Abstract
The transmission of cytomegalovirus (CMV) from mother to fetus can give rise to severe neurodevelopment defects in newborns. One strategy to prevent these congenital defects is prophylactic vaccination in young women. A candidate vaccine antigen is glycoprotein B (gB). This antigen is abundant on the virion surface and is a major target of neutralization responses in human infections. Here, we have evaluated in a challenge model of congenital guinea pig CMV (GPCMV) infection, GPCMV-gB vaccines formulated with the clinically-relevant Adjuvant Systems AS01B and AS02V, or with Freund’s adjuvant (FA). Fifty-two GPCMV-seronegative female guinea pigs were administered three vaccine doses before being mated. GPCMV-challenge was performed at Day 45 of pregnancy (of an estimated 65 day gestation). Pup mortality rates in the gB/AS01B, gB/AS02V, and gB/FA groups were 24% (8/34), 10% (4/39) and 36% (12/33), respectively, and in the unvaccinated control group was 65% (37/57). Hence, efficacies against pup mortality were estimated at 64%, 84% and 44% for gB/AS01B (p<0.001), gB/AS02V (p<0.001) and gB/FA (p=0.014), respectively. Efficacies against GPCMV viremia (i.e DNAemia, detected by PCR) were estimated at 88%, 68% and 25% for the same vaccines, respectively, but were only significant for gB/AS01B (p<0.001), and gB/AS02V (p=0.002). In dams with viremia, viral load was approximately 6-fold lower with vaccination than without. All vaccines were highly immunogenic after two and three doses. In light of these results and of other results of AS01-adjuvanted vaccines in clinical development, vaccine immunogenicity was further explored using human CMV-derived gB antigen adjuvanted with either AS01B or the related formulation AS01E. Both adjuvanted vaccines were highly immunogenic after two doses, in contrast to the lower immunogenicity of the unadjuvanted vaccine. In conclusion, the protective efficacy and immunogenicity of adjuvanted vaccines in this guinea pig model are supportive of investigating gB/AS01 and gB/AS02 in the clinic.
Keywords: Cytomegalovirus, Guinea pig cytomegalovirus, Cytomegalovirus vaccine, Glycoprotein B, Adjuvant, Vaccine efficacy, Guinea pig challenge model
1. Introduction
Maternal infection with human cytomegalovirus (HCMV) during pregnancy occasionally causes severe disease in newborns, and can lead to neurodevelopmental sequelae and sensorineural deafness [1]. Congenital HCMV infection complicates an estimated 40 000 pregnancies annually in the United States [2]. A vaccine capable of protecting newborns from the sequelae of congenital HCMV infection is a major public health priority [3]. However, it is not clear what would constitute an optimal vaccine. Subunit vaccines that target the major envelope glycoprotein gB (gpUL55) have been evaluated for immunogenicity and safety in clinical trials [4–7]. Although there has been no efficacy evaluation of an HCMV vaccine for prevention of congenital infection to date, a clinical study has demonstrated the proof-of-concept of protection against primary CMV infection in women with an adjuvanted gB-based formulation [5].
Efficacy evaluations of vaccines against homologous, species-specific CMVs can be performed using the guinea pig cytomegalovirus (GPCMV) model [8;9]. In contrast to the CMVs of most small animals, CMV in guinea pigs causes maternal viremia and disease, can cross the placenta, and can infect fetuses resulting in still-born offspring [10;11]. Furthermore the anatomy of the guinea pig placenta more closely resembles the human placenta than in other small laboratory animals [12–15]. Preconception immunization of guinea pigs with recombinant GPCMV gB, administered either as a DNA vaccine or purified baculovirus-expressed protein vaccine, has been shown to protect against GPCMV challenge made during pregnancy, as measured by the reduction of pup mortality and infection, and by the reduction of maternal and fetal viral load [16;17]. Moreover, protection conferred by gB subunit vaccination has been found to be highly dependent upon the adjuvant employed. Lower rates of maternal GPCMV-viral load and maternal-fetal transmission have been observed in dams immunized with gB formulated with Freund’s adjuvant (gB/FA) compared with dams immunized with gB formulated with alum (gB/alum) [17]. Although effective in non-clinical vaccines, FA is not a clinically-relevant adjuvant, and observations regarding the efficacy of this adjuvant are of limited applicability to human clinical trials. In contrast, GlaxoSmithKline’s (GSK’s) proprietary Adjuvant Systems AS01B, AS01E and AS02V are clinically relevant and have been included in a variety of candidate vaccines evaluated up to Phase III [18–20]. These three Adjuvant Systems contain the immunostimulants 3-O-desacyl-4′-monophosphoryl lipid A (MPL) and QS-21 and can promote both antibody and cell-mediated immune responses to subunit antigens [18]. Therefore we have performed two preclinical studies to evaluate gB antigens formulated with these Adjuvant Systems.
