Abstract
Vitamin D levels in people of African descent are often described as inadequate or deficient. Whether low vitamin D levels in people of African descent lead to compromised bone or cardiometabolic health is unknown. Clarity on this issue is essential because if clinically significant vitamin D deficiency is present, vitamin D supplementation is necessary. However, if vitamin D is metabolically sufficient, vitamin D supplementation could be wasteful of scarce resources and even harmful. In this review vitamin D physiology is described with a focus on issues specific to populations of African descent such as the influence of melanin on endogenous vitamin D production and lactose intolerance on the willingness of people to ingest vitamin D fortified foods. Then data on the relationship of vitamin D to bone and cardiometabolic health in people of African descent are evaluated.
Introduction
In populations throughout the African diaspora low vitamin D levels are anticipated. However, there is a paucity of data and hence uncertainty about vitamin D status in people living in Africa. Nonetheless, the most critical gap in existing knowledge is whether low levels of vitamin D are healthy and appropriate for African descent populations or whether levels of vitamin D considered to be low by the Institute of Medicine (IOM) and the Endocrine Society represent a health issue1-2.
Historically, vitamin D research has focused on bone metabolism and calcium balance. Our understanding of the contribution of vitamin D to health now extends from skeletal integrity to cardiometabolic health and beyond. The essential discovery was the identification in many cell types that there is a vitamin D receptor (VDR) within both the nucleus and plasma membrane caveolae3. In the nucleus, vitamin D in the form of 1,25-dihydroxyvitamin D (1,25(OH)2D) acts as a steroid hormone, joining with the nuclear VDR to form a transcription-factor complex which controls the expression of over 200 genes3-4. Beyond its genomic activity 1,25(OH)2D interacts with the VDR on the plasma membrane and thereby influences a host of intracellular actions3-5.
In this review we focus on the effect of vitamin D on bone and cardiometabolic conditions specifically: obesity, diabetes, hypertension and cardiovascular disease. To understand the relationship of vitamin D to bone health, we examined data in three groups: sub-Saharan Africans, African-Americans and the African diaspora beyond the United States. Due to the depth of the literature, the cardiometabolic health section is focused on African-Americans but has important implications for sub-Saharan Africa and the African diaspora.
Vitamin D
Vitamin D Sources and Pathway
Vitamin D has two forms, D2 and D3. But vitamin D is not a classic vitamin5-6. By definition, a vitamin is a nutrient that cannot be produced endogenously but is essential for good health and must be ingested. Yet one of the two forms, D3, can be produced in the epidermal layer of sun-exposed skin by conversion from 7-dehydrocholesterol (Figure). However, sun exposure is rarely sufficient to meet vitamin D requirements. Therefore vitamin D must be obtained from either dietary sources or supplements (4). Vitamin D2 is found in fungi such as mushrooms, while vitamin D3 is available in oily fish (e.g. salmon, sardines, herring, anchovies, trout, cod). Vitamin D supplements may provide either D2 or D3. Because of greater potency and a longer duration of action most supplements contain D37-8. In the United States, all forms of milk (fluid, concentrated, dry, evaporated), calcium fortified juice drinks (orange juice) and ready to eat cereals are fortified with vitamin D39-11.
Figure.
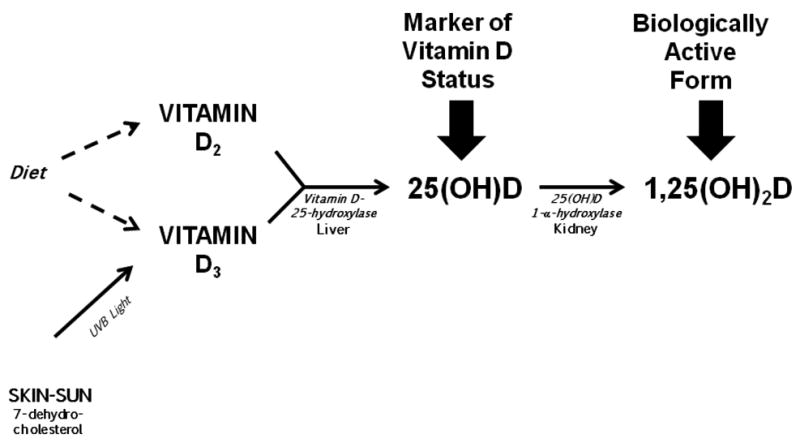
Vitamin D pathway from precursors to active form.
In the liver D2 and D3 become hydroxlyated at the 25-carbon position leading to the formation of the biochemically stable compound, 25-hydroxyvitamin D (25(OH)D) (Figure) 6. Even though 25(OH)D is used clinically as a marker of vitamin D sufficiency, it is not biologically active. To become biologically active, 25(OH)D must undergo a second hydroxylation by 1α-hydroxylase to form 1,25-dihydroxyvitamin D (1,25(OH)2D) (Figure). This enzyme was first identified in the kidney which is the most important physiological source of 1,25(OH)2D, but has subsequently been found in adipose tissue and in a myriad of other tissues including bone and pancreatic cells, vascular tissue and cardiac muscle12-14. Both 25(OH)D and 1,25(OH)2D, are deactivated by hydroxylation at the 24-carbon position, to ultimately form calcitroic acid, which is excreted in the urine 15.
