Abstract
The study of malaria parasites on the Indian subcontinent should help us understand unexpected disease outbreaks and unpredictable disease presentations from Plasmodium falciparum and from Plasmodium vivax infections. The Malaria Evolution in South Asia (MESA) research program is one of ten International Centers of Excellence for Malaria Research (ICEMR) sponsored by the US National Institute of Health. In this second of two reviews, we describe why population structures of Plasmodia in India will be characterized and how we will determine their consequences on disease presentation, outcome and patterns. Specific projects will determine if genetic diversity, possibly driven by parasites with higher genetic plasticity, plays a role in changing epidemiology, pathogenesis, vector competence of parasite populations, and whether innate human genetic traits protect Indians from malaria today. Deep local clinical knowledge of malaria in India will be supplemented by basic scientists who bring new research tools. Such tools will include whole genome sequencing and analysis methods; in vitro assays to measure genome plasticity, RBC cytoadhesion, invasion, and deformability; mosquito infectivity assays to evaluate changing parasite-vector compatibilities; and host genetics to understand protective traits in Indian populations. The MESA-ICEMR study sites span diagonally across India, including a mixture of very urban and rural hospitals, each with very different disease patterns and patient populations. Research partnerships include government-associated research institutes, private medical schools, city and state government hospitals, and hospitals with industry ties. Between 2012-2017, in addition to developing clinical research and basic science infrastructure at new clinical sites, our training workshops will engage new scientists and clinicians throughout South Asia in the malaria research field.
Keywords: malaria, Plasmodium falciparum, Plasmodium vivax, India, South Asia, epidemiology, drug resistance, ICEMR
1. Introduction
This review discusses the study plan for the US-India Program Project titled “Malaria Evolution in South Asia” (MESA). It is part of a broader global effort by the US National Institutes of Health to support ten International Centers of Excellence for Malaria Research (ICEMR) worldwide (Rao, 2011 in this issue). Here, we present some important questions related to malaria evolution in South Asia and we describe the selection of our study sites, research tools being deployed, anticipated new findings, and training plans for long-term health and continuity of multinational collaborations. We also review how our MESA-ICEMR activity dove-tail with exciting in-country malaria research in India. Overall, we hope that the MESA-ICEMR will identify questions and study sites that will help the Indian National Programme and international programs understand and control malaria more efficiently and effectively with the long-term of goal of global malaria eradication (Rao, 2011 in this issue).
2. Malaria in India Today
Before discussing the goals of the MESA-ICEMR, it is worthwhile reviewing what is known and unknown about malaria in India. In an accompanying review in this special issue of Acta Tropica (Kumar et al, 2012), we discuss geographic and clinical diversity of malaria in South Asia and the public health challenges presented by the disease. Below, we briefly review the highlights:
2.1 Number of cases and deaths
Today, of 1.2 billion people in India, 80% live in malaria risk areas. Officially, there are at least 1.5 million malaria cases every year in India and they are divided evenly between P. falciparum (Pf) and P. vivax (Pv) (Fig 4 in this issue Kumar et al, 2012; Singh et al. 2009). Malaria-related economic losses are roughly $0.5 to 1.0 billion USD, annually.
The question of mortality from malaria has significant uncertainties. The Government of India in recent years has reported as few as 1,000 annual deaths attributable to malaria, but WHO estimates are an order of magnitude larger (WHO, 2009). Verbal autopsy studies conducted around 2000 and independent analysis based on reporting inconsistencies of certified deaths suggest that malaria deaths in India may have been as high as 150,000-200,000 per year (Dhingra et al, 2010; Kumar et al, 2011). If the higher estimates of mortality, which are sensitive to rural areas of India far from hospital infrastructures, are correct, the raw number of cases of malaria infections may be significantly higher than 1.5 million. A special commission has been summoned in 2011 by the Government of India to estimate the true malaria burden of India.
2.2 Variations in endemicity
Of the 1-2 million confirmed cases of malaria every year in India, 4% are from high risk areas, 32% from moderate risk areas, and 44% of the patients live in low risk areas for malaria (http://www.searo.who.int/). Based on numbers from the Indian National Vector Borne Disease Control Programme, the areas of low endemicity include large stretches of Northern, Western and Southern parts of the country (Fig 3 in this issue, Kumar et al. 2012). Districts with moderate malaria risk are scattered across central India. The most malaria prone districts, with a Annual Parasite Incidence (API) of >5, can be found in the eastern states of Chhattisgarh, Jharkhand, Orissa, and the Northeastern states, which are located East of Bangladesh near the Myanmar border. Localized hyperendemic districts are found in Orissa, Arunachal, Meghalaya, Chhattisgarh, Assam, Mizoram, Jharkhand, and Andaman and Car Nicobar Islands. In 2008, the 20 districts with the highest malaria burden had API ranging from 21% to 74% with a population of 10.7 million (In this issue, Kumar et al. 2012).
2.3 Parasite species distribution
Overall, India has a 50:50 distribution of P. falciparum and P. vivax, but the areas of concentration of the two species can vary in different parts of India. The plains in the middle of the country, the hilly states to the North, the states in the northwest alongside Pakistan, and the southern states have predominantly P. vivax and less than < 10% P. falciparum infections. The hyperendemic districts of Orissa, the forested districts with traditional ethnic tribes in surrounding states of Jharkhand, Chhattisgahr, West Bengal, and the states of the Northeast (East of Bangladesh) have the highest representation of P. falciparum, frequently reaching or exceeding 90%. The representation of P. falciparum over P. vivax has been increasing in India over the last 30 years (Fig 4 in Kumar et al 2012).
2.4 Clinical severity
In India, the incidence of disease severity differs across the country and by parasite species. Mortality rates from complicated P. falciparum malaria are usually not high if patients are diagnosed and treated early, but can approach 20-30% in the tertiary care centers of hyperendemic districts and during outbreaks in any part of India (Sukla et al. 1995; Harris et al. 2001). In complicated cases, cerebral malaria and multi-organ failures are common.
Advanced clinical complications from the “benign species” P. vivax have been reported from Bikaner, India and also from other sites in recent years (Valecha et al. 1992; Patial et al. 1998; Beg et al. 2002; Kochar et al. 2005). Patient populations with microscopically confirmed and PCR confirmed mono infection of P. vivax can present with one or more symptoms of jaundice, severe anemia, respiratory distress with acidosis, acute renal failure, cerebral dysfunction with multiple convulsions, abnormal bleeding, shock (hypotension), pulmonary edema and hemoglobinuria (Kochar et al. 2005). Among patients with severe vivax malaria, approximately 50% had these complications. In the Rajasthan study, though of limited scope, several pregnant women with P. vivax infection had abortions, still births and preterm deliveries (Kochar et al, 2005). A case in Goa, for the first time, showed histopathological evidence for parasite accumulation in newly effected organs (Kochar et al. 2005; Valecha et al. 2009).
Amongst severe cases, malaria with Acute Respiratory Distress Syndrome (ARDS) is of biggest concern due to the high case fatality rates associated with such presentations (Valecha et al. 1992; Patial et al. 1998; Beg et al. 2002; Kochar et al. 2005)
3. Malaria management in India
3.1 History of malaria control programs
In 1953, the National Malaria Control Programme (NMCP) was launched in India. DDT was used for vector control and chloroquine for treatment. This resulted in spectacular decline in malaria6. Buoyed by the confidence of achieving malaria control in pilot projects of the NMCP, India joined worldwide campaign to eradicate malaria in the late 1950s and early 1960s and converted NMCP to full scale National Malaria Eradication Programme (NMEP). Initially, the malaria eradication campaign achieved great success in India and parasite rates decreased in many regions of the country, but overtime these successes were lost due to a series of obstacles, including operational and financial difficulties (Singh et al. 2009). In 1977, India created a revised plan for malaria control, known as the Modified Plan of Operation (MPO) and the National Institute of Malaria Research (NIMR) was established.
To this day, the Indian Council of Medical Research (ICMR)-sponsored NIMR provides short-term as well as long-term solutions to the problems of malaria through basic, applied and operational field research. Along with the additional ICMR-based Regional Medical Research Centers (RMRC), NIMR provides critical intelligence to the National Vector Bourne Disease Control Program (NVBDC) and local public health entities around India.
3.2 Down and up: Historical numbers
The anxieties of undertaking and completing malaria elimination programs reflect historic estimates of malaria in places like India. In 1947, when India became independent, 75 million malaria cases in a population of 330 million were estimated. Sinton (1935) also estimated between 1.0 and 1.35 million deaths attributable to malaria per annum in India. The incidence of malaria significantly declined to just 100,000 by 1964. That is over a 100-fold decrease in malaria incidents. However, soon, the numbers reversed and malaria staged a comeback. By 1976, malaria cases had touched the 6.4 million mark (Singh et al, 2009). The malaria resurgence was characterized by a continued rise in P. falciparum and, today, nearly 50% of malaria around India is due to this species. While the recent reported incidences of malaria to National Vector Borne Diseases Control Programme (NVBDCP) have declined since 2005 from 1.9 million to 1.6 million cases in 2009, the long term burden has not improved, and the incidence of P. falciparum infections continue to rise over the last 20 years.
4. Malaria research in India
Today, the ten US-sponsored ICEMR programs around the world face very different opportunities and challenges in terms of how they may participate and contribute to malaria research and disease control in their respective regions and areas of expertise. To understand how the projects related to malaria evolution were selected by the MESA-ICEMR, it is worthwhile to first review the exciting science that is already in place or underway in India, a vast country with an advanced research and technology base as well as a rapidly expanding economy. A broad understanding of current malaria research activities in India help place today’s MESA-ICEMR goals in the correct context.
Over the centuries, India has experienced a great burden of malaria and has a rich tradition of malaria research to support public health initiatives (Singh et al. 2009). After all, Ronald Ross, who was born in Almora, India, received a Nobel prize in 1902 for demonstrating in 1897 that mosquitoes transmit malaria parasites.
