Abstract
Phenotypic characterization of Akt1 and Igf2 null mice has revealed roles for each in the regulation of placentation, and fetal and postnatal growth. Insulin-like growth factor 2 (IGF2) is encoded by the Igf2 gene and influences cellular function, at least in part, through activation of an intracellular serine/threonine kinase called AKT1. Akt1 and Igf2 null mice were originally characterized on inbred and mixed genetic backgrounds, prohibiting direct comparisons of their phenotypes. The impact of loss of AKT1 or IGF2 on placental, fetal, and postnatal function were examined following transfer of Akt1 and Igf2 null mutations to an outbred CD1 genetic background. Disruption of IGF2 did not affect AKT expression or activation. Both Akt1−/− and Igf2−/− mice exhibited decreased placental weight, fetal weight and viability. Deregulation of placental growth was similar in Akt1 and Igf2 nulls; however, disruption of Igf2 had a more severe impact on prenatal survival and postnatal growth. Placental structure, including organization of junctional and labyrinth zones and development of the interstitial, invasive, trophoblast lineage, were similar in mutant and wild-type mice. Akt1 and Igf2 null mutations affected postnatal growth. The relative impact of each gene differed during pre-weaning versus post-weaning growth phases. AKT1 had a more significant role during pre-weaning growth, whereas IGF2 was a bigger contributor to post-weaning growth. Akt1 and Igf2 null mutations impact placental, fetal and postnatal growth. Placental phenotypes are similar; however, fetal and postnatal growth patterns are unique to each mutation.
Keywords: AKT1, IGF2, trophoblast, placentation
Introduction
The maternal-fetal interface is a dynamic site where uterine and placental structures cooperate to promote the development of the fetus. These specialized tissues facilitate efficient nutrient delivery. When trophoblast cells develop abnormally their ability to function as a link between mother and fetus is altered and can lead to diseases such as preeclampsia and fetal growth restriction, resulting in pregnancy failure (Pijnenborg et al., 1981; Kaufmann et al., 2003), or longer term adverse effects on postnatal development and adult health (Bateson et al., 2004; Gluckman and Hanson 2004). Rodent models are useful tools for studying mammalian development and pregnancy.
Several studies have linked the phosphatidylinositol 3 kinase (PI3K)/AKT signaling pathway to placental development and postnatal growth. PI3K is a lipid kinase that phosphorylates phosphatidylinositol and phosphoinositides (Engelman et al., 2006). The cellular actions of several growth factors, including insulin-like growth factors (IGFs), are mediated through activation of PI3K (Cantley 2002; Fayard et al., 2005; Manning and Cantley 2007). PI3K initiates a signaling cascade leading to activation of a serine/threonine kinase called AKT (Cantley 2002; Brazil et al., 2004; Manning and Cantley 2007). AKT exists as three isoforms (AKT1, AKT2, AKT3) acting on an overlapping set of substrates involved in many cellular processes including: metabolism, cell cycle, survival, protein synthesis, and differentiation (Coffer et al., 1998; Brazil and Hemmings 2001; Engelman et al., 2006; Gonzalez and McGraw 2009). Small molecule inhibitors of PI3K disrupt activation of AKT in trophoblast stem cells and interfere with their differentiation towards endocrine and invasive phenotypes (Kamei et al., 2001; Kent et al., 2010, 2011). Several mutant mouse models impacting the PI3K/AKT signaling pathway exhibit disruptions in placental and postnatal development. Most notable are Akt1−/− and Igf2−/− mice. Mutations of Akt1 or Igf2 genes result in similar phenotypes, including fetal loss, and decreased placental, fetal and postnatal growth (DeChiara et al., 1990; Lopez et al., 1996; Chen et al., 2001; Cho et al., 2001; Constância et al., 2002; Yang et al., 2003; Plaks et al., 2011).
Akt1−/− and Igf2−/− placentas possess few glycogen positive trophoblast cells (Lopez et al., 1996; Yang et al., 2003). Glycogen cells are initially situated within the junctional zone compartment of the placenta and subsequently move into the uterine decidua beginning at gestation day 13.5 (Ain et al., 2003; Bouillot et al., 2006; Coan et al., 2006). One of the key functions of invasive trophoblast cells is the remodeling of uterine spiral arterioles. This remodeling transforms tightly coiled spiral arterioles into dilated vessels, which facilitates nutrient delivery (Pijnenborg et al., 1981; Kaufmann et al., 2003; Adamson et al., 2002).
In addition to the pregnancy phenotype Akt1−/− mice are unable to support their pups after birth due to multiple lactation defects resulting in a decrease in milk production (Boxer et al., 2006; Maroulakou et al., 2008). AKT1 may also have a role in cell survival, as overexpression of AKT1 in mice delays mammary gland involution (Ackler et al., 2002). Although, IGF2 has been implicated in mammary gland development (Brisken et al., 2002; Hovey et al., 2003), lactational insufficiency has not been reported in Igf2−/− mice.
Collectively, the reported phenotypes for Akt1−/− and Igf2−/− mice are difficult to directly compare because the mutations were evaluated on different genetic backgrounds. The Akt1−/− mutation was analyzed on a C57BL/6 mouse genetic background (Cho et al., 2001), whereas the Igf2−/− mutation was evaluated on a mixed C57BL/6 × 129 mouse genetic background (DeChiara et al., 1990). Individual inbred strains possess strain-specific modifier genes that can impact phenotypes resulting from specific genetic mutations (Montagutelli 2000; Doetschman 2009). In contrast, outbred mouse stocks possess greater allelic variation and are less susceptible to strain-specific modifier genes (Montagutelli 2000; Chia et al., 2005). Outbred mouse stocks also exhibit robust reproductive performance, which can be advantageous when investigating certain genetic mutations affecting fertility. In this report, we transferred Akt1 and Igf2 null mutations to the outbred CD1 mouse by backcrossing. Some similarities and some differences were noted in the placental, fetal, and postnatal phenotypes.