Vitamin D Production and Ingestion in People of African Descent
Melanin Pigmentation
Irradiation of 7-dehydrocholesterol in the epidermal layer of sun-exposed skin leads to the production of vitamin D3. The amount of vitamin D3 produced depends on the balance between the availability of ultraviolet B (UVB) light and the amount of melanin in the epidermis. UVB refers to ultraviolet light with wavelengths between 280-315 nm and is the specific form of ultraviolet light necessary for the conversion of 7-dehydrocholesterol to vitamin D3. UVB exposure is greatest at the equator and declines at higher and lower latitudes. Therefore residents of countries in equatorial Africa receive more UVB than their counterparts in North America, Europe, Australia and even North and South Africa6, 16.
Due to high exposure to UVB, Africans living in equatorial countries should have high endogenous production of vitamin D3. However, melanin in the epidermis competes with 7-dehydrocholesterol for UVB absorption16. Therefore, when skin melanin content is high, longer periods of sun exposure are necessary for vitamin D3 synthesis. Overall the endogenous production of vitamin D3 depends on both latitudinal position and melanin content. As equatorial Africans move to countries at latitudes distant from their country of birth, the competitive effect of increased melanin pigmentation on endogenous vitamin D3 synthesis may be magnified due to less UVB exposure16.
Vitamin D Fortification of Dairy Products and Lactose Intolerance
In the nineteen thirties the Food and Drug Administration (FDA) approved the addition of vitamin D to foods. Consequently many foods were fortified with vitamin D including milk, soda, bread and even beer and hot dogs11. In the nineteen fifties after several serious cases of vitamin D intoxication in England, the FDA decided to limit vitamin D fortification in the United States to milk and cereal products. With this decrease in fortification, vitamin D deficiency in the United States recurred. In 2003 to reverse persistently low vitamin D levels, the FDA ruled that vitamin D could be added to juices that were already fortified with calcium11. Nonetheless, fortified milk remains the major source of dietary vitamin D in the United States10, 17.
Active avoidance of milk and milk products is common in adults of African descent. Lactose, a sugar found in milk, is a disaccharide composed of glucose and galactose. In humans the enzyme, lactase, is responsible for the digestion of this sugar. If the enzyme is active, symptoms associated with the ingestion of milk are minimal or absent. In whites and some Asian populations the activity of the enzyme persists throughout life and lactose can be ingested without symptoms18-19. However in populations of African descent, lactase persistence into adulthood is uncommon and gastrointestinal symptoms occur when any food or beverage with lactose is ingested20. The symptoms of lactose maldigestion include flatulence, abdominal discomfort and diarrhea. In the United States, 80% of African- Americans are lactose maldigesters compared to only 15% of white Americans21. In addition, African-Americans have a high rate of self-diagnosed lactose intolerance and out of fear of symptoms consume few dairy products22. Hence, the avoidance of dairy products due to either real or perceived lactose maldigestion is a major obstacle to providing vitamin D supplemented dairy products to African-Americans22.
III. Vitamin D Thresholds
Even though vitamin D has been recognized as a critical nutrient since the second decade of the twentieth century23, the level of vitamin D considered sufficient has been debated for decades. To address this controversy, the IOM convened the Committee to Review Dietary Reference Intakes for Vitamin D and Calcium and the Endocrine Society established a Task Force to develop an Endocrine Society Guideline1-2. Both groups undertook a rigorous and systematic review of the literature. The IOM and Endocrine Society agree that vitamin D is important for bone health and the role of vitamin D in extraskeletal diseases including cardiometabolic health is uncertain1-2, 24. Nonetheless, there is disagreement that centers on the level of 25(OH)D which must be achieved to ensure bone health. According to IOM guidelines, 25(OH)D levels ≥20 ng/mL are sufficient but the Endocrine Society believes that levels of ≥30 ng/mL are necessary (Table 1). This disagreement may not be relevant to populations of African descent. The majority of studies in African descent populations, report that 25(OH)D levels are <20 ng/mL with little or no adverse effect on bones detectable25-26.
Table. Institute of Medicine and Endocrine Society Vitamin D Thresholds1-2.
| Institute of Medicine | Endocrine Society | ||
|---|---|---|---|
| Vitamin D Status | 25(OH)D (ng/mL) | Vitamin D Status | 25(OH)D (ng/mL) |
| Deficiency | <12 | Deficiency | <20 |
|
| |||
| Inadequacy | 12-19 | Insufficiency | 21-29 |
|
| |||
| Sufficiency | 20-50 | Sufficiency | 30-100 |
|
| |||
| Possible Harm | >50 | ||
IV. Vitamin D Levels in Populations of African Descent
African-Americans
National Health and Nutrition Examination Surveys (NHANES) have consistently found that African-Americans have lower 25(OH)D levels than whites and Mexican-Americans27-30. For example, NHANES 2000 to 2004 data revealed mean 25(OH)D in African-Americans were 16.1 ng/mL versus 22.0 ng/mL in Mexican Americans and 26.7 ng/mL in white Americans30. Or in another words national datasets almost uniformly reveal that mean 25(OH)D levels in African-Americans is <20 ng/mL. According to both the IOM and the Endocrine Society levels of 25(OH)D<20 ng/mL are not sufficient1-2.
