed tumor cells. J Immunol. 2011;187:1166–75. doi: 10.4049/jimmunol.1001670. [DOI] [PubMed] [Google Scholar]
- 83.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 84.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 85.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 86.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 87.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 90.Chen X, Tao Q, Yu H, Zhang L, Cao X. Tumor cell membrane-bound heat shock protein 70 elicits antitumor immunity. Immunol Lett. 2002;84:81–7. doi: 10.1016/S0165-2478(02)00042-1. [DOI] [PubMed] [Google Scholar]
- 91.Dai J, Liu B, Caudill MM, Zheng H, Qiao Y, Podack ER, Li Z. Cell surface expression of heat shock protein gp96 enhances cross-presentation of cellular antigens and the generation of tumor-specific T cell memory. Cancer Immun. 2003;3:1. [PubMed] [Google Scholar]
- 92.Wang MH, Grossmann ME, Young CY. Forced expression of heat-shock protein 70 increases the secretion of Hsp70 and provides protection against tumour growth. Br J Cancer. 2004;90:926–31. doi: 10.1038/sj.bjc.6601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bendz H, Ruhland SC, Pandya MJ, Hainzl O, Riegelsberger S, Braüchle C, Mayer MP, Buchner J, Issels RD, Noessner E. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem. 2007;282:31688–702. doi: 10.1074/jbc.M704129200. [DOI] [PubMed] [Google Scholar]
- 94.Kurotaki T, Tamura Y, Ueda G, Oura J, Kutomi G, Hirohashi Y, Sahara H, Torigoe T, Hiratsuka H, Sunakawa H, et al. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol. 2007;179:1803–13. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- 95.Susumu S, Nagata Y, Ito S, Matsuo M, Valmori D, Yui K, Udono H, Kanematsu T. Cross-presentation of NY-ESO-1 cytotoxic T lymphocyte epitope fused to human heat shock cognate protein 70 by dendritic cells. Cancer Sci. 2008;99:107–12. doi: 10.1111/j.1349-7006.2007.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 97.Del Cid N, Shen L, Belleisle J, Raghavan M. Assessment of roles for calreticulin in the cross-presentation of soluble and bead-associated antigens. PLoS One. 2012;7:e41727. doi: 10.1371/journal.pone.0041727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 99.He Y, Franchi L, Núñez G. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013;190:334–9. doi: 10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]