Results
AKT and IGF2 expression in the mouse placenta
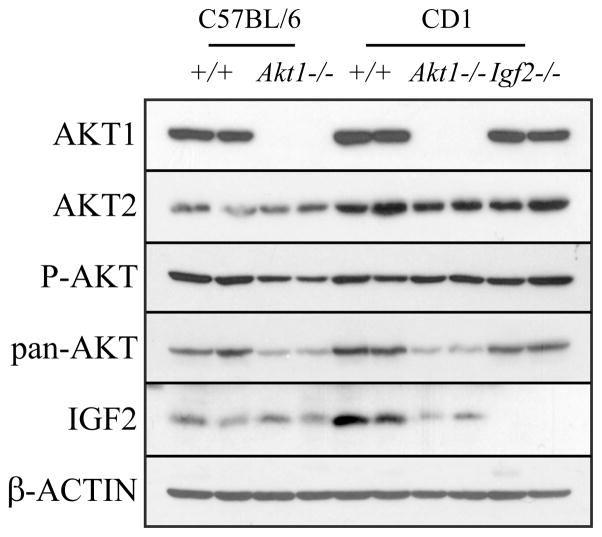
IGF2 and AKT regulate cell proliferation, survival, and differentiation (Cantley 2002; Fayard et al., 2005; Manning and Cantley 2007; McDonald et al., 2007). IGF2 is a member of the insulin/relaxin growth factor family and is capable of activating AKT signaling pathways (Claeys et al., 2002; Lu et al., 2005; McDonald et al., 2007). Akt1−/− and Igf2−/− mutations were successfully transferred to the CD1 genetic background. Gestation day 17.5 placentas were examined for their expression of AKT isoforms, AKT activation, and expression of IGF2 (Fig. 1). For all experiments, homozygous wild-type or homozygous mutant breeding pairs were used to generate wild-type and mutant placentas and offspring.
Fig. 1. Western blot analysis of AKT and IGF2 expression and AKT activation in wild-type, Akt1 null, and Igf2 null placentas.
Whole cell lysates were prepared from wild-type, Akt1−/− or Igf2 −/− gestational day 17.5 placentas from mice with a C57BL/6 or CD1 genetic background. Antibodies to AKT1, AKT2, phosphorylated AKT Ser 473 (P-AKT), total AKT (pan-AKT), and IGF2 were used in the analyses. Western blot analysis for β-ACTIN was included as a loading control.
As expected, AKT1 was absent from Akt1−/− placentas (Fig. 1). Total AKT was decreased in Akt1 null placentas, indicating that the predominant AKT isoform in the placenta is AKT1. Akt1−/− C57BL/6 placentas showed a modest decrease in AKT activation (P-AKT), whereas P-AKT levels in CD1 placentas were not affected by the Akt1 null mutation, suggesting that AKT2 and AKT3 isoforms may be more effective in compensating for the AKT1 deficiency on the CD1 genetic background. Placental AKT2 protein concentrations were not affected by Akt1 gene disruption.
Western blot analysis of Igf2−/− placentas showed no detectable expression of IGF2 protein confirming the null mutation (Fig. 1). Most importantly, disruption of IGF2 did not affect AKT protein expression or AKT activation.
These results verify the integrity of the null mice and demonstrate AKT isoform compensation and a disconnection between IGF2 and AKT activation within the placenta.
Placental and fetal growth in Akt1 and Igf2 mutant embryos
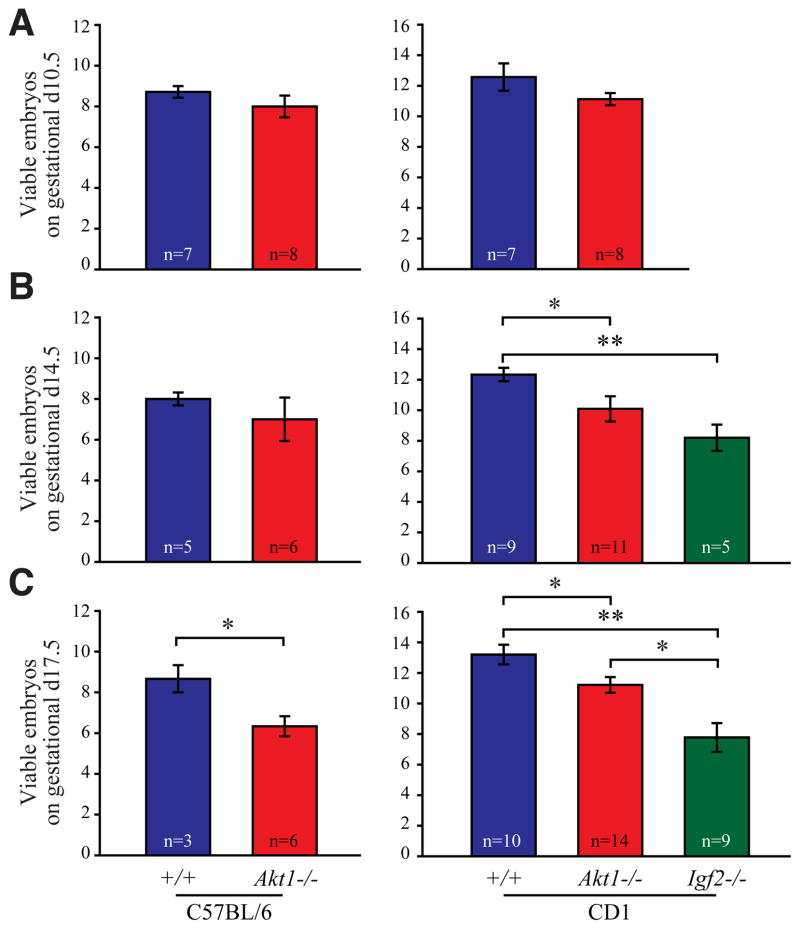
We next evaluated the impact of disruption of Akt1 or Igf2 genes on placental and fetal development. Viability of placental-fetal sites was monitored at gestation days 10.5, 14.5, and 17.5 (Fig. 2). Placental-fetal loss for C57BL/6 Akt1 nulls was significantly greater than for wild-type controls on gestation day 17.5 (Fig. 2C), whereas placental-fetal loss for CD1 Akt1 and CD1 Igf2 nulls was significantly greater than for wild-type controls on both gestation days 14.5 and 17.5 (Fig. 2 B,C).
Fig. 2. Embryonic/fetal survival is decreased in Akt1 and Igf2 nulls.