Africans and African Immigrants
Data on vitamin D levels in healthy African adults is scant. Therefore, a consensus has not been reached on the vitamin D status of Africans. We were able to identify 11 studies which reported vitamin D levels in Africans or African immigrants. Four studies reported vitamin D levels in equatorial Africans, three in South Africans and four in Africans immigrants to Europe or the United States31-41.
Three of the four studies from equatorial Africa suggest Africans have high vitamin D levels but one clearly reported low levels31, 37-39. For example, Aspray et al. analyzed vitamin D status in 113 Gambian women who ranged in age from young adult to elderly, and reported that mean 25(OH)D levels were ≥29 ng/mL in all age groups31. In another study from Gambia, Prentice reported that in 103 pregnant women, mean 25(OH)D levels were 41.2 ng/dL and no woman had levels <20 ng/mL39. Luxwolda et al. measured vitamin D levels in 60 Tanzanian men and women with traditional tribal lifestyles and found mean 25(OH)D to be 46.0 ng/mL37. However, Njemini et al. reported in a cohort of 152 Cameroonians older than 60 years that while the overall mean level of 25(OH)D was 21.1 ng/mL, 24% of the Cameroonian had levels of 25(OH)D with a mean of 12.5 ng/dL38.
Data from three studies of South Africans reveal one study reporting sufficient levels of 25(OH)D and two studies suggesting low levels32-33, 36. Kruger et al. reported that in 658 black South African women from both rural and urban areas the mean levels in all groups of the D3 form of 25(OH)D were between 25.1 and 31.0 ng/mL36. However, Charlton et al. reported in 173 coloured South Africans (i.e. mixed black and white ancestry) living in Cape Town that mean 25(OH)D levels were 14.8 ng/mL32. Similarly, Daniels et al. reported in 139 black South African nurses that mean 25(OH)D were about 19.0 ng/mL33. Reviewing these 7 studies together, we postulate that vitamin D levels might be lower in black South Africans than equatorial Africans. If true, this is consistent with the hypothesis that distance from the equator is associated with less UVB rays and therefore less endogenous vitamin D production in Africans.
The four studies which report vitamin D levels in African immigrants were based on very small samples but uniformly report low levels34-35, 40-41. In the Netherlands, Van der Meer et al. determined 25(OH)D levels in a multi-ethnic cohort which included 57 African immigrants who had a median 25(OH)D level of 13.2 ng/mL41. Emilion et al. reported that 70% of 165 African women residing in France had 25(OH)D levels less than 20 ng/mL35. In the United Kingdom, Dibba et al. reported in a sample of 20 Gambian adults that mean 25(OH)D levels in men were 10.5 ng/mL and in women were 18.8 ng/mL34. Lastly, in a study of 71 veiled East African women who immigrated to the west coast of the United States, 53.2% of the women had 25(OH)D levels less than 15 ng/mL40. Altogether these studies suggest that the majority of sub-Saharan Africans who have immigrated to Europe and the United States have 25(OH)D which would be considered by either IOM or the Endocrine Society to be inadequate or deficient.
Vitamin D and Bone Health
Race Differences in Bone Mineral Density
In the United States, the National Osteoporosis Risk Assessment (NORA) study evaluated the frequency of osteoporosis in 197,848 post-menopausal women from five groups: African-American, white, Hispanic, Native American and Asians and found that bone mineral density (BMD) was significantly higher in African-Americans than all other groups42. Furthermore, post-menopausal African-American women had an incidence of fracture and prevalence of osteoporosis which was nearly 50% lower than white women42. Other studies have also confirmed that African-American women have a higher peak bone mass, higher BMD, lower bone turnover and a lower incidence of non-spinal fracture than white women43-44. These findings are also true in men, as African-American men are consistently found to have higher BMD than Hispanic and white men45-47.
We identified two studies that examined either BMD or fracture rate or both in Africans. Aspray et al. compared BMD in 195 rural women living in Gambia to 391 age-matched white British women living in England and found that bone mineral content was lower in Gambian than white women. As the rate of traumatic bone fracture is lower in Gambian than English women, the authors speculate that factors beyond bone mineral content contribute to better bone quality in Africans48. Additional support for a lower fracture rate in Africans than whites can be found in a comparison of the rate of fracture at two sites, hip and forearm in 746,700 people living in Ibadan, Nigeria and 416,500 people living in England. All fractures were radiologically confirmed and the risk ratio was 20 times lower in the Africans than in the whites49.