Presently, India has more than 200 health research institutions (ICMR- WHO Health Research Directory, 2007). There are ongoing initiatives in India to study the prevalence and susceptibility patterns of present day malaria (Dhangadamajhi et al., 2009; Dhiman et al., 2010; Sharma, 2009; Singh 2009; Singh et al., 2010a, 2011; Valecha, 2009), complemented with molecular epidemiology of parasites, especially relative to known drug resistance markers (Awasthi et al., 2011; Choudry and Sharma, 2009; Dhangadamajhi et al., 2009; Garg et al., 2009; Gupta et al., 2010; Lumb et al., 2009a, 2009b, 2011; Mixson-Hayden et al., 2010; Mohanty et al., 2009a; Prajapati et al., 2011; Rajesh et al., 2007; Rout et al., 2009). The basic molecular sciences are well balanced with detailed studies on clinical presentations and pathogenesis of malaria in India (Baliga et al., 2011; Kochar et al., 2010; Mahajan et al., 2011; Rout et al., 2011; Tanwar, 2011). The capacity for in-country human clinical trials is in place (Mullick et al., 2011; Valecha et al., 2010a; Valecha et al., 2010b). Many strong groups are also undertaking synthetic chemistry as well as natural product chemistry to develop new antimalarial drugs (Kumar et al., 2011b; Lakshmi et al 2011; Mishra et al, 2011). In-country efforts to evaluate vaccine candidates and to develop tools for malaria vaccinology are well developed (Chauhan et al., 2010; Imam et al., 2011; Mayor et al., 2009; Ozarkar et al., 2007; Parween et al., 2011). Basic science studies from malaria labs cover all the major disciplines including biochemistry and cell biology (Balusu et al, 2011; Gaur and Chitnis, 2011; Nagaraj et al., 2010a, 2010b; Kumar et al., 2011a; Pattakotu et al., 2011; Prusty et al.,2010; Sahar et al., 2011; Singh 2010b; Vaid, 2010), computation and structural biology (Chitnis and Sharma, 2009; Gil et al., 2009; Gogia et al., 2011; Maity et al., 2011; Mohanty 2009b; Rawat et al., 2011), human genetics (Balgir 2008; Deepa, et al., 2011; Dey et al., 2009; Sarkar et al., 2010), biophysics (Bambardekar et al., 2008), parasite genomics (Acharya et al., 2009, 2011; Ranjan et al., 2011) and other new technologies (Sharma et al., 2010, 2011). The initiatives listed above are not a comprehensive list of malaria research activities in India, but rather a glimpse for perspective.
The projects mentioned above emerge from diverse organizations within and outside the structure of the Government of India. The Indian Council of Medical Research directly supports the National Institute of Health (NIMR) and the Regional Medical Research Centres (RMRC). The Ministry of Health supports the National Vector Borne Disease Control Program (NVBDCP) including its research activities. Other important contributors include the All India Institute of Medical Sciences (Delhi), the Armed Forces Medical College (Pune), the Central Drug Research Institute (Lucknow), and the Defence Research and Development Establishment (Gwalior). Premier research institutes with academic components across the country contribute to interactions between malaria basic science and applied malaria research. These include Jawaharlal Nehru University (Delhi), Jawaharlal Nehru Centre for Advanced Scientific Research (Bangalore), University of Delhi, India Institute of Chemical Biology (Kolkata), the Institute of Life Sciences (Bhubaneshwar), the Indian Institute of Science (Bangalore), the Indian Institute of Technology (Delhi, Mumbai), the Indian Statistical Institute (Kolkata), Pune University, Postgraduate Institute of Medical Education & Research (Chandigarh), and the Tata Institute for Fundamental Studies (Mumbai). Non-governmental, international organizations have played a major role for several decades in public health as well as basic science initiatives, especially the International Centre for Genetic Engineering and Biotechnology (Delhi) and the World Health Organization – Southeast Asia Office (Delhi).
Many hospitals and medical colleges, which frequently also serve as tertiary care centers for many states of India, see a vast number of malaria patients and participate in clinical research. Amongst the more visible in the last few decades have been the Sardar Patel Medical College (Bikaner, Rajasthan), the Ispat General Hospital run by the Steel Authority of India Limited (Rourkela, Orissa), the Calcutta School of Tropical Medicine (Kolkata, West Bengal), Seth G. S. Medical College and K. E. M. Hospital (Mumbai, Maharashtra), TN Medical College and BYL Nair Ch. Hospital (Mumbai, Maharashtra), S.C.B. Medical College (Cuttack, Orissa), and the Postgraduate Institute of Medical Education and Research (Chandigarh). While these institutions are staffed with highly experienced infectious disease specialists and have provided fantastic descriptions of malaria in India, many such healthcare institutions have had limited opportunities to directly conduct or participate in clinically-relevant basic science research with public health implications. During the days of scarce resources, basic science tended to be centralized, as was the expertise to formulate appropriate questions for clinical and basic research. While the Regional Medical Research Centers (RMRC) provide well-equipped, modern research laboratories away from cities, the majority of medical schools in India still lack the necessary basic scientific infrastructure for malaria research.
All this is now changing. Government organizations such as the Indian Council of Medical Research (ICMR) and the Department of Biotechnology are aggressively funding biomedical research across India. Within the ICMR structure to promote biomedical research, extramural research is promoted through the Centres for Advanced Research in select departments of medical colleges, universities and non-ICMR research institutes. Task force studies, often in multicentric structures, with defined targets, specific time frames, standardized and uniform methodologies are also possible. Open-ended research is conducted on the basis of applications for grants-in-aid much like R01s in the US. Career development in biomedical research is promoted through research fellowships, visiting fellowships, research studentships, and various training programmes and workshops. For all such reasons, there is excitement that clinicians will enjoy increasing opportunities to engage regularly with the best malaria scientists within India and outside India too.
5. Opportunities for joint learning, cooperation and collaboration
With this background, it is possible to articulate how the NIH-supported MESA-ICEMR will fit in and contribute to the larger malaria research activities in South Asia. The MESA-ICEMR program will hire and train research staff in India and meet international standards for sample management, data management, and data sharing. Even though the program will not have the reach of the Indian national disease control and surveillance programs, the select sites of the MESA-ICEMR span across critical parts of India where there are significant uncertainties about disease prevalence and severity.
5.1 Broad goals
The two most important goals of the MESA-ICEMR program are to:
Establish a network of dedicated and reliable clinical sites for malaria research across India that offer contrasting views of the disease in the country.
Understand variations in the genetic makeup of Indian malaria parasites and the effects of these genetic variations on severity of disease outcomes.
5.2 Operationalization of clinical and scientific objectives
The MESA-ICEMR will combine the best of basic sciences and clinical research by:
Bringing together Indian physicians and scientists, alongside US physicians and scientists with interest in medical research, to study the molecular basis of pathogenesis, virulence, and treatment efficacy.
Bringing “state of the science” technologies to MESA-ICEMR sites and facilitating engagement between Indian clinicians and both Indian and US technologists. Amongst other things, this will include whole-genome sequencing and DNA microarrays to reconstruct evolutionary history of parasites at the ICEMR study sites, microfluidic tools to understand the role of cytoadhesion and RBC deformability limits in pathogenesis, stem-cell technologies to understand invasion and growth of P. vivax in reticulocyte-like cells, and mosquito assays to define vector compatibilities.
5.3 Training opportunities
Beyond specific new scientific questions in India and new tools to address the questions, we are an integral part of the larger US-supported ICEMR programs committed to development of long-term, sustainable relationships across the globe:
The MESA-ICEMR team will sponsor national workshops to allow scientists and clinicians from across South Asia to exchange information and to initiate joint studies with members of the ICEMR teams.
The MESA-ICEMR team will facilitate opportunities for South Asian scientists and clinicians to apply for national and international funding, to leverage activities supported directly by the ICEMR, and facilitate international collaborations.
6. Overarching hypothesis of the MESA-ICEMR: ARMD parasites
The MESA-ICEMR has a defined, overarching scientific hypothesis that brings together each individual, different scientific project and allows us to reach the broader public health and operational goals articulated above.
6.1 The public health connection
Across South Asia, large variation in species-dominance from the NE to Southern states and frequent epidemics of severe malaria sometimes associated with natural disasters such as earthquakes or floods are observed, oftentimes for unexplained reasons. It is of both intellectual and practical interest to understand and predict where it may be easy to carry out malaria eradication operations, where it would be difficult to sustain such campaigns, and where current elimination programs are unlikely to make a dent without aggressive additional resourcing. Most importantly, it would be very useful to know if aggressive, but incomplete, eradication campaigns may actually fuel the emergence of more robust and, possibly, more virulent populations of malaria parasites.
6.2 A fight for relevant knowledge
There is little doubt that increasing allocation of resources and access to public health support will make a major dent in malaria disease, as was seen in the 1960s. However, the globally declared goal is not just to control malaria, but to eradicate it. Beyond more money and improved infrastructure, limitations in our understanding of malaria as a complex, population-based disease may limit the successful control of malaria and, particularly, true elimination. For example, people cannot live under bed nets all day and every day, and mosquitoes can evolve to alter their biting habits. Shrinking parasite population sizes appear to help public health causes, but they could also place greater burdens on the pathogens to change at faster rates and possibly contribute to higher virulence. Initially, such evolution may be to acquire drug resistance. Once the capacity for faster genetic change is in place, it is not difficult to see how the ability to change would favor acquisition of new niches on a continental scale, within vectors, possibly to favor propagation of the disease, and even within human hosts, possibly presenting new disease outcomes. In the fight against malaria, it is vital to understand the capacity of parasite populations to change.