Pregnant wild-type and Akt1 nulls on C57BL/6 and CD1 genetic backgrounds were prepared, sacrificed on gestation days 10.5 (A), 14.5 (B), and 17.5 (C), and viable fetuses counted. Similar analyses were performed on CD1 Igf2 nulls. Bars represent the mean ± standard error of the mean (SEM). Numbers of litters evaluated are indicated. Values significantly different from controls are indicated with an asterisk (*P<0.05, **P<0.001).
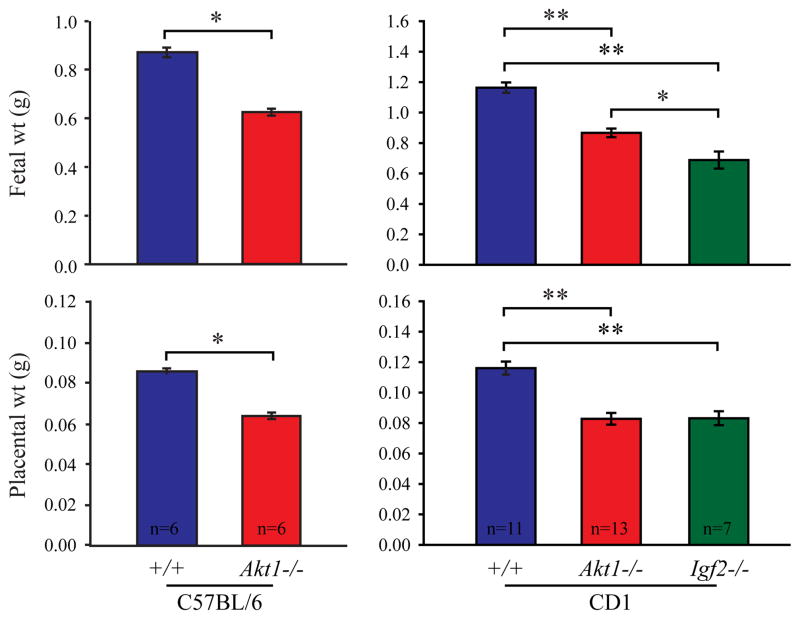
Placental and fetal growth responses were monitored on gestation day 17.5 (Fig. 3). Placental and fetal weights were significantly lower in Akt1−/− and Igf2−/− when compared to wild-type. The magnitude of the placental weight decrease was comparable in Akt1 and Igf2 nulls; whereas Igf2 nulls exhibited a significantly greater fetal growth restriction than did Akt1 nulls (Fig. 3).
Fig. 3. Akt−/− and Igf2−/− fetuses and placentas are growth restricted.
Pregnant wild-type and Akt1 nulls on C57BL/6 and CD1 genetic backgrounds were prepared and sacrificed on gestation day 17.5. Fetuses and placentas were dissected and weighed. Similar analyses were performed on CD1 Igf2 nulls. Bars represent the mean ± SEM. Numbers of litters evaluated are indicated. Values significantly different from controls are indicated with an asterisk (*P<0.05, **P<0.001).
The results suggest that AKT1 and IGF2 contribute to the health and growth of the placenta and fetus.
Placentation in Akt1 and Igf2 nulls
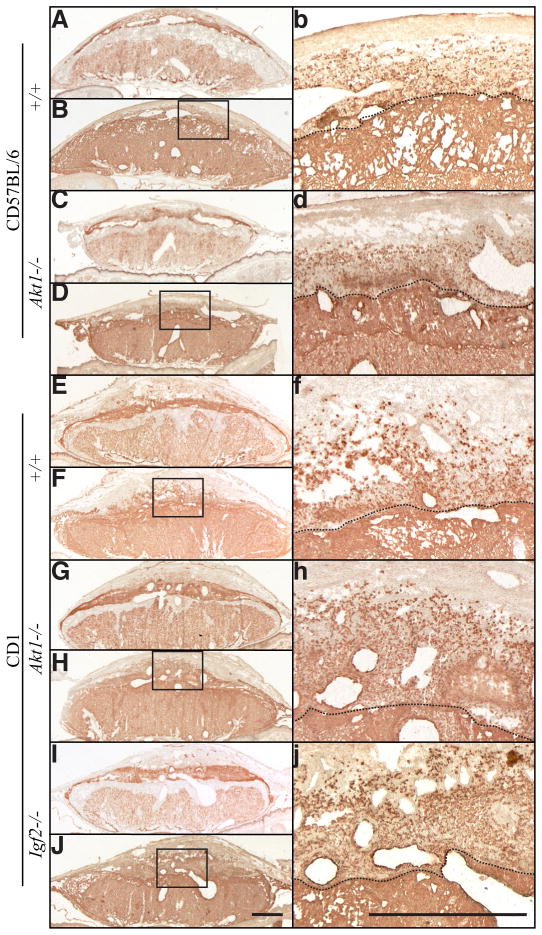
The mature mouse hemochorial placentation site is organized into three compartments: i) labyrinth zone (site of maternal-fetal exchange); ii) junctional zone (situated at the uterine interface); iii) mesometrial compartment, which includes the uterine decidua, invasive trophoblast, and the metrial gland. These compartments can be easily distinguished using Isolectin B4 to identify the compartments (labyrinth zone: positive; junctional zone: negative; uterine decidua: positive) and cytokeratin immunostaining to locate the intrauterine invasive trophoblast cells. Growth restricted null and wild-type placentation sites were histologically examined at gestation day 17.5 (Fig. 4). Placentation sites from each group contained recognizable labyrinth zone, junctional zone, and uterine mesometrial compartments. Ratios of junctional zone/labyrinth zone among the null and wild-type placentation sites did not significantly differ. Interstitial invasive trophoblast cells were identified throughout the uterine decidua of mutant and wild-type placentation sites (Fig. 4). The relative abundance of invasive trophoblast cells and their depth of invasion were also comparable among all strains.
Fig. 4. Organization of the placentation site in wild-type and Akt1 and Igf2 nulls.
Isolectin B4 binding (A,C,E,G,I) and cytokeratin immunocytochemistry (B,D,F,H,J) were performed on placentation sites from gestation day 17.5 pregnant wild-type and Akt1 nulls on C57BL/6 and CD1 genetic backgrounds. Similar analyses were performed on CD1 Igf2 nulls. High magnification of the boxed areas in (B,D,F,H,J) are shown in images labeled with the respective lower case letters. The dashed line indicates the decidua-junctional zone interface. Scale bars, 1 mm.
The absence of functional AKT or IGF2 affects the size of the placenta but does not affect the structural organization of the placentation site.