In the first study, GPCMV gB vaccines formulated with AS01B or AS02V, were evaluated in comparison with gB/FA, in the guinea pig challenge model of congenital CMV infection. Vaccine efficacies were estimated from maternal protection against GPCMV viremia and offspring mortality, in addition to measuring vaccine immunogenicity. In the second study, the immunogenicity of the human (H)CMV-derived gB antigen (hgB) formulated with AS01B or its related formulation AS01E was evaluated in guinea pigs.
2. Materials and methods
2.1. Animal husbandry
Study 1 was performed at the University of Minnesota (Minneapolis, MN, USA). Inbred adult strain-2 guinea pigs for preparation of salivary gland passaged-GPCMV stocks were obtained from the University of Minnesota. Age-matched young female and breeder male Hartley guinea pigs were obtained from Harlan Laboratories (Indianapolis, IN, USA). All animals were confirmed to be GPCMV-seronegative by ELISA [17]. Animals were housed under conditions approved by the American Association of Accreditation of Laboratory Animal Care, in accordance with institutional animal use committee policies at the University of Minnesota. Study 2 was performed using age-matched female Hartley guinea pigs (obtained from Charles River, Lyon, France) at GSK Vaccines (Rixensart, Belgium) in accordance with the Belgian national guidelines for animal experimentation.
2.2. CMV stocks
GPCMV (strain no. 22122, ATCC VR682) used in neutralization assays was propagated in guinea pig fibroblast lung cell cultures (GPL; ATCC CCL 158) maintained in F-12 medium supplemented with 10% fetal calf serum (FCS, Fisher Scientific), 10,000 IU/l penicillin, 10 mg/l streptomycin (Gibco-BRL) and 7.5% NaHCO3 (Gibco-BRL). Salivary gland-passaged GPCMV stocks (SG virus) used for animal challenge studies were prepared by sequential passage in strain-2 guinea pigs. HCMV (AD169 strain) from Novasep was propagated in MRC5 fibroblast cell line culture (obtained from Novasep).
2.3. Vaccines
Viral stocks of recombinant baculovirus expressing GPCMV gB were maintained in Spondoptera frugiperda (Sf9) cell cultures. GPCMV gB was produced in Trichoplusia ni cells infected at high multiplicity with recombinant baculovirus. Infected-cell lysates were subjected to lectin column chromatography and elution as previously described [17;21]. Although one-step purification by lectin column chromatography did not purify GPCMV gB to complete homogeneity, the preparation was highly enriched for gB, as gauged by SDS-PAGE and western blot analysis (data not shown) [17;21]. The HCMV gB (hgB) antigen, a recombinant fusion protein consisting of the extracellular domain of the native gB (AD169 strain) and peptide sequences from glycoprotein gD of Herpes Simplex virus 2 (HSV2), was manufactured through a vaccine development and production agreement between GSK Biologicals SA and Henogen SA, Belgium. The hgB antigen had been purified to >95% homogeneity from transfected Chinese Hamster Ovary cells. One 1 mL vaccine dose contained 50 μg GPCMV gB antigen, or 15 μg of hgB antigen. The rationale for selecting the antigen quantities was based upon previously published and unpublished experience in the (independent) evaluations of immunogenicities and/or protective efficacies following vaccinations with these antigens [17;21]. AS01B and AS02V in one vaccine dose each contained 50 μg of MPL and 50 μg of QS-21, whereas AS01E in one vaccine dose contained 25 μg of MPL and 25 μg of QS-21. AS01 variants also contain liposome whereas AS02V is an oil-in-water emulsion. AS01B, AS01E and AS02V were obtained from GSK Vaccines (Rixensart, Belgium). QS-21 was from Antigenics Inc, a wholly owned subsidiary of Agenus Inc., Lexington, MA, USA. For the gB/FA vaccination regimen, complete FA was used in the first vaccine dose and incomplete FA in the second and third doses. Standard complete and incomplete FA were obtained from Sigma/Aldrich. Adjuvants were mixed with antigen immediately prior to injection.