6.3 Seeds of an overarching hypothesis
We have previously shown that parasites from Southeast Asia can acquire resistance to new antimalarial compounds at frequencies that are 1,000-10,000 times higher than parasites from Africa or South America (Rathod et al, 1997). These findings were consistence with later molecular epidemiology studies that definitively showed the movement of drug resistance traits from South east Asia to other regions of the world including Africa (Wootton et al, Nature, 2002; Roper et al, Science,2004; Dondorp et al, N Engl J Med 2009).
It is known that resistance traits for individual drugs have spread from SE Asia to Africa, through India. However, it is not clear whether hypermutating parasites occur outside SE Asia. Using newly developed in vitro technologies, the presence of potentially hypermutating malaria parasites will be studied in South Asia. Additionally, the potential contribution of such parasites to disease and to parasite population diversity will be studied. Sudden malaria epidemics occur in unexpected places throughout India, every year. An ability to survey and monitor parasite populations on the basis of genome plasticity would provide unprecedented tools for accessing disease threats for public health policy makers, first in India, but later in other parts of the world.
6.4 ARMD malaria parasites: a formal hypothesis
We hypothesize that some parasite subpopulations in South Asia acquire high drug resistance through enhanced genome plasticity. This Accelerated Resistance to Multiple Drugs, or ARMD, trait may then in turn cause increased virulence through new mechanisms of pathogenesis, improved propagation across the subcontinent, and possibly decreased innate resistance in humans.
6.5 Why consider the ARMD hypothesis?
The scientific approach demands new hypotheses for explaining facts when existing knowledge is inadequate to explain large gaps in our understanding of the universe. In malaria research and public health, it is the inadequacy of our current disease models to regularly explain, let alone predict, why certain villages or cities unexpectedly experience a potent disease outbreak, but other nearby communities do not, despite the fact that they experience the same rains and often harbor the same capacity to support vectors and parasites. We cannot explain why some adults in hyperendemic areas come down with severe malaria, even though they may have had sufficient immunity in previous years or even if they carry host genes that are thought to protect against malaria.
Developing alternate possible explanations for existing facts and developing appropriate tests to determine if the new explanations can account for current observations is at the very core of the scientific method. This is particularly important in the public health arena where the difference between good science and pedestrian data-gathering can affect lives, and prioritization of precious resources. As the chemist Linus Pauling said, “The scientist, if he is to be more than a plodding gatherer of bits of information, needs to exercise an active imagination.” (‘Imagination in Science’, Tomorrow (Dec 1943), 38-9. Quoted In Barbara Marinacci (ed.), Linus Pauling In His Own Words: Selected Writings, Speeches, and Interviews (1995), 82.).
6.6 What if the ARMD hypothesis is wrong?
Science celebrates efforts that reveal new empowering principles of knowledge and share new light on an old problem. We still enjoy the rewards of breakthroughs from the 1800s, whether it is concepts like germ theory or the role of mosquitoes in malaria propagation. Today in malaria research and in the public health arena, new powerful hypothesis are needed to have a complete understanding of malaria, ideas that have great predictive power in guiding resources to control disease outbreaks and disease severity.
However, during this process, there are no guarantees that a highly plausible hypothesis will be right. Breakthroughs in science would not occur if the community was constantly worried that a reasonable hypothesis may be wrong. Breakthroughs come from various iterations of a hypothesis or multiple hypotheses that are well-tested, some of which are eliminated until one cannot be shot down. “A discovery is rarely, if ever, a sudden achievement, nor is it the work of one man; a long series of observations, each in turn received in doubt and discussed in hostility, are familiarized by time, and lead at least to the gradual disclosure of truth”. - Sir Berkley Moynihan, 1865-1936 in Kuhn, Thomas S. (1962), The Structure of Scientific Revolutions, Chicago, IL: University of Chicago Press. 2nd edition 1970. 3rd edition 1996. It is always possible that large variations in disease presentations are due to other considerations: For instance, co-infections with Hepatitis, TB, HIV and/or other enteric diseases could complicate malaria infections and disease presentations (Baliga, K.V., et al 2011). Once the malaria research infrastructure is in place (with properly equipped facilities, trained staff, and good administrative structures), alternate ideas may be pursued as necessary based on preliminary data from current samples and planned activities.
6.7 A low risk–high payoff initiative
Beyond the philosophy of good science, in the current context of the ICEMR program, a well-designed plan to test the ARMD trait will yield many benefits including (i) high quality study-sites and highly trained investigators in a strategically important part of the malaria world, (ii) development of new tools to characterize the genetic structure of malaria parasite populations, (iii) development of new phenotypic assays that capture the essence of clinical variation in malaria, (iv) identification of molecular events associated with virulence in malaria parasites, and (v) a more complete understanding of the human capacity for innate resistance to malaria.
The hypothesis that ARMD parasites can drive unpredictability in diseases comes with standard risks of imaginative science directed at unknown puzzles, but the plan to test the hypothesis is not risky at all. Regardless of the outcome of the test of the hypothesis, the efforts will yield powerful advances in understanding malaria presentations in South Asia with direct benefits to national and international disease control programs.
7. A research plan
To penetrate into the diverse types of malaria presentations in India, we have identified a mix of diverse study sites ranging from the very urban to very rural. Our study sites span from the northeast of South Asia (including the states of Assam, Tripura, and West Bengal) across the middle of India (through Ranchi, Jharkhand and Wardha, Maharashtra) to the western coastal cities (Panaji, Goa and Mumbai, Maharashtra). The sites cover a variety of geographies: Kolkata and Mumbai are large urban centers with over 10 million people including many migrant workers from other parts of India. Ranchi, Dibrugarh, and Agartala have high levels of hemoglobin disorders and other potential protective traits common in tribal areas. Wardha represents a rural community with a more homogeneous human population, but still very high levels of severe malaria.
Our interactions will touch diverse aspects of modern biomedical research in India including the Government of India (Indian Council of Medical Research institutes, NIMR and RMRC), a semiautonomous research institute (NIBMG), both state-run and private medical schools (Goa Medical College, Goa; Jawaharlal Nehru Medical College, Wardha; Maharashtra; Assam Medical College, Dibrugarh; Calcutta School of Tropical Medicine, Kolkata; Agartala Medical College), those run by city municipal corporations (Kasturba Hospital and Topiwala Medical College & BYL Nair Hospital, Mumbai), and a private, rural hospital (Shalini Memorial Hospital, Ranchi, Jharkhand).
To help fill some important gaps in our knowledge of malaria in India and to test the specific hypothesis that rapidly changing malaria parasites (ARMD parasites) may be responsible for the uneven and changing profile of the disease in India, the MESA-ICEMR will undertake four different, but tightly integrated, projects.
7.1. Epidemiology
Our goal is to study prevalence and accurately map the profile of malaria in a variety of sites across India that capture very different epidemiology, particularly in regions where deep and complete epidemiological studies have been lacking. Our center includes sites where there are varying and often changing ratios of P. falciparum and P. vivax, where district data point to different presentations of disease severity and deaths from malaria, and where our understanding of vector specificities and preferences are incomplete. We will also determine the drug susceptibility and resistance profiles of parasites from clinically defined patients at our different study sites. We also assay for phenotypic characteristics that are expected to influence malaria disease presentations across India. The background data on patient clinical history, the quality of drugs they use, and their responses under standard care will provide an independent crosscheck for information from others, including the national disease control program.
An important aspect of the MESA-ICEMR partner selection was and continues to be the engagement of experienced, practicing academic clinicians who have not previously practiced basic science research, and established basic scientists who have not worked on malaria at endemic sites. Through such partnerships, we hope to gain new insights into previously unexplored and nontraditional areas of malaria research.
7.2 Parasite Plasticity
Traditional antimalarials are beginning to fail in many parts of India (Shah, et al, 2011). Artemisinin-based combination therapies are common in India, but there is evidence for artemisinin resistance in neighboring Southeast Asia (Dondorp, A.M., et al 2009, 2010) The in vitro capacity for parasites from India to change genetically may be correlated to disease severity in donor patients and to genome sequences of the parasites. We hypothesize that some parasite subpopulations in South Asia acquire high drug resistance through enhanced genome plasticity. This Accelerated Resistance to Multiple Drugs (ARMD) trait may then, in turn, cause increased virulence through new mechanisms by which disease is caused, improved propagation across the subcontinent and, possibly, decreased innate resistance in humans.
To test this hypothesis, stretching from NE India to Western India, we will compare clinical presentations of malaria to in vitro measurements of genome plasticity for parasites from those patients (Rathod et al, 1994; Rathod et al, 1997). Given that parasites will be isolated from patients with prospectively obtained clinical records and treatment information, large data sets will be useful to determine whether the ARMD status of a parasite population helps drive their overall evolutionary success with respect to drug resistance, increased virulence, vector compatibilities, and capacity to infect a wider pool of humans with different levels of innate or acquired resistance.
We will also use parasite genomics and genomic associations to identify chromosomal regions that drive success of the most disease-enhancing forms of malaria (Dharia, et al, 2009; Ganesan et al, 2008; Gardiner, et al, 2002; Gupta, B., et al 2010; Trimnell, A.R., et al, 2006). Beyond understanding the nature of how parasites drive genetic variation, the genomic studies are also expected to yield molecular insights into the mutational outcomes that confer select traits: the basis for resistance to specific drugs (Mixon-Hayden, et al, 2010; Roper, C., et al, 2004; Wootton, J.C., et al, 2002), the determinants of vector compatibility, and the basis for broader infectivity (Rao, T.R., 1983; Sharma, S.K., 2004, 2006; Sharma, V.P., 1991) and virulence in previously exposed adults (Beg, M.a., et al 2002; Kochar, D.K., et al 2010; Patial, R.K., et al 1998; Rout, R., et al, 2009; Singh, N., et al, 2011; Shukla, R.P., et al 1995; Tanwar, A. R., 2011).