Postnatal development in Akt1 and Igf2 nulls
AKT1 and IGF2 have been implicated as regulators of postnatal viability, growth, and development (DeChiara et al., 1990; Cho et al., 2001). In our experimentation, genetic background influenced postnatal survival. Female C57BL/6 mice possessing the Akt1 null mutation were not able to support postnatal offspring survival (Table 1). Such findings are consistent with earlier reports indicating a vital role for AKT1 in mammary gland development and lactation (Boxer et al., 2006; Maroulakou et al., 2008). In contrast, a high percentage of female CD1 mice possessing the Akt1 null mutation (88%) were able to sustain sufficient lactation to support postnatal growth and survival (Table 1). Female CD1 wild-type and Igf2 null mice exhibited effective maternal support for their offspring (Table 1).
TABLE 1.
EFFECTS OF AKT1, IGF2, AND GENETIC BACKGROUND ON POSTNATAL SURVIVAL
| Genotype | Number of litters examined | Litter sizea | Percent viable littersa | Sex ratio (male:female)a |
|---|---|---|---|---|
| C57BL/6 +/+ | 10 | 6.8 ± 0.49 | 100 | 30:38 |
| C57BL/6 Akt1−/− | 11 | 4b | 9.1c | 2:2b |
| CD1+/+ | 10 | 12.30 ± 1.07 | 100 | 63:60 |
| CD1 Akt−/− | 26 | 8.56 ± 0.52d | 88.5 | 105:91 |
| CD1 Igf2−/− | 6 | 6.83 ± 0.48d | 100 | 19:22 |
Determined on day 20 postpartum;
Based on data from the one litter with live pups on day 20 postpartum;
Significantly different from all other groups, P<0.005;
Significantly different from CD1+/+, P<0.005.
Offspring sex ratios can be influenced by maternal factors (Rosenfeld et al., 2003; Rosenfeld et al., 2004). Although, disruption of Akt1 or Igf2 genes affected the maternal environment, these manipulations did not alter offspring sex ratios (Table 1).
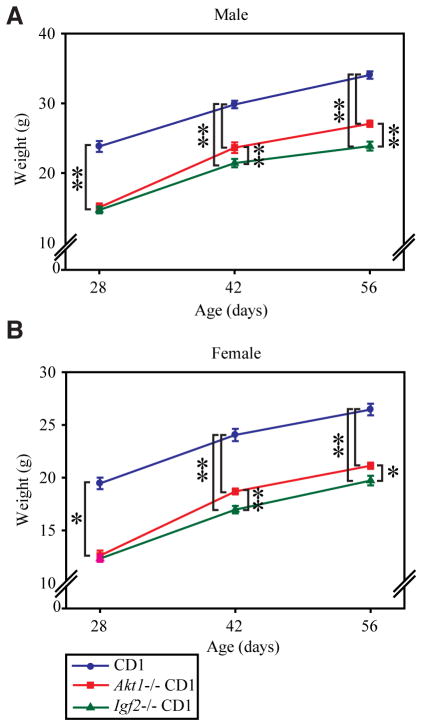
Akt1 and Igf2 null male and female progeny showed a significant decrease in postnatal growth (Fig. 5). At the time of weaning, there were no significant differences between Akt1 and Igf2 null pup weights (Fig. 5). However, post-weaning weight gain was significantly less in Igf2−/− than in Akt1−/− offspring (Fig. 5).
Fig. 5. Akt1 and Igf2 null mice exhibit decreased postnatal growth.
Body weights of wild-type, Akt1−/−, and Igf2−/− pups on a CD1 genetic background were measured at 28, 42 and 56 days after birth. Bars represent the mean ± SEM. 18–31 pups from 4–8 litters were weighed for each genotype and sex at each time point: males (A) and females (B). Values significantly different from controls are indicated with an asterisk (*P<0.05, **P<0.001).
In summary, the effects of AKT1 on pre-weaning postnatal survival are influenced by maternal genetic background. Both AKT1 and IGF2 contribute to post-weaning growth, with IGF2 possessing a greater role.
Discussion
AKT1 and IGF2 contribute to the regulation of placentation and prenatal and postnatal growth. These insights were largely derived from experimentation with mice possessing null mutations for Akt1 and Igf2 genes (DeChiara et al., 1990; Lopez et al., 1996; Chen et al., 2001; Cho et al., 2001; Constância et al., 2002; Yang et al., 2003). Reported phenotypes of mice with the respective null alleles are similar but difficult to directly compare because genetic backgrounds of the mutant mice in the previous analyses differed. In the present report, we have investigated placentation and postnatal growth phenotypes in CD1 mice possessing Akt1 or Igf2 null alleles.
Disruptions in either the Akt1 gene or the Igf2 gene result in similar placental phenotypes. Placentation sites were smaller for both mutants; however, the organization of the mouse placentation site into recognizable compartments (labyrinth zone, junctional zone, and uterine mesometrial region) was not affected by either mutation. Previous reports described deficiencies in glycogen positive trophoblast cell numbers from both Akt1 and Igf2 null placentas (Lopez et al., 1996; Yang et al., 2003). Glycogen positive trophoblast cells accumulate in the junctional zone after midgestation and subsequently invade into the uterine decidua (Ain et al., 2003; Bouillot et al., 2006; Coan et al., 2006), inferring that Akt1 and Igf2 signaling pathways may regulate development of the interstitial invasive trophoblast lineage (Simmons et al., 2005). This notion was further supported by in vitro experiments demonstrating roles for AKT and IGF2 in trophoblast invasion (McKinnon et al., 2001; Qiu et al., 2004a; Qiu et al., 2004b; Miller et al., 2005). In the present report, we did not observe a deficit in interstitial trophoblast invasion accompanying either mutation. Cytokeratin immunostaining proved to be an effective means for identifying the interstitially invasive trophoblast cells. The defect exhibited in the Akt1 and Igf2 null placentas is not in the derivation of the “glycogen/interstitial invasive” trophoblast lineage or their ability to invade into the uterus but instead in their ability to accumulate glycogen. PI3K/AKT and IGF2 signaling can regulate key enzymes promoting cellular glycogen storage (Binoux 1995; Phillips et al., 1998; Whiteman et al., 2002; Woodgett, 2005; Liang et al., 2010). The similarities in placentation phenotypes suggest that AKT1 and IGF2 are acting as part of a single sequential pathway or equivalent parallel pathways.