2.4. Experimental design
For Study 1, female Hartley guinea pigs were immunized with 3 subcutaneous doses, at monthly intervals, as previously described [17]. Blood samples were taken immediately prior to the second and third immunizations, 30 days after the third immunization, and 10 days after viral challenge. Mating was performed immediately after the third vaccine dose by co-caging one female and one male guinea pig for 2 weeks and the day that mating occurred was designated as pregnancy Day 0. GPCMV challenge was performed on ~45-day pregnant animals by subcutaneous injection of 1.5 × 105 pfu of GPCMV. Animals were observed daily up to parturition to determine pregnancy outcome, as previously described [16;17]. For Study 2, guinea pigs were intramuscularly injected twice, 28 days apart. Blood was taken 28 and 14 days after the first and the second immunization, respectively. In both studies, serum samples were prepared from blood using routine methodology.
2.5. ELISA, neutralization, and antibody avidity analyses
For Study 1, the ELISA was performed using plates coated with GPCMV antigens and a control antigen, as previously described [17]. ELISA titers were defined as the reciprocal of the highest dilution that produced an absorbance (OD450) of at least 0.10, and twice the absorbance against the control antigen. GPCMV neutralizating titers were determined as previously described [17]. The avidity index (AI) of antibodies in a serum sample was determined from the ratio of the OD450 of GPCMV-bound antibodies (on plates coated with polypeptides extracted from GPCMV-infected fibroblasts) in the presence and absence of 4 M urea [22]. Antibodies were detected using the Radim diagnostic kit according to the manufacturer’s specifications, with the exception that an HRP-conjugated rabbit anti-guinea pig serum (Accurate Pharmaceuticals) was used as the secondary antibody. The AI was calculated as an average of values taken from those dilutions (1:160, 1:320, 1:640, and 1:1280) which gave measurements within the linear range of the ELISA assay (0.5–1.0 OD units) [22].
For Study 2, the ELISA was performed using HCMV gB as a coating antigen (0.4 μg/well). Anti-gB antibody titers were calculated in comparison with a reference pool of positive sera to which an arbitrary titer of 175000 ELISA units (EU)/mL had been attributed. The microneutralization assay was adapted from a previously published assay [23] and performed using MRC-5 cells as an infection substrate. After overnight incubation with a mixture of HCMV virus (30 TCID50/50μl; strain AD169, Henogen SA, Belgium) and diluted serum samples, cells were fixed and virus infection was indicated by the detection of Immediate Early 1 protein (IE1) using a monoclonal antibody (MAB810R, Chemicon) and routine immunohistochemistry. The neutralizing titers were expressed as the reciprocal of the serum dilution inducing a reduction of 50% of infected cells relative to cells incubated with virus alone (calculation by point to point regression analysis).
2.6. Real-time PCR
Viremia was defined as the detection of GPCMV genomic DNA (DNAemia) and viral load was defined as the concentration of GPCMV genomic DNA in maternal blood, measured 10 days post-challenge. Maternal-fetal GPCMV transmission was confirmed by detection of GPCMV genomic DNA in the spleen and liver of necropsied pups. GPCMV genomic DNA was detected using quantitative real-time PCR. The following primers, based on the GPCMV gB gene sequence [24], were used: Forward primer, 5′-CTTCGTGGTTGAACGGG-3′; Reverse primer, 5′-GTAGTCGAAAGGACGTTGC-3′; Probe 1, 5′-TGGTGACCTTCGTTACCAATCCGTTTGGA-fluorescein; Probe 2, 5′-LC Red 640-CTTCGTGGTGTTCCTGTTCTGCGT-Phosphate. DNA was extracted from whole blood or (neonatal) liver/spleen homogenates using Roche MagNa Pure LC Total Nucleic Acid Isolation Kit according to manufacturer’s instructions. The PCR reaction mixtures were prepared using the Lightcycler Fast Start Master hybridization probes (Roche Applied Sciences) supplemented with 2.5 mM MgCl2, 0.5 μM primers and 0.2 μM probes. PCR was performed in the Lightcycler instrument. The limit of detection of the assay was consistently between 5 and 10 copies/reaction.