7.3. Pathogenesis
Malaria pathogenesis involves a complex set of physiological changes in the parasite and the host, and not always in a predictable way (Miller, L.H., et al 2002). Much od what we know about pathogenesis is based on studies in Africa, and much less from the Indian subcontinent. In the ICEMR program, parasites will be collected from patients with diverse disease presentations in children, in adults and in pregnant mothers. Parasites will be characterized in vitro for capacity for genetic change, but also with respect to pathogenesis-related phenotypes. The Pathogenesis project questions and experiments will fall into two general groups. One set of studies will be on biochemical alterations of invasive merozoite parasites or mature parasite-infected red blood cells whose surfaces are altered. Such changes can affect properties such as cytoadherance to host ligands to avoid host immune responses, the ability of sera to interfere with cytoadherance, and increased or more efficient invasion mechanisms. Variations in these processes will be studied using classic cytoadhesion studies (Avril, et al, 2008), newer microfluidic approaches (Herricks et al, 2011), and invasion assays of host cells modified with the most current methods to manipulate differentiation of proginator erythroid lineages (Crosnier, et al., 2011).
A second set of studies will focus on variations in the physical characteristics of infected red blood cells and possible variations in spleenic clearances of parasitized RBCs. These studies will utilize variations of microfluidic devices that capture limits of deformability of thousands of circulating red blood cells in any patient (Herricks, et al, 2009).
Upon invasion, malaria parasites affect the shape, rigidity and minimal cell diameter of an infected cell (Cranston, et al 1984; Miller, L.H., et al 1971), making them more susceptible to filtration (David, P. H. et al, 1983; Dondorp, A.M., et al, 2002). Differences in splenic filtration may control the number of infected erythrocytes that survive in peripheral circulation, which may affect the pathological progression during malaria infection (Dondorf, A.M., et al 1997, 2002). Stringent filtration of infected erythrocytes may lead to a more slow progression of infection leading to anemia. Less stringent filtration may lead to the more rapid onset of acute symptoms by allowing parasite load to increase at a higher rate. The rate of infected erythrocyte pitting or splenic filtration may further relate to hemoglobinopathies such as the sickle cell trait or thalassemia; many of which appear to have been selected by malaria parasites.
Our group has developed novel microfluidic assays that allow us to indirectly observe splenic filtration by measuring surface area, volume and minimum cylindrical diameter of large numbers of infected and uninfected erythrocytes ex vivo from malaria infections (Herricks, et al, 2009). In this project, we will investigate if splenic filtration is a modifier of disease severity. Specifically, we will investigate if splenic filtration capacities are innate or altered during the course of malaria infection, how splenic filtration function may be modulated by hemoglobinopathies, and investigate if there are differences in splenic filtration between individuals and how this is associated with infection outcome (hyperparasitemia or severe malaria anemia).
7.4 Host Genetics
Given the distribution of genetic diversity in India (Majumder P.P., 2010), the MESA-ICEMR team will also determine the relationships between the genetic background of human hosts and malaria disease severity. The goal of the human genetics studies will be to identify host genomic factors involved in protection from P. falciparum and P. vivax infections or disease. The studies will help identify polymorphisms in genes from immunological and inflammatory pathways of individuals who present with severe and asymptomatic infections either with the Accelerated Resistance to Multiple Drugs (ARMD) trait or without. Additionally, genetic polymorphisms at the whole-genome level associated with susceptibility to Plasmodium infections and delayed clearance of parasitemia after use of common antimalarials will be identified.
The proportions of malaria cases due to P. falciparum and P. vivax vary considerably across geographical regions of India. Mutations in various human globin genes, the glucose-6-phosphate dehydrogenase gene, and the Duffy blood-group locus are known to reduce malaria susceptibility or to alter the clinical course of this disease (Abu-Zeid, YA, et al 1992; Balgir, RS, et al, 2008; Sarkar, et al 2010; Williams, et al, 2005). Mechanism of protection may also involve innate or adaptive immunity, cytoadherence, and inflammation, with pregnancy malaria often best described (Anderson, P. et al, 2008; Avril, M. et al, 2008; Bockhorst, J., et al 2007; Duffy, P.E., Fried, M. 2003; Fried, M., and Duffy, P.E. 1996; Fried, M., et al 1998; Kyriacou, H.M., et al, 2006; Marsh, K., et al, 1989; O’Neil-Dunne, I., et al, 2001; Schnitzer, B., et al, 1972).
There are two alternate approaches to identify human genes that confer protection against infectious disease. The first is to identify suspected candidates based on knowledge of malaria-associated alterations in human physiology and immunity (Abu-Zeid, YA, et al 1992; Balgir, RS, et al, 2008; Sarkar, et al 2010; Williams, et al, 2005). This approach has the advantage that most scientists have faith in molecular or cellular precedents. The disadvantage is that this approach limits discovery of new genes that confer resistance since the approach only looks at a few candidate genes. The second approach is to take an open ended genome-wide search at what confers protection (Jallow, M., et al 2009). Historically, the disadvantage has been that this approach is very expensive and technology has limited the resolution at which the genome could be probed. Recently, the advantages have been winning out as causes of complex diseases are being deciphered successfully and the cost and technology allow development of genome-wide association techniques (Ozaki, K., et al, 2002; Richards, J.B., et al 2008; Majumder, P.P., 2009; Scott, L.J.,2007).
Variations in traditional, specific human gene candidates for protection against infections will be examined along with Genome-Wide Association Studies to find new genes in Indian populations that may also confer protection. It is of particular interest to see if protective traits in humans weaken when the parasite populations bear the ARMD trait compared to when they do not.
The MESA-ICEMR will also investigate contributions of innate and adaptive immunity functioning with genetic hemoglobinopathies and red cell disorders such as HbS, HbE, HbC, and glucose-6-phoshate deficiency to confer protection against malaria.
8. Summary
The ten worldwide ICEMR Program Projects vary tremendously in terms of disease distribution in each country, the type of research currently ongoing or previously conducted in the region, the existing and missing research structures in-country, and what new things can be accomplished by leveraging ICEMR resources to build in-country capacity. At least at the start of our ICEMR operations, it is important to get a handle on variations in malaria presentations within India. In future, as based on our findings and as resources allow, our studies will be expanded to additional neighboring countries. In an accompanying review in this issue (Kumar, A., et al 2012), we discussed in some detail the prevalence and control measures against malaria in South Asia, but also the tremendous economic and social growth that South Asia is experiencing and the healthcare expectations and challenges that come with such growth. In the present review, we have presented a context in which the activities of the MESA-ICEMR will fit within and strengthen the broader malaria research activities in South Asia and how pursuit of a specific hypothesis, the ability of malaria parasites to change rapidly, may affect malaria epidemiology in different parts of South Asia. We believe these activities will provide significance relevant data to control and surveillance programs and will help expand malaria research capabilities and efficacy in the region.
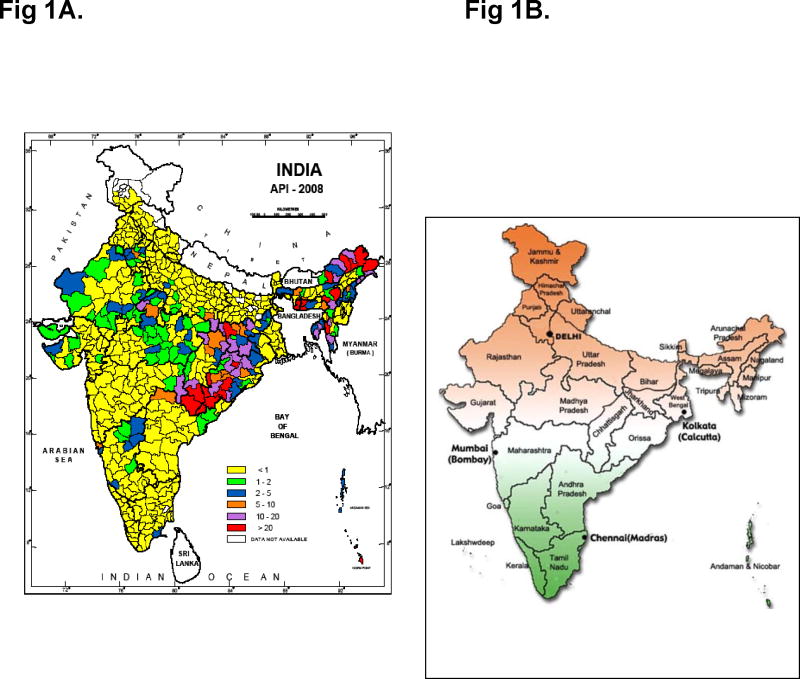
Fig. 1.
Annual Parasite Incidence (malaria cases/1000 population) in India for the year 2008 (Left, National Vector Borne Disease Control Programme, New Delhi). State map of India (right, http://blog.lookindia.in)
Fig. 2.
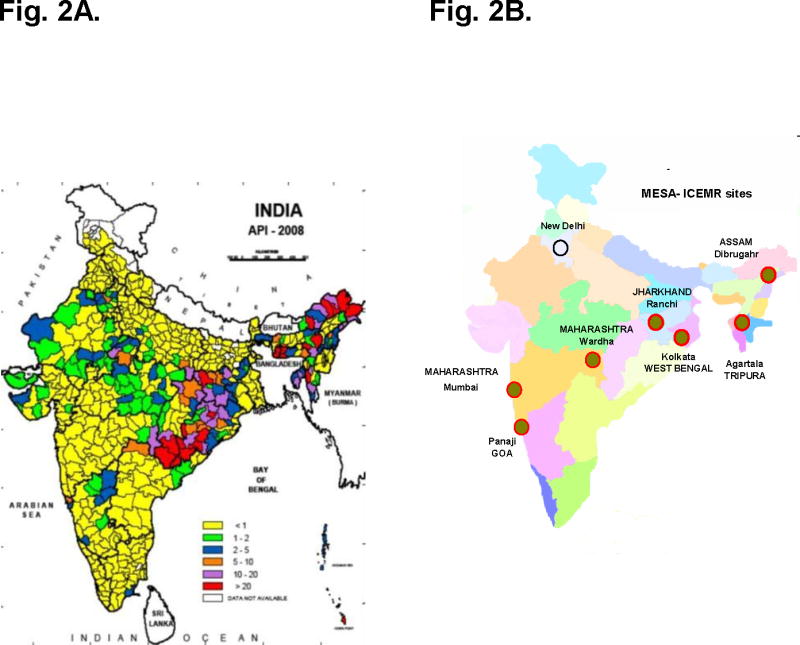
Fig. 2A. Annual Parasite Incidence (malaria cases/1000 population) in India for the year 2008 (Left, National Vector Borne Disease Control Programme, New Delhi).