Postnatal pre-weaning development was more severely affected by a deficiency in the Akt1 gene than a deficiency of the Igf2 gene. This phenotype is likely related to the actions of AKT1 on lactation. Previous reports indicated that AKT1 contributes to development of a competent lactating mammary gland (Boxer et al., 2006; Maroulakou et al., 2008). Based on our findings, the relative importance of AKT1 in lactation is dictated by the genetic background of the mutant mice. As previously demonstrated, AKT1 disruption on a C57BL/6 genetic background profoundly affects maternal pre-weaning offspring support. Pups die shortly following birth. In contrast, the Akt1 null mutation on a CD1 genetic background results in a much less severe phenotype, resulting in a high rate of offspring survival. Even though, CD1 Akt1 null pre-weaning development was superior to C57BL/6 Akt1 null pre-weaning development, it was inferior to CD1 Igf2 null pre-weaning development. Igf2 null fetuses are significantly smaller than Akt1 null fetuses at gestation day 17.5 but caught up to Akt1 null offspring by postnatal day 28. Thus, C57BL/6 and CD1 genetic backgrounds may differ in their abilities to compensate for a deficiency in AKT1.
AKT1 and IGF2 are important factors regulating fetal and postnatal growth (Efstratiadis 1998; Hill et al., 1998; Simmen et al., 1998; Allan et al., 2001; Han and Carter 2001; Whiteman et al., 2002; Randhawa 2008). Deficiencies in IGF2 have more profound effects on fetal and post-weaning growth than do AKT1 deficiencies (present study). These phenotypic differences are probably attributed to the spectrum of signaling pathways activated by IGF2 and necessitated by developmental changes in nutritional sources and metabolic demands. Insulin and IGF1 receptor tyrosine kinases mediate IGF2 activation of several intracellular signaling cascades, which include but are not limited to PI3K/AKT, and are also not restricted to AKT1 (Laviola et al., 2007; Werner et al., 2008; Gallagher and LeRoith 2010). IGF2 is also capable of modulating cellular function through interactions with IGF receptor 2 (Hawkes et al., 2007; Brown et al., 2009; El-Shewy and Luttrell 2009). Thus, the breadth of IGF2 regulation of fetal and postnatal growth probably extends to other signaling mechanisms beyond activation of AKT1.
Genetic background influences phenotypes of genetically manipulated mice (Doetschman 2009). Some aspects of the Akt1 null phenotype differed when evaluated on the C57BL/6 versus the outbred CD1 genetic backgrounds. These phenotypic differences indicate the existence of allelic variation within the mouse capable of modifying AKT1 signaling. The CD1 outbred mouse provided a means of demonstrating the existence of these modifiers and could be used to search for quantitative trait loci associated with AKT1 signaling; however, the outbred mouse may not be the most effective tool for this purpose. Genetic backgrounds of outbred mouse stocks are not well characterized and breeding practices can affect their stability (Chia et al., 2005). In contrast, inbred mouse strains possess well-characterized and stable genomes. Screening inbred mouse strains for phenotypic variations in AKT1 signaling may represent a more effective approach for determining the most appropriate parent strains for congenic analysis and subsequent identification of modifier genes (Doetschman 2009). Characterization of recombinant inbred mouse strains represents an alternative strategy for investigating genetic modifiers affecting AKT1 signaling (Threadgill et al., 2011)
In summary, AKT1 and IGF2 possess similar effects on placentation; however, they differentially affect fetal and postnatal growth. IGF2 has a prominent role in fetal and post-weaning growth, while AKT1 via its actions on lactational competence contributes significantly to pre-weaning growth. It is also important to acknowledge that AKT1 and IGF2 are not the sole regulators of placentation and fetal and postnatal growth; but instead are part of complex regulatory networks (Efstratiadis 1998; Simmons and Cross 2005; Cianfarani et al., 2007; Randhawa 2008; Knofler 2010).
Materials and Methods
Reagents
Antibodies specific for pan-AKT, phospho-Ser 473 AKT, AKT1, and AKT2 were obtained from Cell Signaling Technology (Danvers, MA). Antibodies to IGF2 and β-ACTIN (ACTB; clone AC-15) were obtained from Millipore (Billerica, MA) and Sigma/Aldrich (St. Louis, MO), respectively. Rat monoclonal anti-mouse cytokeratin antibody TROMA-1 is from the Developmental Studies Hybridoma Repository (Iowa City, IA). Reagents for SDS-PAGE were obtained from Bio-Rad Laboratories Inc (Richmond, CA). ECL Western blotting detection reagents were purchased from Millipore. All other reagents were purchased from Sigma unless otherwise noted.
Animals, genotyping, and tissue dissections and collection
CD1 and C57BL/6 wild-type mice were obtained from Charles River (Wilmington, MA) and Jackson Laboratories (Bar Harbor, ME), respectively. Dr. Morris Birnbaum, University of Pennsylvania (Philadelphis, PA) provided the Akt1 null mice (Cho et al., 2001) and Dr. Argiris Efstratiadis, Columbia University (New York, NY) provided the Igf2 null mice (DeChiara et al., 1990). The Akt1 null mutation was on a C57BL/6 genetic background and the Igf2 null mutation was on a mixed genetic background. Akt1 and Igf2 null mutations were systematically transferred to a CD1 genetic background through backcrossing to CD1 wild-type mice for more than six generations. Genomic DNA was extracted from tail biopsies and PCR was used to assess the genotype. The following primers were used to detect the listed alleles:
Akt1 wild-type allele
Forward primer: 5′AGCTCTTCTTCCACCTGTCTC3′
Reverse primer: 5′GCTCCATAAGCACACCTTCAGG3′ (Cho et al., 2001)
Igf2 wild-type allele
Forward primer: 5′GTGGGTGTGGTTAAGCTGCAA3′
Reverse primer: 5′GTCCGAACAGACAAACTGAA3′
and null alleles were determined by presence of the neomycin cassette
Forward primer: 5′GATGTTTCGCTTGGTGGTCG3′
Reverse primer: 5′GCTTGGGTGGAGAGGCTATT3′.