Relationship by race of Vitamin D Levels to Fracture Risk or Bone Mineral Density
The relationship of vitamin D to fracture risk is complex. In fact, a disconcerting observation has been made between vitamin D levels and fracture risk in African-American women. Cauley et al. examined by race the relationship of 25(OH)D to fracture incidence in the Women's Health Initiative Observational Study (WHI-OS)50. Using a nested case-control study design, 25(OH)D levels in women who had fractures after enrollment, were compared to age- and race-matched controls who did not. Unexpectedly, higher 25(OH)D levels were associated with an increased hip fracture risk in African-American women compared to a lower fracture risk in white women. As a result, the authors caution that the relationship between 25(OH)D and fracture risk may vary by race and that vitamin D supplementation might be counterproductive in African-Americans50.
Other studies identified which relate vitamin D to BMD are cross-sectional. In a South African study, Daniels et al. reported that 25(OH)D levels were lower but BMD of the femoral neck was significantly higher in black women than white women, even after adjusting for weight and bone size33. Interestingly, 1,25(OH)D was higher in the black than the white women33. This latter finding may ultimately have etiologic significance.
In African-Americans, three studies were identified and found no relationship between BMD and 25(OH)D levels. First, Gutierrez et al. in an analysis of NHANES 2003-2004 data, reported a high correlation between low BMD and low 25(OH)D levels in whites and Mexican-Americans but not in African-Americans. In a study by Hannan et al. of 1114 white, Hispanic and African-American men living in Boston, a positive correlation was observed between 25(OH)D and BMD in whites, but not in either the African-American or Hispanic men46. In addition, Freedman et al. evaluated 25(OH)D levels in 340 African-American diabetics enrolled in the African-American Diabetes Heart Study and reported no association with BMD51. Together these studies demonstrate that a lack of an association between BMD and 25(OH)D levels is a consistent finding in African-Americans.
Factors Affecting Bone Health Independent of Vitamin D
The lack of a convincing relationship between low 25(OH) D levels and adverse bone health in people of African descent necessitates a search for factors that may affect bone health independent of vitamin D. The key to this conundrum may lie in racial differences in skeletal sensitivity to parathyroid hormone (PTH)25, 52. African-Americans require lower serum 25(OH)D levels to suppress PTH. Furthermore, African-Americans appear to have skeletal resistance to PTH 28, 53-54. For example, Cosman et al. gave 33 pre-menopausal women (15 African-American and 18 white) continuous infusions of PTH for 24 hours and monitored serum and urine indices of bone turnover and calcium metabolism53. After the PTH infusion, markers of bone resorption were significantly lower in African-Americans than whites, suggesting that African-American women have decreased sensitivity to PTH induced bone resorption53.
The Relationship of Vitamin D to Obesity, Diabetes, Hypertension and Cardiometabolic Health
Independent of race there is great controversy about whether low vitamin D contributes to the high prevalence of obesity, diabetes and cardiometabolic disease55-58. Even though both Africans and African-Americans have low vitamin D levels and disproportionately high rates of obesity, diabetes and cardiometabolic disease, the literature examining an association is much larger in African-Americans. It is essential to review these investigations to determine whether racial differences in vitamin D are causative or simply coexist with racial disparities in obesity, diabetes, hypertension and cardiometabolic disease.
Obesity
Vitamin D is fat soluble and sequestered in the adipose tissue59. Therefore it is not surprising that an inverse relationship exists between 25(OH)D levels and BMI60-61. As the prevalence of obesity is higher in African-Americans than whites, it is possible that obesity alone contributes to race differences in vitamin D levels. However, even after controlling for total body fat, African-Americans still have lower vitamin D than whites60-61. With weight loss vitamin D levels increase in both African-Americans and whites but it is unknown if the degree of increase in 25(OH)D after weight loss is race-dependent62.
Type 2 Diabetes
Vitamin D levels are low in African-Americans and the prevalence of type 2 diabetes is high. Therefore investigations have been undertaken to determine if there is an etiological association. However cross-sectional, prospective and supplementation studies do not provide convincing evidence that low vitamin D has a role in African-Americans in the development of diabetes. For example, Scraggs et al. examined Third NHANES (1988-1994) data and found an inverse association between vitamin D levels and diabetes in 2766 whites and 1726 Hispanics but not in 1736 African-Americans63.
Prospective studies of the effect of low 25(OH)D on the development of diabetes have actually provided conflicting results but most notably are characterized by low enrollment of African-Americans. For example, Song et al. performed a meta-analysis of 21 prospective studies with a total of 76,220 participants and found a significant inverse relationship between baseline vitamin D levels and diabetes incidence58. However, only 0.01%, or 647 or the 76,220 participants were African-American. Of these 647 African-Americans, 419 were enrolled in the Diabetes Prevention Program (DPP) and 228 were postmenopausal women participating in the WHI Clinical Trials and Observational Study.