Fig. 2B. Distribution of MESA- ICEMR study sites across India. (right)
Fig. 3.
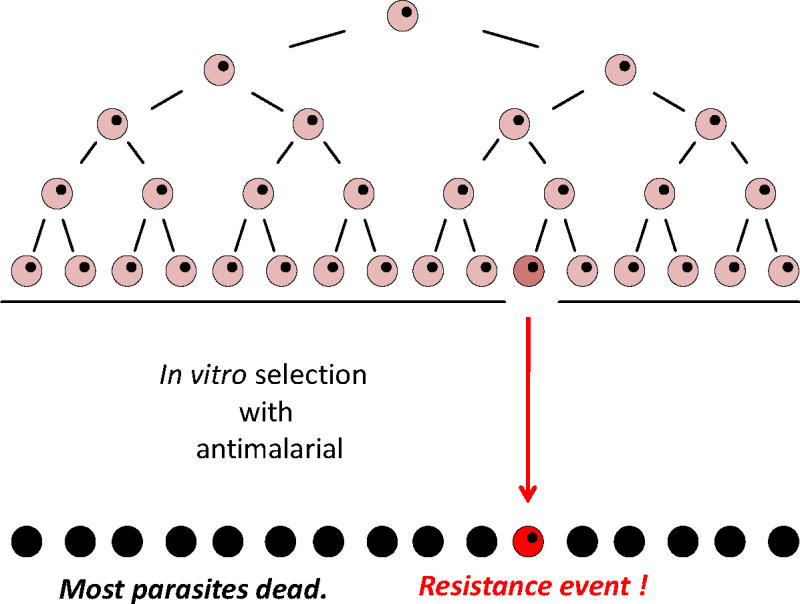
A generic scheme to illustrate how genetic plasticity is measured by selection of resistant parasites from a minimum number of freshly expanded P. falciparum cells required to reproducibly select for resistance to a new test antimalarial.
Fig. 4.
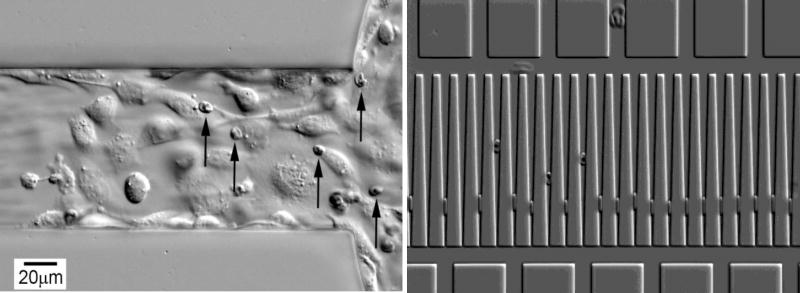
Illustration of two different microfluidic platforms to characterize physical properties of P. falciparum-infected RBCs from individual patients. (A) Cytoadhesion of surface attached mammalian endothelial cells to parasitized erythrocytes under flow. Unattached, uninfected RBCs are flowing too fast to be seen. (B) Entrapment of infected erythrocytes (upper set) compared to uninfected RBCs (Lower set). The penetration of individual cells into the wedges captures information on surface area and volume, which in turn is related to the deformability of RBCs.
Highlights.
A US-India team will study the origin of genetic diversity of malaria parasites
Genetic diversity may be driven by plasticity of some parasite populations
Plasticity will be directly assessed using de novo acquisition of drug resistance
High diversity may drive epidemiological trends, pathogenesis, vector compatibility, and human infectivity
Genes promoting virulent traits will be identified using genomic tools
Acknowledgments
The Program Project on “Malaria Evolution in South Asia” is an International Center of Excellence for Malaria Research (ICEMR) supported by the US National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIH/NIAID) grant U19 AI089688.
Abbreviations
- ICEMR
International Center of Excellence for Malaria Research
- NVBDCP
National Vector Borne Disease Control Programme
- MESA
Malaria Evolution in South Asia
- NIMR
National Institute of Malaria Research
- NIBMG
National Institute of Biomedical Genomics
- GWAS
Genome-Wide Association Studies
- ARMD
Accelerated Resistance to Multiple Drugs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Zeid YA, Abdulhadi NH, Theander TG, Hviid L, Saeed BO, Jepsen S, Jensen JB, Bayoumi RA. Seasonal changes in cell mediated immune responses to soluble Plasmodium falciparum antigens in children with haemoglobin AA and haemoglobin AS. Trans R Soc Trop Med Hyg. 1992;86:20–22. doi: 10.1016/0035-9203(92)90422-9. [DOI] [PubMed] [Google Scholar]
- Acharya P, Pallavi R, Chandran S, Chakravarti H, Middha S, Acharya J, Kochar S, Kochar D, Subudhi A, Boopathi AP, Garg S, Das A, Tatu U. A glimpse into the clinical proteome of human malaria parasites Plasmodium falciparum and Plasmodium vivax. Proteomics Clin Appl. 2009;3:1314–1325. doi: 10.1002/prca.200900090. [DOI] [PubMed] [Google Scholar]
- Acharya P, Pallavi R, Chandran S, Dandavate V, Sayeed SK, Rochani A, Acharya J, Middha S, Kochar S, Kochar D, Ghosh SK, Tatu U. Clinical Proteomics of the Neglected Human Malarial Parasite Plasmodium vivax. PLoS One. 2011;6:e26623. doi: 10.1371/journal.pone.0026623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Nielsen MA, Resende M, Rask TS, Dahlback M, Theander T, Lund O, Salanti A. Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog. 2008;4:e42. doi: 10.1371/journal.ppat.0040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril M, Kulasekara BR, Gose SO, Rowe C, Dahlback M, Duffy PE, Fried M, Salanti A, Misher L, Narum DL, Smith JD. Evidence for globally shared, cross-reacting polymorphic epitopes in the pregnancy-associated malaria vaccine candidate VAR2CSA. Infect Immun. 2008;76:1791–1800. doi: 10.1128/IAI.01470-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi G, Prasad GB, Das A. Population genetic analyses of Plasmodium falciparum chloroquine receptor transporter gene haplotypes reveal the evolutionary history of chloroquine-resistant malaria in India. Int J Parasitol. 2011;41:705–709. doi: 10.1016/j.ijpara.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Balgir RS. Hematological profile of twenty-nine tribal compound cases of hemoglobinopathies and G-6-PD deficiency in rural Orissa. Indian J Med Sci. 2008;62:362–371. [PubMed] [Google Scholar]
- Baliga KV, Uday Y, Sood V, Nagpal A. Acute febrile hepato-renal dysfunction in the tropics: co-infection of malaria and leptospirosis. J Infect Chemother. 2011;17:694–697. doi: 10.1007/s10156-011-0220-7. [DOI] [PubMed] [Google Scholar]
- Bambardekar K, Dharmadhikari AK, Dharmadhikari JA, Mathur D, Sharma S. Measuring erythrocyte deformability with fluorescence, fluid forces, and optical trapping. J Biomed Opt. 2008;13:064021. doi: 10.1117/1.3037342. [DOI] [PubMed] [Google Scholar]
- Bulusu V, Jayaraman V, Balaram H. Metabolic fate of fumarate, a side product of the purine salvage pathway in the intraerythrocytic stages of Plasmodium falciparum. J Biol Chem. 2011;286:9236–9245. doi: 10.1074/jbc.M110.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson JG, Rogerson SJ, Cooke BM, Reeder JC, Chai W, Lawson AM, Molyneux ME, Brown GV. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat Med. 2000;6:86–90. doi: 10.1038/71582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, Smego RA., Jr Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg. 2002;67:230–232. doi: 10.4269/ajtmh.2002.67.230. [DOI] [PubMed] [Google Scholar]
- Bockhorst J, Lu F, Janes JH, Keebler J, Gamain B, Awadalla P, Su XZ, Samudrala R, Jojic N, Smith JD. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol Biochem Parasitol. 2007;155:103–112. doi: 10.1016/j.molbiopara.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Chitnis CE, Sharma A. Targeting the Plasmodium vivax Duffy-binding protein. Trends Parasitol. 2008;24:29–34. doi: 10.1016/j.pt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Choudhary V, Sharma YD. Extensive heterozygosity in flanking microsatellites of Plasmodium falciparum Na+/H+ exchanger (pfnhe-1) gene among Indian isolates. Acta Trop. 2009;109:241–244. doi: 10.1016/j.actatropica.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Chauhan VS, Yazdani SS, Gaur D. Malaria vaccine development based on merozoite surface proteins of Plasmodium falciparum. Hum Vaccin. 2010;6 doi: 10.4161.hv.6.9.12468. [DOI] [PubMed] [Google Scholar]
- Cranston HA, Boylan CW, Carroll GL, Sutera SP, Williamson JR, Gluzman IY, Krogstad DJ. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science. 1984;223:400–403. doi: 10.1126/science.6362007. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, Rayner JC, Wright GJ. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–7. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Gupta RK, Thakor HG, Sonal GS, Dhillon GP. New perspectives of malaria control in India under World Bank Project. J Indian Med Assoc. 2009;107:870. 879-880, 882-873. [PubMed] [Google Scholar]
- Das S, Shevade S, LaCount DJ, Jarori GK. Plasmodium falciparum enolase complements yeast enolase functions and associates with the parasite food vacuole. Mol Biochem Parasitol. 2011;179:8–17. doi: 10.1016/j.molbiopara.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David PH, Hommel M, Miller LH, Udeinya IJ, Oligino LD. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A. 1983;80:5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa, Alwar VA, Rameshkumar K, Ross C. ABO blood groups and malaria related clinical outcome. J Vector Borne Dis. 2011;48:7–11. [PubMed] [Google Scholar]
- Dey S, Guha M, Alam A, Goyal M, Bindu S, Pal C, Maity P, Mitra K, Bandyopadhyay U. Malarial infection develops mitochondrial pathology and mitochondrial oxidative stress to promote hepatocyte apoptosis. Free Radic Biol Med. 2009;46:271–281. doi: 10.1016/j.freeradbiomed.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Dharia NV, Sidhu AB, Cassera MB, Westenberger SJ, Bopp SE, Eastman RT, Plouffe D, Batalov S, Park DJ, Volkman SK, Wirth DF, Zhou Y, Fidock DA, Winzeler EA. Use of high-density tiling microarrays to identify mutations globally and elucidate mechanisms of drug resistance in Plasmodium falciparum. Genome Biol. 2009;10:R21. doi: 10.1186/gb-2009-10-2-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhangadamajhi G, Kar SK, Ranjit MR. High prevalence and gender bias in distribution of Plasmodium malariae infection in central east-coast India. Trop Biomed. 2009;26:326–333. [PubMed] [Google Scholar]
- Dhangadamajhi G, Rout BK, Kar SK, Ranjit MR. Genetic diversity of Plasmodium vivax in a hyperendemic area predominated by Plasmodium falciparum; a preliminary study. Trop Biomed. 2010;27:578–584. [PubMed] [Google Scholar]