Timed pregnancies were generated by cohabitation of female and male animals. The presence of a copulatory plug was designated day 0.5 of pregnancy. Mouse placentation sites were collected on gestational days 10.5, 14.5, and 17.5. Tissue for histological sections were frozen in heptane and stored at −80°C until sections (10 μm) were prepared with a cryostat. Tissue samples for RNA and protein extraction were frozen in liquid nitrogen and stored at −80°C. RNA was extracted from tissues using TRIzol (Invitrogen Carlsbad, CA). Whole tissue lysates were prepared using radioimmuno-precipitation buffer (RIPA: 10 mM Tris-HCl, pH 7.2, 1% Triton X-100 or 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 5 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin). The animals were housed in an environmentally controlled facility, with lights on from 0600–2000 h and were allowed free access to food and water. The University of Kansas Animal Care and Use Committee approved protocols for the care and use of animals.
Western blotting analysis
Samples were resolved by SDS-PAGE. Separated proteins were electrophoretically transferred to nitrocellulose. Filters were probed with the designated antibodies overnight at 4°C. Blots were incubated with horseradish peroxidase-conjugated antibodies to rabbit IgG for 1 h at room temperature. Antibodies were used at the following dilutions: pan-AKT (1:2000), phospho-Ser 473 AKT (1:2000), AKT1 (1:1000), AKT2 (1:1000), IGF2 (1:2000) and β-ACTIN (1:8000). Blots were incubated with horseradish peroxidase-conjugated antibodies to rabbit (Cell Signaling, 1:2000) or mouse (Sigma, 1:2000) IgG for 1 h at room temperature.
Immunohistochemistry and lectin-binding histochemistry
Trophoblast cells were monitored with a rat monoclonal anti-mouse cytokeratin antibody, TROMA-1 (Alam et al., 2007). Tissue sections were fixed in ice-cold 4% paraformaldehyde and blocked in 10% normal goat serum for 30 min at room temperature. Primary antibody incubation was for 1 h at room temperature with antibodies specific for cytokeratin (1:20). Sections were incubated with biotin labeled secondary antibodies for 30 min at room temperature and avidin conjugated peroxidase for 30 min at room temperature followed by color development with an AEC kit (Zymed Laboratories, San Francisco, CA).
The basic architecture of the mouse placenta was visualized using biotinylated Griffonia simplicifolia lectin (Isolectin B4; Vector Laboratories, Peterborough, UK) (Alam et al., 2007). Tissue sections were fixed in ice-cold 4% paraformaldehyde, blocked in 10% normal goat serum for 30 min at room temperature, and incubated with Isolectin B4 (5 μg/ml) for 1 h at room temperature. Avidin conjugated peroxidase was added for 30 min at room temperature followed by color development with an AEC kit (Zymed Laboratories, San Francisco, CA).
Morphologic measurements of the sizes of the placental zones and depth of trophoblast invasion were determined from tissue sections of gestation day 17.5 placentation sites using National Institutes of Health Image J software (Konno et al., 2007; Rosario et al., 2008). Placental zones were identified using Isolectin B4 staining and the depth of trophoblast invasion was determined by cytokeratin immunostaining. Cross-sectional surface area measurements were made from a histologic plane at the center of each placentation site perpendicular to the flat fetal surface of the placenta (Konno et al., 2007). Definitions of each compartment within the placentation site (labyrinth zone, junctional zone, and uterine mesometrial compartment have been described (Alam et al., 2007). Labyrinth zone and uterine mesometrial compartments bind Isolectin B4, whereas the junctional zone is negative.
All tissues were counterstained with Mayer’s hematoxylin. Bright field and fluorescence images were captured using Leica MZFLIII equipped with CCD cameras (Leica Microsystems GmbH, Welzlar, Germany).
Statistical analysis
Statistical comparisons of two means were performed with Student’s t-test or Welch’s t-test. Comparisons of multiple groups were evaluated with analysis of variance. The source of variation from significant F-ratios was determined with Tukey’s HSD Multiple Comparison Test. Statistical analyses were performed using the R Statistical Package (http://www.r-project.org/). Chi-square tests were used to determine whether there was a significant difference in the survival of litters between the different mouse strains.
Acknowledgments
The authors thank Dr. Morris Birnbaum (University of Pennsylvania, Philadelphia, PA) and Dr. Argiris Efstratiadis (Columbia University, New York, NY) for providing the Akt1 and Igf2 null mice, respectively. We would also like to thank Brent Canham and Dr. Pengli Bu for technical assistance and advice.
Abbreviations used in this paper
- ACTB
β-actin
- IGF2
insulin-like growth factor 2
- PI3K
phosphatidylinositol 3 kinase
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
- ACKLER S, AHMAD S, TOBIAS C, JOHNSON MD, GLAZER RI. Delayed mammary gland involution in MMTV-AKT1 transgenic mice. Oncogene. 2002;21:198–206. doi: 10.1038/sj.onc.1205052. [DOI] [PubMed] [Google Scholar]
- ADAMSON SL, LU Y, WHITELEY KJ, HOLMYARD D, HEMBERGER M, PFARRER C, CROSS JC. Interactions between Trophoblast Cells and the Maternal and Fetal Circulation in the Mouse Placenta. Dev Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- AIN R, CANHAM LN, SOARES MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- ALAM SMK, KONNO T, DAI G, LU L, WANG D, DUNMORE JH, GODWIN AR, SOARES MJ. A uterine decidual cell cytokine ensures pregnancy-dependent adaptations to a physiological stressor. Development. 2007;134:407–415. doi: 10.1242/dev.02743. [DOI] [PubMed] [Google Scholar]