The Diabetes Prevention Program is a multicenter, placebo-controlled trial evaluating the efficacy of drug treatment versus placebo and intensive lifestyle intervention for the prevention of type 2 diabetes64. Pittas et al. specifically analyzed the data in the placebo and intensive lifestyle arms from the Diabetes Prevention Program 64. They reported in the 2,039 participants of whom 419 were African-American that higher vitamin D levels were associated with a lower incidence of diabetes in both the placebo and intensive lifestyle group and that this was observation was independent of race. While the authors specify that race had little effect, grouping the 419 African-Americans together with the entire cohort might have obscured race differences in the association of vitamin D with diabetes incidence. Nonetheless, an opposite conclusion was made by Robinson et al. in a post hoc analysis of data from the WHI study in which 5140 women were evaluated and 228 of the women were African-American65. These investigators reported low vitamin D levels were not associated with an increased risk of developing of diabetes.
Supplementation studies are a powerful way to determine if raising low vitamin D levels can slow or prevent the development of diabetes. A meta-analyses of 15 studies participants performed by George et al. revealed that currently there is no convincing evidence for a beneficial effect of vitamin D supplementation on glucose metabolism56. However, the number of African-Americans included in the evaluation by George et al. was a small fraction of the total number. Nonetheless, we identified two vitamin D supplementation studies which enrolled African-Americans and were not included in the meta-analyses. Harris et al. undertook a 3 month randomized placebo controlled trial to detect the effect of 4000 IU/d of vitamin D3 on glucose homeostasis in 89 overweight African-Americans66. Using serial oral glucose tolerance tests these investigators determined that vitamin D supplements significantly raised 25(OH)D levels but did influence degree of glycemia. Davidson et al. undertook a one year double-blind randomized placebo controlled trial study in which 109 African-Americans and Hispanics with prediabetes and 25(OH)D<30 ng/ml were enrolled to specifically detect the effect of high dose vitamin D supplements (88,865IU/week) on glucose parameters67. They concluded that vitamin D supplementation raised their 25(OH)D levels but had no impact on glucose or insulin dynamics. In short, the evidence in studies overall, and in African-Americans specifically, do not demonstrate that raising vitamin D levels will reverse or prevent the development of hyperglycemia or diabetes.
Hypertension and Cardiovascular Disease
Hypertension
Cross-sectional studies do not universally demonstrate an association between low vitamin D levels and hypertension in African-Americans. NHANES data supports an association between hypertension and Vitamin D in African-Americans but the Insulin Resistance Atherosclerosis Family Study does not68-71. In an analysis of 12,644 adults from NHANES III (1988-1994) data, Scragg et al. reported African-Americans had lower 25(OH)D levels and higher BP than whites71. Furthermore, the race difference in 25(OH)D levels explained half of the increased prevalence of hypertension in African-Americans. In addition Fiscella et al. in analysis of NHANES 2001-2006 data, reported that the difference in systolic blood pressure between African-Americans and whites was reduced by 26% after controlling for differences in vitamin D status68. Gupta et al. in an analysis of 621 African-Americans enrolled in NHANES 2001-2006 reported that low vitamin D levels were associated with high rates of prehypertension and prediabetes69. However, in an examination of data from the Insulin Resistance Atherosclerosis Family Study, Schmitz et al. revealed an inverse association between 25(OH)D levels and blood pressure in 1334 Hispanic and African-American adults which did not remain significant after adjusting for BMI70.
Again, supplementation studies are a logical, clinically relevant way to investigate the effect of vitamin D on blood pressure. Most, but not all studies, conducted to determine the effect Vitamin D supplementation have been performed in whites and suggest a weakly antihypertensive effect57, 72. Two supplementation studies which enrolled exclusively African-American participants have also reported modest, yet significant anti-hypertensive or vaso-dilatory effects of vitamin D supplementation. For 3 months Forman et al. assigned 283 African-Americans to a daily regimen of placebo or a vitamin D3 supplement, and found for each 1 ng/mL increase in serum 25(OH)D, there was a 0.2 mmHg decrease in systolic blood pressure73. Harris et al. examined vascular endothelial function with brachial artery flow-mediated dilation in an randomized, controlled trial in which 57 non-hypertensive African-Americans received either 60,000 IU monthly for 4 months (equivalent to ∼2,000 IU daily) of vitamin D3 or placebo74. The authors reported that flow mediated dilation improved significantly in the group receiving vitamin D supplementation. In contrast to these two studies, which suggest a positive benefit, Pittas et al. examined data reported from the WHI Randomized Trial in which women were given both low dose vitamin D3 (400IU/d) and calcium carbonate (1000 mg/d) and noted an increase in incident hypertension among the African-American women57, 75.
Cardiovascular Disease
As with hypertension, a role for low vitamin D in the etiology of cardiovascular disease is uncertain in general and even less clear in African-Americans than whites. A prospective analyses of cardiovascular mortality in NHANES III 1988-1994 participants followed for about 10 years, led Fiscella and Franks to conclude that low levels of vitamin D in African-Americans contributed significantly to their excess cardiovascular mortality68. Yet Michos et al. also evaluated NHANES III 1988-1994 data for a 15 year period and made the opposite conclusion, specifically that low vitamin D levels did not contribute to increased risk of death from stroke in African-Americans76.