- Dhiman S, Goswami D, Rabha B, Gopalakrishnan R, Baruah I, Singh L. Malaria epidemiology along Indo-Bangladesh border in Tripura State, India. Southeast Asian J Trop Med Public Health. 2010;41:1279–1289. [PubMed] [Google Scholar]
- Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, Bassani DG, Suraweera W, Laxminarayan R, Peto R. Adult and child malaria mortality in India: a nationally representative mortality survey. The Lancet. 2010;376:1768–1774. doi: 10.1016/S0140-6736(10)60831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Angus BJ, Hardeman MR, Chotivanich KT, Silamut K, Ruangveerayuth R, Kager PA, White NJ, Vreeken J. Prognostic significance of reduced red blood cell deformability in severe falciparum malaria. Am J Trop Med Hyg. 1997;57:507–511. doi: 10.4269/ajtmh.1997.57.507. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, et al. Artemisinin Resistance in Plasmodium falciparum Malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Nyanoti M, Kager PA, Mithwani S, Vreeken J, Marsh K. The role of reduced red cell deformability in the pathogenesis of severe falciparum malaria and its restoration by blood transfusion. Trans R Soc Trop Med Hyg. 2002;96:282–286. doi: 10.1016/s0035-9203(02)90100-8. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Yeung S, White L, Nguon C, Day NPJ, Socheat D, von Seidlein L. Artemisinin resistance: current status and scenarios for containment. Nature Reviews Microbiology. 2010;8:272–280. doi: 10.1038/nrmicro2331. (2010) [DOI] [PubMed] [Google Scholar]
- Duffy PE, Fried M. Antibodies That Inhibit Plasmodium falciparum Adhesion to Chondroitin Sulfate A Are Associated with Increased Birth Weight and the Gestational Age of Newborns. Infect Immun. 2003;71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- Garg S, Saxena V, Kanchan S, Sharma P, Mahajan S, Kochar D, Das A. Novel point mutations in sulfadoxine resistance genes of Plasmodium falciparum from India. Acta Trop. 2009;110:75–79. doi: 10.1016/j.actatropica.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Ganesan K, Ponmee N, Jiang L, Fowble JW, White J, Kamchonwongpaisan S, Yuthavong Y, Wilairat P, Rathod PK. A genetically hard-wired metabolic transcriptome in Plasmodium falciparum fails to mount protective responses to lethal antifolates. PLoS Pathog. 2008;4:e1000214. doi: 10.1371/journal.ppat.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur D, Chitnis CE. Molecular interactions and signaling mechanisms during erythrocyte invasion by malaria parasites. Curr Opin Microbiol. 2011;14:422–428. doi: 10.1016/j.mib.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Gill J, Chitnis CE, Sharma A. Structural insights into chondroitin sulphate A binding Duffy-binding-like domains from Plasmodium falciparum: implications for intervention strategies against placental malaria. Malar J. 2009;8:67. doi: 10.1186/1475-2875-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogia S, Balaram H, Puranik M. Hypoxanthine guanine phosphoribosyltransferase distorts the purine ring of nucleotide substrates and perturbs the pKa of bound xanthosine monophosphate. Biochemistry. 2011;50:4184–4193. doi: 10.1021/bi102039b. [DOI] [PubMed] [Google Scholar]
- Gupta B, Awasthi G, Das A. Malaria parasite genome scan: insights into antimalarial resistance. Parasitol Res. 2010;107:495–499. doi: 10.1007/s00436-010-1917-8. [DOI] [PubMed] [Google Scholar]
- Harris VK, Richard VS, Mathai E, Sitaram U, Kumar KV, Cherian AM, Amelia SM, Anand G. A study on clinical profile of falciparum malaria in a tertiary care hospital in south India. Indian J Malariol. 2001;38:19–24. [PubMed] [Google Scholar]
- Herricks T, Antia M, Rathod PK. Deformability limits of Plasmodium falciparum-infected red blood cells. Cell Microbiol. 2009;11:1340–1353. doi: 10.1111/j.1462-5822.2009.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herricks T, Seydel KB, Turner G, Molyneux M, Heyderman R, Taylor T, Rathod PK. A microfluidic system to study cytoadhesion of Plasmodium falciparum infected erythrocytes to primary brain microvascularendothelial cells. Lab Chip. 2011;11:2994–3000. doi: 10.1039/c1lc20131j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICMR-WHO Health Research Directory. 2007 ( http://www.whoindia.org/linkfiles/medical_ethics_directory_of_health_research_institutions.pdf)
- Imam M, Devi YS, Verma AK, Chauhan VS. Comparative Immunogenicities of full-length Plasmodium falciparum merozoite surface protein 3 and a 24-kilodalton N-terminal fragment. Clin Vaccine Immunol. 2011;18:1221–1228. doi: 10.1128/CVI.00064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallow M, Teo YY, Small KS, Rockett KA, Deloukas P, Clark TG, Kivinen K, Bojang KA, Conway DJ, Pinder M, Sirugo G, Sisay-Joof F, Usen S, Auburn S, Bumpstead SJ, Campino S, Coffey A, Dunham A, Fry AE, Green A, Gwilliam R, Hunt SE, Inouye M, Jeffreys AE, Mendy A, Palotie A, Potter S, Ragoussis J, Rogers J, Rowlands K, Somaskantharajah E, Whittaker P, Widden C, Donnelly P, Howie B, Marchini J, Morris A, SanJoaquin M, Achidi EA, Agbenyega T, Allen A, Amodu O, Corran P, Djimde A, Dolo A, Doumbo OK, Drakeley C, Dunstan S, Evans J, Farrar J, Fernando D, Hien TT, Horstmann RD, Ibrahim M, Karunaweera N, Kokwaro G, Koram KA, Lemnge M, Makani J, Marsh K, Michon P, Modiano D, Molyneux ME, Mueller I, Parker M, Peshu N, Plowe CV, Puijalon O, Reeder J, Reyburn H, Riley EM, Sakuntabhai A, Singhasivanon P, Sirima S, Tall A, Taylor TE, Thera M, Troye-Blomberg M, Williams TN, Wilson M, Kwiatkowski DP. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat Genet. 2009;41:657–665. doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochar DK, Das A, Kochar A, Middha S, Acharya J, Tanwar GS, Gupta A, Pakalapati D, Garg S, Saxena V, Subudhi AK, Boopathi PA, Sirohi P, Kochar SK. Thrombocytopenia in Plasmodium falciparum, Plasmodium vivax and mixed infection malaria: a study from Bikaner (Northwestern India) Platelets. 2010;21:623–627. doi: 10.3109/09537104.2010.505308. [DOI] [PubMed] [Google Scholar]
- Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A. Plasmodium vivax malaria. Emerg Infect Dis. 2005;11:132–134. doi: 10.3201/eid1101.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chery L, et al. Malaria in South Asia: Prevalence and Control. Acta Tropica. 2012 doi: 10.1016/j.actatropica.2012.01.004. (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Dua VK, Rathod PK. Malaria-attributed death rates in India. Lancet. 2011;377:991–992. doi: 10.1016/S0140-6736(11)60379-6. author reply 994-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Chaubey S, Shah P, Tanveer A, Charan M, Siddiqi MI, Habib S. Interaction between sulphur mobilisation proteins SufB and SufC: evidence for an iron-sulphur cluster biogenesis pathway in the apicoplast of Plasmodium falciparum. Int J Parasitol. 2011a;41:991–999. doi: 10.1016/j.ijpara.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kumar N, Khan SI, Atheaya H, Mamgain R, Rawat DS. Synthesis and in vitro antimalarial activity of tetraoxane-amine/amide conjugates. Eur J Med Chem. 2011b;46:2816–2827. doi: 10.1016/j.ejmech.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77:69–78. [PubMed] [Google Scholar]
- Kyriacou HM, Stone GN, Challis RJ, Raza A, Lyke KE, Thera MA, Kone AK, Doumbo OK, Plowe CV, Rowe JA. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol Biochem Parasitol. 2006;150:211–218. doi: 10.1016/j.molbiopara.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi V, Srivastava S, Mishra SK, Srivastava MN, Srivastava K, Puri SK. Antimalarial activity in Xylocarpus granatum (Koen) Nat Prod Res. 2011 doi: 10.1080/14786419.2010.535000. [DOI] [PubMed] [Google Scholar]
- Lumb V, Das MK, Mittra P, Ahmed A, Kumar M, Kaur P, Dash AP, Singh SS, Sharma YD. Emergence of an unusual sulfadoxine-pyrimethamine resistance pattern and a novel K540N mutation in dihydropteroate synthetase in Plasmodium falciparum isolates obtained from Car Nicobar Island, India, after the 2004 Tsunami. J Infect Dis. 2009a;199:1064–1073. doi: 10.1086/597206. [DOI] [PubMed] [Google Scholar]