- ALLAN GJ, FLINT DJ, PATEL K. Insulin-like growth factor axis during embryonic development. Reproduction. 2001;122:31–39. doi: 10.1530/rep.0.1220031. [DOI] [PubMed] [Google Scholar]
- BATESON P, BARKER D, CLUTTON-BROCK T, DEB D, D’UDINE B, FOLEY RA, GLUCKMAN P, GODFREY K, KIRKWOOD T, LAHR MM, MCNAMARA J, METCALFE NB, MONAGHAN P, SPENCER HG, SULTAN SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- BINOUX M. The IGF system in metabolism regulation. Diabete Metab. 1995;21:330–337. [PubMed] [Google Scholar]
- BOUILLOT S, RAMPON C, TILLET E, HUBER P. Tracing the glycogen cells with protocadherin 12 during mouse placenta development. Placenta. 2006;27:882–888. doi: 10.1016/j.placenta.2005.09.009. [DOI] [PubMed] [Google Scholar]
- BOXER RB, STAIRS DB, DUGAN KD, NOTARFRANCESCO KL, PORTOCARRERO C, KEISTER BA, BELKA GK, CHO H, RATHMELL JC, THOMPSON C, BIRNBAUM MJ, CHODOSH LA. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab. 2006;4:475–490. doi: 10.1016/j.cmet.2006.10.011. [DOI] [PubMed] [Google Scholar]
- BRAZIL DP, HEMMINGS BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- BRAZIL DP, YANG Z-Z, HEMMINGS BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- BRISKEN C, AYYANNAN A, NGUYEN C, HEINEMAN A, REINHARDT F, TAN J, DEY SK, DOTTO GP, WEINBERG RA, JAN T. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell. 2002;3:877–887. doi: 10.1016/s1534-5807(02)00365-9. [DOI] [PubMed] [Google Scholar]
- BROWN J, JONES EY, FORBES BE. Interactions of IGF-II with the IGF2R/cation-independent mannose-6-phosphate receptor mechanism and biological outcomes. Vitam Horm. 2009;80:699–719. doi: 10.1016/S0083-6729(08)00625-0. [DOI] [PubMed] [Google Scholar]
- CANTLEY LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- CHEN WS, XU PZ, GOTTLOB K, CHEN ML, SOKOL K, SHIYANOVA T, RONINSON I, WENG W, SUZUKI R, TOBE K, KADOWAKI T, HAY N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIA R, ACHILLI F, FESTING MFW, FISHER EMC. The origins and uses of mouse outbred stocks. Nat Genet. 2005;37:1181–1186. doi: 10.1038/ng1665. [DOI] [PubMed] [Google Scholar]
- CHO H, THORVALDSEN JL, CHU Q, FENG F, BIRNBAUM MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- CIANFARANI S, GEREMIA C, PUGLIANIELLO A, MAIORANA A, GERMAN D. Late effects of disturbed IGF signaling in congenital diseases. Endocr Dev. 2007;11:16–27. doi: 10.1159/000111054. [DOI] [PubMed] [Google Scholar]
- CLAEYS I, SIMONET G, POELS J, VAN LOY T, VERCAMMEN L, DE LOOF A, VANDEN BROECK J. Insulin-related peptides and their conserved signal transduction pathway. Peptides. 2002;23:807–816. doi: 10.1016/s0196-9781(01)00666-0. [DOI] [PubMed] [Google Scholar]
- COAN PM, CONROY N, BURTON GJ, FERGUSON-SMITH AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn. 2006;235:3280–3294. doi: 10.1002/dvdy.20981. [DOI] [PubMed] [Google Scholar]
- COFFER PJ, JIN J, WOODGETT JR. Protein kinase B (c-Akt): a multi-functional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335 (Pt 1):1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSTÂNCIA M, HEMBERGER M, HUGHES J, DEAN W, FERGUSON-SMITH A, FUNDELE R, STEWART F, KELSEY G, FOWDEN A, SIBLEY C, REIK W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- DECHIARA TM, EFSTRATIADIS A, ROBERTSEN EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- DOETSCHMAN T. Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol. 2009;530:4223–433. doi: 10.1007/978-1-59745-471-1_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSTRATIADIS A. Genetics of mouse growth. Int J Dev Biol. 1998;42:955–976. [PubMed] [Google Scholar]
- EL-SHEWY HM, LUTTRELL LM. Insulin-like growth factor-2/mannose-6 phosphate receptors. Vitam Horm. 2009;80:667–697. doi: 10.1016/S0083-6729(08)00624-9. [DOI] [PubMed] [Google Scholar]
- ENGELMAN JA, LUO J, CANTLEY LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- FAYARD E, TINTIGNAC LA, BAUDRY A, HEMMINGS BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- GALLAGHER EJ, LEROITH D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–618. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLUCKMAN PD, HANSON MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- GONZALEZ E, MCGRAW TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN VK, CARTER AM. Control of growth and development of the fetoplacental unit. Curr Opin Pharmacol. 2001;1:632–640. doi: 10.1016/s1471-4892(01)00108-4. [DOI] [PubMed] [Google Scholar]
- HAWKES C, AMRITRAJ A, MACDONALD RG, JHAMANDAS JH, KAR S. Heterotrimeric G proteins and the single-transmembrane domain IGF-II/M6P receptor: functional interaction and relevance to cell signaling. Mol Neurobiol. 2007;35:329–345. doi: 10.1007/s12035-007-0021-2. [DOI] [PubMed] [Google Scholar]
- Hill DJ, Petrik J, Arany E. Growth factors and the regulation of fetal growth. Diabetes Care. 1998;21(Suppl 2):B60–B69. [PubMed] [Google Scholar]
- HOVEY RC, HARRIS J, HADSELL DL, LEE AV, ORMANDY CJ, VONDERHAAR BK. Local insulin-like growth factor-II mediates prolactin-induced mammary gland development. Mol Endocrinol. 2003;17:460–471. doi: 10.1210/me.2002-0214. [DOI] [PubMed] [Google Scholar]
- KAMEI T, JONES SR, CHAPMAN BM, MCGONIGLE KL, DAI G, SOARES MJ. The phosphatidylinositol 3-kinase/Akt signaling pathway modulates the endocrine differentiation of trophoblast cells. Mol Endocrinol. 2002;16:1469–1481. doi: 10.1210/mend.16.7.0878. [DOI] [PubMed] [Google Scholar]