Similarly, the Multi-Ethnic Study of Atherosclerosis investigators found in a cohort of 6436 adults followed for nearly 9 years that lower 25(OH)D levels were associated with a high rate of coronary heart disease risk in whites and Chinese, but a low risk in African-Americans and Hispanics 77. In addition, Freedman et al. in an evaluation of 340 African-Americans enrolled in the African-American Diabetes Heart Study found a positive association between 25(OH)D levels and calcified plaque in the aorta and carotid arteries51. This finding by Freedman et al. raises concern that vitamin D supplementation could have an adverse effect on cardiovascular health in African-Americans and well-designed prospective studies are essential.
Conclusion
The cardinal reason for providing vitamin D supplementation is to ensure bone health. But existing data in African-Americans and Africans do not support that bone health is compromised or even influenced by low vitamin D levels. Furthermore a convincing role for low vitamin D in the development of diabetes, hypertension or adverse cardiovascular health in people of African descent is not supported by existing data. In addition analyses of the WHI and the African-American Diabetes Study data raise the possibility that vitamin D supplementation could have adverse effects on bone, blood pressure and atherosclerotic disease in the aorta and carotid arteries. In short, even though people of African descent have vitamin D levels which are below the established norms of other populations, people of African descent do not appear to be vitamin D deficient. Key factors that may explain this include race differences in skeletal sensitivity to PTH, VDR receptor binding proteins and levels of 1 α-hydroxlyase78.
Overall, identifying the vitamin D levels which are associated with best health in populations of African descent has worldwide implications for health care, health maintenance and health policy. If people of African descent are vitamin D deficient, it is essential to provide supplementation. However, if vitamin D levels are sufficient in African descent population at levels which are lower than in whites, vitamin D supplementation has the potential to lead not only to medical complications but also to iatrogenic vitamin D toxicity. Lessons from over-supplementation of vitamin D in England in the mid-twentieth century must be remembered. Solid prospective studies designed to determine thresholds for vitamin D which are associated with best health in populations of African descent are critical. In African descent populations until there is better understanding of the clinical significance of vitamin D thresholds set by IOM and Endocrine Society-it is premature to describe 25(OH)D levels <20 ng/mL as “inadequate”, “insufficient” or “deficient” as these words imply that supplementation would be appropriate. The best evidence available does not yet support on a population basis this type of intervention.
Acknowledgments
All the authors were supported by the Intramural Research Program of NIDDK.
Abbreviations
- BMD
Bone mineral density
- FDA
Federal drug administration
- IOM
Institue of Medicine
- NHANES
Ntional Health and Nutrition Examination Survey
- PTH
Parathyroid hormone
- VDR
Vitamin D receptor
- UVB
Ultraviolet B
- WHI-OS
Women's health initiative—observational study
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 3.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006 Dec;147(12):5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 4.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998 Oct;78(4):1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 5.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004 Nov;89(11):5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J. Bioavailability of Vitamin D2 and D3 in Healthy Volunteers, a randomised placebo-controlled trial. J Clin Endocrinol Metab. 2013 Sep 3; doi: 10.1210/jc.2012-4287. [DOI] [PubMed] [Google Scholar]
- 9.Biancuzzo RM, Young A, Bibuld D, et al. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr. 2010 Jun;91(6):1621–1626. doi: 10.3945/ajcn.2009.27972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1710S–1716S. doi: 10.1093/ajcn/80.6.1710S. [DOI] [PubMed] [Google Scholar]
- 11.Tangpricha V, Koutkia P, Rieke SM, Chen TC, Perez AA, Holick MF. Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am J Clin Nutr. 2003 Jun;77(6):1478–1483. doi: 10.1093/ajcn/77.6.1478. [DOI] [PubMed] [Google Scholar]
- 12.Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004 Feb 27;215(1-2):31–38. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Byrne ME, Chang E, et al. 1alpha,25-Dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008 Nov;112(1-3):122–126. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somjen D, Weisman Y, Kohen F, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005 Apr 5;111(13):1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 15.Reddy GS, Tserng KY. Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry. 1989 Feb 21;28(4):1763–1769. doi: 10.1021/bi00430a051. [DOI] [PubMed] [Google Scholar]
- 16.Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007 Oct;57(4):588–593. doi: 10.1016/j.jaad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Black LJ, Seamans KM, Cashman KD, Kiely M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr. 2012 Jun;142(6):1102–1108. doi: 10.3945/jn.112.158014. [DOI] [PubMed] [Google Scholar]
- 18.Byers KG, Savaiano DA. The myth of increased lactose intolerance in African-Americans. J Am Coll Nutr. 2005 Dec;24(6 Suppl):569S–573S. doi: 10.1080/07315724.2005.10719505. [DOI] [PubMed] [Google Scholar]