- Lumb V, Das MK, Singh N, Dev V, Khan W, Sharma YD. Multiple origins of Plasmodium falciparum dihydropteroate synthetase mutant alleles associated with sulfadoxine resistance in India. Antimicrob Agents Chemother. 2011;55:2813–2817. doi: 10.1128/AAC.01151-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb V, Das MK, Singh N, Dev V, Wajihullah, Sharma YD. Characteristics of genetic hitchhiking around dihydrofolate reductase gene associated with pyrimethamine resistance in Plasmodium falciparum isolates from India. Antimicrob Agents Chemother. 2009b;53:5173–5180. doi: 10.1128/AAC.00045-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb V, Sharma YD. Novel K540N mutation in Plasmodium falciparum dihydropteroate synthetase confers a lower level of sulfa drug resistance than does a K540E mutation. Antimicrob Agents Chemother. 2011;55:2481–2482. doi: 10.1128/AAC.01394-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan RC, Narain K, Mahanta J. Anaemia & expression levels of CD35, CD55 & CD59 on red blood cells in Plasmodium falciparum malaria patients from India. Indian J Med Res. 2011;133:662–664. [PMC free article] [PubMed] [Google Scholar]
- Maity K, Venkata BS, Kapoor N, Surolia N, Surolia A, Suguna K. Structural basis for the functional and inhibitory mechanisms of beta-hydroxyacyl-acyl carrier protein dehydratase (FabZ) of Plasmodium falciparum. J Struct Biol. 2011;176:238–249. doi: 10.1016/j.jsb.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Majumder PP. The human genetic history of South Asia. Curr Biol. 2010;20(4):R184–7. doi: 10.1016/j.cub.2009.11.053. [DOI] [PubMed] [Google Scholar]
- Majumder PP, Staats HF, Sarkar-Roy N, Varma B, Ghosh T, Maiti S, Narayanasamy K, Whisnant CC, Stephenson JL, Wagener DK. Genetic determinants of immune-response to a polysaccharide vaccine for typhoid. Hugo J. 2009;3(1-4):17–30. doi: 10.1007/s11568-010-9134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- Mayor A, Rovira-Vallbona E, Srivastava A, Sharma SK, Pati SS, Puyol L, Quinto L, Bassat Q, Machevo S, Mandomando I, Chauhan VS, Alonso PL, Chitnis CE. Functional and immunological characterization of a Duffy binding-like alpha domain from Plasmodium falciparum erythrocyte membrane protein 1 that mediates rosetting. Infect Immun. 2009;77:3857–3863. doi: 10.1128/IAI.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- Miller LH, Usami S, Chien S. Alteration in the rheologic properties of Plasmodium knowlesi-infected red cells. A possible mechanism for capillary obstruction. J Clin Invest. 1971;50:1451–1455. doi: 10.1172/JCI106629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K, Dash AP, Dey N. Andrographolide: A Novel Antimalarial Diterpene Lactone Compound from Andrographis paniculata and Its Interaction with Curcumin and Artesunate. J Trop Med. 2011;2011:579518. doi: 10.1155/2011/579518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mixson-Hayden T, Jain V, McCollum AM, Poe A, Nagpal AC, Dash AP, Stiles JK, Udhayakumar V, Singh N. Evidence of selective sweeps in genes conferring resistance to chloroquine and pyrimethamine in Plasmodium falciparum isolates in India. Antimicrob Agents Chemother. 2010;54:997–1006. doi: 10.1128/AAC.00846-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Swain S, Singh DV, Mahapatra N, Kar SK, Hazra RK. A unique methodology for detecting the spread of chloroquine-resistant strains of Plasmodium falciparum, in previously unreported areas, by analyzing anophelines of malaria endemic zones of Orissa, India. Infect Genet Evol. 2009a;9:462–467. doi: 10.1016/j.meegid.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Mohanty S, Saklani S, Mahajan M. In silico characterization of genetic homology in nuclear-encoded apicoplast-targeted genes between Plasmodium falciparum & P. vivax. Indian J Med Res. 2009b;129:520–524. [PubMed] [Google Scholar]
- Mullick S, Das S, Guha SK, Bera DK, Sengupta S, Roy D, Saha P, Biswas A, Das M, Ray K, Kundu PK, Maji AK. Efficacy of Chloroquine and Sulphadoxine-Pyrimethamine either alone or in combination before introduction of ACT as first-line therapy in uncomplicated Plasmodium falciparum malaria in Jalpaiguri District, West Bengal, India. Trop Med Int Health. 2011 doi: 10.1111/j.1365-3156.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- Nagaraj VA, Arumugam R, Prasad D, Rangarajan PN, Padmanaban G. Protoporphyrinogen IX oxidase from Plasmodium falciparum is anaerobic and is localized to the mitochondrion. Mol Biochem Parasitol. 2010a;174:44–52. doi: 10.1016/j.molbiopara.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Nagaraj VA, Prasad D, Arumugam R, Rangarajan PN, Padmanaban G. Characterization of coproporphyrinogen III oxidase in Plasmodium falciparum cytosol. Parasitol Int. 2010b;59:121–127. doi: 10.1016/j.parint.2009.12.001. [DOI] [PubMed] [Google Scholar]
- O'Neil-Dunne I, Achur RN, Agbor-Enoh ST, Valiyaveettil M, Naik RS, Ockenhouse CF, Zhou A, Megnekou R, Leke R, Taylor DW, Gowda DC. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect Immun. 2001;69:7487–7492. doi: 10.1128/IAI.69.12.7487-7492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Ohnishi Y, Iida A, Sekine A, Yamada R, Tsunoda T, Sato H, Hori M, Nakamura Y, Tanaka T. Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nat Genet. 2002;32:650–654. doi: 10.1038/ng1047. [DOI] [PubMed] [Google Scholar]
- Ozarkar AD, Prakash D, Deobagkar DN, Deobagkar DD. Prediction of B cell and T cell epitopes of DBLalpha domain in Plasmodium falciparum malaria vaccine candidate var gene. Protein Pept Lett. 2007;14:528–530. doi: 10.2174/092986607780989967. [DOI] [PubMed] [Google Scholar]
- Palanichamy MG, Sun C, Agrawal S, Bandelt HJ, Kong QP, Khan F, Wang CY, Chaudhuri TK, Palla V, Zhang YP. Phylogeny of mitochondrial DNA macrohaplogroup N in India, based on complete sequencing: implications for the peopling of South Asia. Am J Hum Genet. 2004;75:966–978. doi: 10.1086/425871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise ME, Lewis LS, Ayisi JG, Nahlen BL, Slutsker L, Muga R, Sharif SK, Hill J, Steketee RW. A rapid assessment approach for public health decision-making related to the prevention of malaria during pregnancy. Bulletin of the World Health Organization. 2003;81:316–323. [PMC free article] [PubMed] [Google Scholar]
- Parija SC. PCR for diagnosis of malaria. Indian J Med Res. 2010;132:9–10. [PubMed] [Google Scholar]
- Parween S, Gupta PK, Chauhan VS. Induction of humoral immune response against PfMSP-1(19) and PvMSP-1(19) using gold nanoparticles along with alum. Vaccine. 2011;29:2451–2460. doi: 10.1016/j.vaccine.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Patial RK, Kapoor D, Mokta JK. Cerebral dysfunction in vivax malaria: a case report. Indian J Med Sci. 1998;52:159–160. [PubMed] [Google Scholar]
- Patakottu BR, Singh PK, Malhotra P, Chauhan VS, Patankar S. In vivo analysis of translation initiation sites in Plasmodium falciparum. Mol Biol Rep. 2011 doi: 10.1007/s11033-011-0971-3. [DOI] [PubMed] [Google Scholar]
- Prusty D, Dar A, Priya R, Sharma A, Dana S, Choudhury NR, Rao NS, Dhar SK. Single-stranded DNA binding protein from human malarial parasite Plasmodium falciparum is encoded in the nucleus and targeted to the apicoplast. Nucleic Acids Res. 2010;38:7037–7053. doi: 10.1093/nar/gkq565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar T, Reddy KS, Bharadwaj M, Pandey AK, Singh S, Chitnis CE, Gaur D. Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PLoS One. 2011;6:e17102. doi: 10.1371/journal.pone.0017102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati SK, Joshi H, Dev V, Dua VK. Molecular epidemiology of Plasmodium vivax anti-folate resistance in India. Malar J. 2011;10:102. doi: 10.1186/1475-2875-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh V, Elamaran M, Vidya S, Gowrishankar M, Kochar D, Das A. Plasmodium vivax: genetic diversity of the apical membrane antigen-1 (AMA-1) in isolates from India. Exp Parasitol. 2007;116:252–256. doi: 10.1016/j.exppara.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Ranjan R, Chugh M, Kumar S, Singh S, Kanodia S, Hossain MJ, Korde R, Grover A, Dhawan S, Chauhan VS, Reddy VS, Mohmmed A, Malhotra P. Proteome analysis reveals a large merozoite surface protein-1 associated complex on the Plasmodium falciparum merozoite surface. J Proteome Res. 2011;10:680–691. doi: 10.1021/pr100875y. [DOI] [PubMed] [Google Scholar]
- Rawat M, Vijay S, Gupta Y, Dixit R, Tiwari PK, Sharma A. Sequence homology and structural analysis of plasmepsin 4 isolated from Indian Plasmodium vivax isolates. Infect Genet Evol. 2011;11:924–933. doi: 10.1016/j.meegid.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Rout R, Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. High CR1 level and related polymorphic variants are associated with cerebral malaria in eastern-India. Infect Genet Evol. 2011;11:139–144. doi: 10.1016/j.meegid.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Rout R, Mohapatra BN, Kar SK, Ranjit M. Genetic complexity and transmissibility of Plasmodium falciparum parasites causing severe malaria in central-east coast India. Trop Biomed. 2009;26:165–172. [PubMed] [Google Scholar]