- KAUFMANN P, BLACK S, HUPPERTZ B. Endovascular Trophoblast Invasion: Implications for the Pathogenesis of Intrauterine Growth Retardation and Preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- KENT LN, KONNO T, SOARES MJ. Phosphatidylinositol 3 kinase modulation of trophoblast cell differentiation. BMC Dev Biol. 2010;10:97. doi: 10.1186/1471-213X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENT LN, RUMI MAK, KUBOTA K, LEE DS, SOARES MJ. FOSL1 is integral to establishing the maternal-fetal interface. Mol Cell Biol. 2011;31:4801–4813. doi: 10.1128/MCB.05780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOFLER M. Critical growth factor and signaling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54:269–280. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONNO T, REMPEL LA, ARROYO JA, SOARES MJ. Pregnancy in the brown Norway rat: a model for investigating the genetics of placentation. Biol Reprod. 2007;76:709–718. doi: 10.1095/biolreprod.106.056481. [DOI] [PubMed] [Google Scholar]
- LAVIOLA L, NATALICCHIO A, GIORGINO F. The IGF-I signaling pathway. Curr Pharm Des. 2007;13:663–669. doi: 10.2174/138161207780249146. [DOI] [PubMed] [Google Scholar]
- LIANG L, GUO WH, ESQUILIANO DR, ASAI M, RODRIGUEZ S, GIRAUD J, KUSHNER JA, WHITE MF, LOPEZ MF. Insulin-like growth factor 2 and the insulin receptor, but not insulin, regulate fetal hepatic glycogen synthesis. Endocrinology. 2010;151:741–747. doi: 10.1210/en.2009-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPEZ MF, DIKKES P, ZURAKOWSKI D, VILLA-KOMAROFF L. Insulin-like growth factor II affects the appearance and glycogen content of glycogen cells in the murine placenta. Endocrinology. 1996;137:2100–2108. doi: 10.1210/endo.137.5.8612553. [DOI] [PubMed] [Google Scholar]
- LU C, LAM HN, MENON RK. New members of the insulin family: regulators of metabolism, growth and now.. reproduction. Pediatr Res. 2005;57:70R–73R. doi: 10.1203/01.PDR.0000159573.55187.CA. [DOI] [PubMed] [Google Scholar]
- MANNING BD, CANTLEY LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAROULAKOU IG, OEMLER W, NABER SP, KLEBBA I, KUPERWASSER C, TSICHLIS PN. Distinct roles of the three Akt isoforms in lactogenic differentiation and involution. J Cell Physiol. 2008;217:468–477. doi: 10.1002/jcp.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD TJ, NIJLAND MJ, NATHANIELSZ PW. The insulin-like growth factor system and the fetal brain: effects of poor maternal nutrition. Rev Endocr Metab Disord. 2007;8:71–84. doi: 10.1007/s11154-007-9044-2. [DOI] [PubMed] [Google Scholar]
- MCKINNON T, CHAKRABORTY C, GLEESON LM, CHIDIAC P, LALA PK. Stimulation of human extravillous trophoblast migration by IGF-II is mediated by IGF type 2 receptor involving inhibitory G protein(s) and phosphorylation of MAPK. J Clin Endocrinol Metab. 2001;86:3665–3674. doi: 10.1210/jcem.86.8.7711. [DOI] [PubMed] [Google Scholar]
- MILLER AG, APLIN JD, WESTWOOD M. Adenovirally mediated expression of insulin-like growth factors enhances the function of first trimester placental fibroblasts. J Clin Endocrinol Metab. 2005;90:379–385. doi: 10.1210/jc.2004-1052. [DOI] [PubMed] [Google Scholar]
- MONTAGUTELLI X. Effect of the genetic background on the phenotype of mouse mutations. J Am Soc Nephrol. 2000;11:S101–S105. [PubMed] [Google Scholar]
- PHILLIPS LS, PAO CI, VILLAFUERTE BC. Molecular regulation of insulin-like growth factor-I and its principal binding protein, IGFBP-3. Prog Nucleic Acid Res Mol Biol. 1998;60:195–265. doi: 10.1016/s0079-6603(08)60894-6. [DOI] [PubMed] [Google Scholar]
- PIJNENBORG R, BLAND JM, ROBERTSON WB, DIXON G, BROSENS I. The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta. 1981;2:303–316. doi: 10.1016/s0143-4004(81)80027-6. [DOI] [PubMed] [Google Scholar]
- PLAKS V, BERKOVITZ E, VANDOORNE K, BERKUTZKI T, DAMARI GM, HAFFNER R, DEKEL N, HEMMINGS BA, NEEMAN M, HARMELIN A. Survival and size are differentially regulated by placental and fetal PKBalpha/AKT1 in mice. Biol Reprod. 2011;84:537–545. doi: 10.1095/biolreprod.110.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDHAWA RS. The insulin-like growth factor system and fetal growth restriction. Pediatr Endocrinol Rev. 2008;6:235–240. [PubMed] [Google Scholar]
- ROSARIO GX, KONNO T, SOARES MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIU Q, YANG M, TSANG BK, GRUSLIN A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004a;128:355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- QIU Q, YANG M, TSANG BK, GRUSLIN A. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod. 2004b;10:677–684. doi: 10.1093/molehr/gah088. [DOI] [PubMed] [Google Scholar]
- RANDHAWA RS. The insulin-like growth factor system and fetal growth restriction. Pediatr Endocrinol Rev. 2008;6:235–240. [PubMed] [Google Scholar]
- ROSENFELD CS, ROBERTS RM. Maternal diet and other factors affecting offspring sex ratio: a review. Biol Reprod. 2004;71:1063–1070. doi: 10.1095/biolreprod.104.030890. [DOI] [PubMed] [Google Scholar]
- ROSENFELD CS, GRIMM KM, LIVINGSTON KA, BROKMAN AM, LAMBERSON WE, ROBERTS RM. Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc Natl Acad Sci USA. 2003;100:4628–4632. doi: 10.1073/pnas.0330808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMMEN FA, BADINGA L, GREEN ML, KWAK I, SONG S, SIMMEN RC. The porcine insulin-like growth factor system: at the interface of nutrition, growth and reproduction. J Nutr. 1998;128:315S–320S. doi: 10.1093/jn/128.2.315S. [DOI] [PubMed] [Google Scholar]
- SIMMONS DG, CROSS JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- THREADGILL DW, MILLER DR, CHURCHILL GA, DE VILLENA FP. The collaborative cross: a recombinant inbred mouse population for the systems genetic era. ILAR J. 2011;52:24–31. doi: 10.1093/ilar.52.1.24. [DOI] [PubMed] [Google Scholar]
- WERNER H, WEINSTEIN D, BENTOV I. Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways. Arch Physiol Biochem. 2008;114:17–22. doi: 10.1080/13813450801900694. [DOI] [PubMed] [Google Scholar]
- WHITEMAN EL, CHO H, BIRNBAUM MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- WOODGETT JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- YANG Z-Z, TSCHOPP O, HEMMINGS-MIESZCZAK M, FENG J, BRODBECK D, PERENTES E, HEMMINGS BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]