- 19.Sahi T. Genetics and epidemiology of adult-type hypolactasia. Scand J Gastroenterol Suppl. 1994;202:7–20. doi: 10.3109/00365529409091740. [DOI] [PubMed] [Google Scholar]
- 20.Vesa TH, Marteau P, Korpela R. Lactose intolerance. J Am Coll Nutr. 2000 Apr;19(2 Suppl):165S–175S. doi: 10.1080/07315724.2000.10718086. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis JK, Miller GD. Overcoming the barrier of lactose intolerance to reduce health disparities. J Natl Med Assoc. 2002 Feb;94(2):55–66. [PMC free article] [PubMed] [Google Scholar]
- 22.Nicklas TA, Qu H, Hughes SO, et al. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am J Clin Nutr. 2011 Jul;94(1):191–198. doi: 10.3945/ajcn.110.009860. [DOI] [PubMed] [Google Scholar]
- 23.Wolf G. The discovery of vitamin D: the contribution of Adolf Windaus. J Nutr. 2004 Jun;134(6):1299–1302. doi: 10.1093/jn/134.6.1299. [DOI] [PubMed] [Google Scholar]
- 24.Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012 Jun;33(3):456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008 Aug;88(2):545S–550S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman BI, Register TC. Effect of race and genetics on vitamin D metabolism, bone and vascular health. Nat Rev Nephrol. 2012 Aug;8(8):459–466. doi: 10.1038/nrneph.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012 Mar;142(3):498–507. doi: 10.3945/jn.111.151977. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011 Jun;22(6):1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kant AK, Graubard BI. Race-ethnic, family income, and education differentials in nutritional and lipid biomarkers in US children and adolescents: NHANES 2003-2006. Am J Clin Nutr. 2012 Sep;96(3):601–612. doi: 10.3945/ajcn.112.035535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr. 2008 Dec;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aspray TJ, Yan L, Prentice A. Parathyroid hormone and rates of bone formation are raised in perimenopausal rural Gambian women. Bone. 2005 Apr;36(4):710–720. doi: 10.1016/j.bone.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Charlton KE, Labadarios D, Lombard CJ, Louw ME. Vitamin D status of older South Africans. S Afr Med J. 1996 Nov;86(11):1406–1410. [PubMed] [Google Scholar]
- 33.Daniels ED, Pettifor JM, Schnitzler CM, Moodley GP, Zachen D. Differences in mineral homeostasis, volumetric bone mass and femoral neck axis length in black and white South African women. Osteoporos Int. 1997;7(2):105–112. doi: 10.1007/BF01623684. [DOI] [PubMed] [Google Scholar]
- 34.Dibba B, Prentice A, Laskey MA, Stirling DM, Cole TJ. An investigation of ethnic differences in bone mineral, hip axis length, calcium metabolism and bone turnover between West African and Caucasian adults living in the United Kingdom. Ann Hum Biol. 1999 May-Jun;26(3):229–242. doi: 10.1080/030144699282732. [DOI] [PubMed] [Google Scholar]
- 35.Emilion E, Emilion R. Estimation of the 25(OH) vitamin D threshold below which secondary hyperparathyroidism may occur among African migrant women in Paris. Int J Vitam Nutr Res. 2011 Jul;81(4):218–224. doi: 10.1024/0300-9831/a000073. [DOI] [PubMed] [Google Scholar]
- 36.Kruger MC, Kruger IM, Wentzel-Viljoen E, Kruger A. Urbanization of black South African women may increase risk of low bone mass due to low vitamin D status, low calcium intake, and high bone turnover. Nutr Res. 2011 Oct;31(10):748–758. doi: 10.1016/j.nutres.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Luxwolda MF, Kuipers RS, Kema IP, Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr. 2012 Nov 14;108(9):1557–1561. doi: 10.1017/S0007114511007161. [DOI] [PubMed] [Google Scholar]
- 38.Njemini R, Meyers I, Demanet C, Smitz J, Sosso M, Mets T. The prevalence of autoantibodies in an elderly sub-Saharan African population. Clin Exp Immunol. 2002 Jan;127(1):99–106. doi: 10.1046/j.1365-2249.2002.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prentice A, Jarjou LM, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatr. 2009 Aug;98(8):1360–1362. doi: 10.1111/j.1651-2227.2009.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed SD, Laya MB, Melville J, Ismail SY, Mitchell CM, Ackerman DR. Prevalence of vitamin D insufficiency and clinical associations among veiled East African women in Washington State. J Womens Health (Larchmt) 2007 Mar;16(2):206–213. doi: 10.1089/jwh.2006.0089. [DOI] [PubMed] [Google Scholar]
- 41.van der Meer IM, Boeke AJ, Lips P, et al. Fatty fish and supplements are the greatest modifiable contributors to the serum 25-hydroxyvitamin D concentration in a multiethnic population. Clin Endocrinol (Oxf) 2008 Mar;68(3):466–472. doi: 10.1111/j.1365-2265.2007.03066.x. [DOI] [PubMed] [Google Scholar]
- 42.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005 Feb;20(2):185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 43.Aloia JF, Vaswani A, Yeh JK, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int. 1996 Dec;59(6):415–423. doi: 10.1007/BF00369203. [DOI] [PubMed] [Google Scholar]
- 44.Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005 May 4;293(17):2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 45.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007 Jul;18(7):943–953. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 46.Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008 Jan;93(1):40–46. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in rate of decline in bone mass in older men: the Baltimore men's osteoporosis study. J Bone Miner Res. 2005 Jul;20(7):1228–1234. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 48.Aspray TJ, Prentice A, Cole TJ, Sawo Y, Reeve J, Francis RM. Low bone mineral content is common but osteoporotic fractures are rare in elderly rural Gambian women. J Bone Miner Res. 1996 Jul;11(7):1019–1025. doi: 10.1002/jbmr.5650110720. [DOI] [PubMed] [Google Scholar]