- Rao M. The International Centres of Excellence for Malaria research. Acta Tropica. 2011 doi: 10.1016/j.actatropica.2011.07.009. (in press, this issue) [DOI] [PubMed] [Google Scholar]
- Rao TR. The Anophilines of India. Malaria Research Centre, Indian Council of Medical Research 1983 [Google Scholar]
- Rathod PK, Khosla M, Gassis S, Young RD, Lutz C. Selection and characterization of 5-fluoroorotate-resistant Plasmodium falciparum. Antimicrob Agents Chemother. 1994;38:2871–2876. doi: 10.1128/aac.38.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod PK, McErlean T, Lee PC. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, Andrew T, Falchi M, Gwilliam R, Ahmadi KR, Valdes AM, Arp P, Whittaker P, Verlaan DJ, Jhamai M, Kumanduri V, Moorhouse M, van Meurs JB, Hofman A, Pols HA, Hart D, Zhai G, Kato BS, Mullin BH, Zhang F, Deloukas P, Uitterlinden AG, Spector TD. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371:1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Biswas NK, Dey B, Mukhopadhyay D, Majumder PP. A large, systematic molecular-genetic study of G6PD in Indian populations identifies a new non-synonymous variant and supports recent positive selection. Infect Genet Evol. 2010;10:1228–1236. doi: 10.1016/j.meegid.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Schnitzer B, Sodeman T, Mead ML, Contacos PG. Pitting function of the spleen in malaria: ultrastructural observations. Science. 1972;177:175–177. doi: 10.1126/science.177.4044.175. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NK, Dhillon GP, Dash AP, Arora U, Meshnick SR, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011;11:57–64. doi: 10.1016/S1473-3099(10)70214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VP. Hidden burden of malaria in Indian women. Malar J. 2009;8:281. doi: 10.1186/1475-2875-8-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. A new global malaria eradication strategy: implications for malaria research from an Indian perspective. Trans R Soc Trop Med Hyg. 2009;103:1202–1203. doi: 10.1016/j.trstmh.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Singh N, Shukla MM, Chand G, Bharti PK, Singh MP, Shukla MK, Mehra RK, Sharma RK, Dash AP. Epidemic of Plasmodium falciparum malaria in Central India, an area where chloroquine has been replaced by artemisinin-based combination therapy. Trans R Soc Trop Med Hyg. 2011;105:133–139. doi: 10.1016/j.trstmh.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Singh N, Shukla MM, Shukla MK, Mehra RK, Sharma S, Bharti PK, Singh MP, Singh A, Gunasekar A. Field and laboratory comparative evaluation of rapid malaria diagnostic tests versus traditional and molecular techniques in India. Malar J. 2010a;9:191. doi: 10.1186/1475-2875-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Alam MM, Pal-Bhowmick I, Brzostowski JA, Chitnis CE. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010b;6:e1000746. doi: 10.1371/journal.ppat.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid A, Ranjan R, Smythe WA, Hoppe HC, Sharma P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood. 2010;115:2500–2507. doi: 10.1182/blood-2009-08-238972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MK, Agarwal GS, Rao VK, Upadhyay S, Merwyn S, Gopalan N, Rai GP, Vijayaraghavan R, Prakash S. Amperometric immunosensor based on gold nanoparticles/alumina sol-gel modified screen-printed electrodes for antibodies to Plasmodium falciparum histidine rich protein-2. Analyst. 2010;135:608–614. doi: 10.1039/b918880k. [DOI] [PubMed] [Google Scholar]
- Sharma MK, Rao VK, Merwyn S, Agarwal GS, Upadhyay S, Vijayaraghavan R. A novel piezoelectric immunosensor for the detection of malarial Plasmodium falciparum histidine rich protein-2 antigen. Talanta. 2011;85:1812–1817. doi: 10.1016/j.talanta.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Chattopadhyay R, Chakrabarti K, Pati SS, Srivastava VK, Tyagi PK, Mahanty S, Misra SK, Adak T, Das BS, Chitnis CE. Epidemiology of malaria transmission and development of natural immunity in a malaria-endemic village, San Dulakudar, in Orissa state, India. Am J Trop Med Hyg. 2004;71:457–465. [PubMed] [Google Scholar]
- Sharma SK, Tyagi PK, Padhan K, Upadhyay AK, Haque MA, Nanda N, Joshi H, Biswas S, Adak T, Das BS, Chauhan VS, Chitnis CE, Subbarao SK. Epidemiology of malaria transmission in forest and plain ecotype villages in Sundargarh District, Orissa, India. Trans R Soc Trop Med Hyg. 2006;100:917–925. doi: 10.1016/j.trstmh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sharma VP, et al. In: Forest malaria in South East Asia. Centre MR, editor. New Delhi: 1991. [Google Scholar]
- Shukla RP, PAC, Mathur A. Investigations of malaria outbreak in Rajasthan. Indian J Malariology. 1995;32 [PubMed] [Google Scholar]
- Singh N, Shukla MM, Sharma VP. Epidemiology of malaria in pregnancy in central India. Bulletin of the World Health Organization. 1999;77:567–572. [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hora R, Belrhali H, Chitnis CE, Sharma A. Structural basis for Duffy recognition by the malaria parasite Duffy-binding-like domain. Nature. 2006;439:741–744. doi: 10.1038/nature04443. [DOI] [PubMed] [Google Scholar]
- Singh V, Mishra N, Awasthi G, Dash AP, Das A. Why is it important to study malaria epidemiology in India? Trends Parasitol. 2009;25:452–457. doi: 10.1016/j.pt.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Sinton JA. What malaria costs India. Government Press of India Press; Delhi: 1936. [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- Tanwar GS, Khatri PC, Sengar GS, Kochar A, Kochar SK, Middha S, Tanwar G, Khatri N, Pakalapati D, Garg S, Das A, Kochar DK. Clinical profiles of 13 children with Plasmodium vivax cerebral malaria. Ann Trop Paediatr. 2011;31:351–356. doi: 10.1179/1465328111Y.0000000040. [DOI] [PubMed] [Google Scholar]
- Trimnell AR, Kraemer SM, Mukherjee S, Phippard DJ, Janes JH, Flamoe E, Su XZ, Awadalla P, Smith JD. Global genetic diversity and evolution of var genes associated with placental and severe childhood malaria. Mol Biochem Parasitol. 2006;148:169–180. doi: 10.1016/j.molbiopara.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Valecha N, Bagga A, Chandra J, Sharma D. Cerebral symptoms with P. vivax malaria. Indian Pediatr. 1992;29:1176–1178. [PubMed] [Google Scholar]
- Valecha N, Joshi H, Eapen A, Ravinderan J, Kumar A, Prajapati SK, Ringwald P. Therapeutic efficacy of chloroquine in Plasmodium vivax from areas with different epidemiological patterns in India and their Pvdhfr gene mutation pattern. Trans R Soc Trop Med Hyg. 2006;100:831–837. doi: 10.1016/j.trstmh.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Valecha N, Joshi H, Mallick PK, Sharma SK, Kumar A, Tyagi PK, Shahi B, Das MK, Nagpal BN, Dash AP. Low efficacy of chloroquine: time to switchover to artemisinin-based combination therapy for falciparum malaria in India. Acta Trop. 2009;111:21–28. doi: 10.1016/j.actatropica.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Valecha N, Looareesuwan S, Martensson A, Abdulla SM, Krudsood S, Tangpukdee N, Mohanty S, Mishra SK, Tyagi PK, Sharma SK, Moehrle J, Gautam A, Roy A, Paliwal JK, Kothari M, Saha N, Dash AP, Bjorkman A. Arterolane, a new synthetic trioxolane for treatment of uncomplicated Plasmodium falciparum malaria: a phase II, multicenter, randomized, dose-finding clinical trial. Clin Infect Dis. 2010a;51:684–691. doi: 10.1086/655831. [DOI] [PubMed] [Google Scholar]
- Valecha N, Phyo AP, Mayxay M, Newton PN, Krudsood S, Keomany S, Khanthavong M, Pongvongsa T, Ruangveerayuth R, Uthaisil C, Ubben D, Duparc S, Bacchieri A, Corsi M, Rao BH, Bhattacharya PC, Dubhashi N, Ghosh SK, Dev V, Kumar A, Pukrittayakamee S. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One. 2010b5:e11880. doi: 10.1371/journal.pone.0011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valecha N, Pinto RG, Turner GD, Kumar A, Rodrigues S, Dubhashi NG, Rodrigues E, Banaulikar SS, Singh R, Dash AP, Baird JK. Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am J Trop Med Hyg. 2009;81:758–762. doi: 10.4269/ajtmh.2009.09-0348. [DOI] [PubMed] [Google Scholar]
- WHO. Global malaria control and elimination: report of a technical review. 2009. [Google Scholar]
- Williams TN, Mwangi TW, Roberts DJ, Alexander ND, Weatherall DJ, Wambua S, Kortok M, Snow RW, Marsh K. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2:e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, Magill AJ, Su XZ. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]