- 49.Adebajo AO, Cooper C, Evans JG. Fractures of the hip and distal forearm in West Africa and the United Kingdom. Age Ageing. 1991 Nov;20(6):435–438. doi: 10.1093/ageing/20.6.435. [DOI] [PubMed] [Google Scholar]
- 50.Cauley JA, Danielson ME, Boudreau R, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women's Health Initiative (WHI) J Bone Miner Res. 2011 Oct;26(10):2378–2388. doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freedman BI, Wagenknecht LE, Hairston KG, et al. Vitamin d, adiposity, and calcified atherosclerotic plaque in african-americans. J Clin Endocrinol Metab. 2010 Mar;95(3):1076–1083. doi: 10.1210/jc.2009-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985 Aug;76(2):470–473. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cosman F, Morgan DC, Nieves JW, et al. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. 1997 Jun;12(6):958–966. doi: 10.1359/jbmr.1997.12.6.958. [DOI] [PubMed] [Google Scholar]
- 54.Cosman F, Shen V, Morgan D, et al. Biochemical responses of bone metabolism to 1,25-dihydroxyvitamin D administration in black and white women. Osteoporos Int. 2000;11(3):271–277. doi: 10.1007/s001980050292. [DOI] [PubMed] [Google Scholar]
- 55.Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011 Jul;96(7):1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 56.George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012 Aug;29(8):e142–150. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 57.Pittas AG, Chung M, Trikalinos T, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010 Mar 2;152(5):307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song Y, Wang L, Pittas AG, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013 May;36(5):1422–1428. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blum M, Dolnikowski G, Seyoum E, et al. Vitamin D(3) in fat tissue. Endocrine. 2008 Feb;33(1):90–94. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coney P, Demers LM, Dodson WC, Kunselman AR, Ladson G, Legro RS. Determination of vitamin D in relation to body mass index and race in a defined population of black and white women. Int J Gynaecol Obstet. 2012 Oct;119(1):21–25. doi: 10.1016/j.ijgo.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanoff LB, Parikh SJ, Spitalnik A, et al. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese Black Americans. Clin Endocrinol (Oxf) 2006 May;64(5):523–529. doi: 10.1111/j.1365-2265.2006.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rock CL, Emond JA, Flatt SW, et al. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity (Silver Spring) 2012 Nov;20(11):2296–2301. doi: 10.1038/oby.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004 Dec;27(12):2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 64.Pittas AG, Nelson J, Mitri J, et al. Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program. Diabetes Care. 2012 Mar;35(3):565–573. doi: 10.2337/dc11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson JG, Manson JE, Larson J, et al. Lack of association between 25(OH)D levels and incident type 2 diabetes in older women. Diabetes Care. 2011 Mar;34(3):628–634. doi: 10.2337/dc10-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris SS, Pittas AG, Palermo NJ. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab. 2012 Sep;14(9):789–794. doi: 10.1111/j.1463-1326.2012.01605.x. [DOI] [PubMed] [Google Scholar]
- 67.Davidson MB, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013 Feb;36(2):260–266. doi: 10.2337/dc12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med. 2010 Jan-Feb;8(1):11–18. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta AK, Brashear MM, Johnson WD. Low vitamin D levels, prediabetes and prehypertension in healthy African American adults. Nutr Metab Cardiovasc Dis. 2012 Oct;22(10):877–882. doi: 10.1016/j.numecd.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 70.Schmitz KJ, Skinner HG, Bautista LE, et al. Association of 25-hydroxyvitamin D with blood pressure in predominantly 25-hydroxyvitamin D deficient Hispanic and African Americans. Am J Hypertens. 2009 Aug;22(8):867–870. doi: 10.1038/ajh.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007 Jul;20(7):713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 72.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009 Oct;27(10):1948–1954. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- 73.Forman JP, Scott JB, Ng K, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013 Apr;61(4):779–785. doi: 10.1161/HYPERTENSIONAHA.111.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harris RA, Pedersen-White J, Guo DH, et al. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011 May;24(5):557–562. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Margolis KL, Ray RM, Van Horn L, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women's Health Initiative Randomized Trial. Hypertension. 2008 Nov;52(5):847–855. doi: 10.1161/HYPERTENSIONAHA.108.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michos ED, Reis JP, Post WS, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition. 2012 Apr;28(4):367–371. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013 Jul 10;310(2):179–188. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Norris KC, Williams SF. Race/ethnicity, serum 25-hydroxyvitamin D, and heart disease. JAMA. 2013 Jul 10;310(2):153–155. doi: 10.1001/jama.2013.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]