2.7. Statistical analyses
Statistical analyses used SAS software (SAS Institute Inc., NC, USA). The incidence of viremia in the dams was analyzed using Fisher’s exact test. The incidence of mortality in the pups was analyzed using a generalized linear mixed model with binomial distribution (SASGLIMMX) because pup mortality events within a litter were considered as dependent events. Significance was ascribed to p-values < 0.05/3 (=0.017) using a Bonferroni correction to account for multiple comparisons with the control group. Parametric analyses of immunogenicity and PCR variables were performed on log10 transformed data. The Shapiro-Wilk test, Skewness and Kurtosis calculations were used to specify limits of acceptable normality. Differences were identified by ANOVA followed by Tukey’s test. All comparisons were two-tailed. Significance was ascribed to p-values <0.05 (and in the case of antibody titers and concentrations, to ≥2-fold differences). Titers and concentration values are described to two-significant figures in the text. Mean and standard deviation pup-weight calculations considered pup weights in a litter as dependent variables.
3. Results
3.1. Disposition
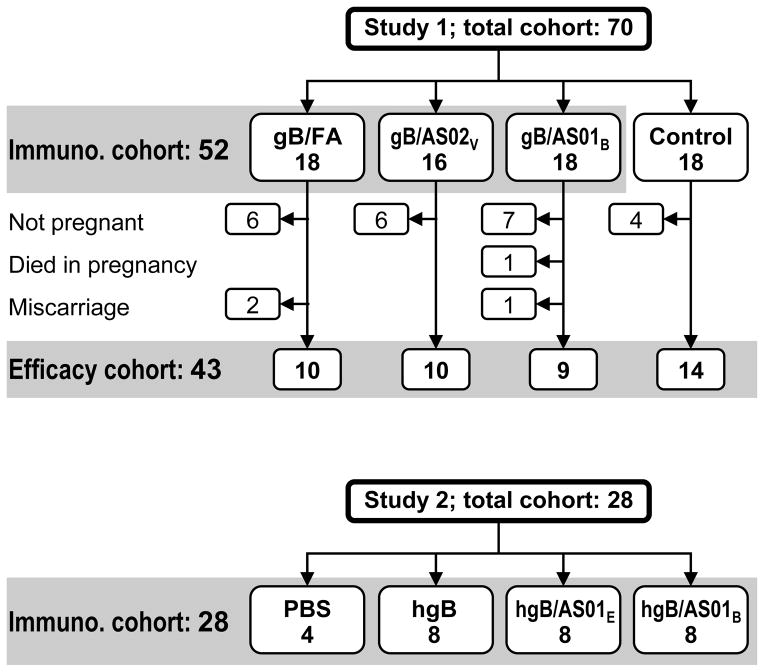
In Study 1, 52 guinea pigs completed the 3-dose vaccination course; 18 animals received gB/FA, 16 received gB/AS02V, and 18 received gB/AS01B. All vaccinated animals and eighteen unvaccinated controls were mated. The majority of animals in each group became pregnant (Figure 1A) and these animals were challenged with GPCMV, after ~45 days of the anticipated 65 day gestation period. Two animals miscarried in the gB/FA group and one in the gB/AS01B group. These two animals were not included in the pregnancy outcome analyses since miscarriage <7 days after viral challenge is not considered to be related to viral infection [17] and is compatible with the relatively high (~8.5%) spontaneous miscarriage rate in guinea pigs [9]. One animal in the gB/AS01B group died of an unexpected bowel perforation. This animal had GPCMV viraemia (1.9×105 GPCMV genome copies/mL) and carried four fetuses, all of which had no signs of GPCMV infection. For the remaining pregnant animals (9–14 per group), parturition occurred at least 7 days after GPCMV challenge.
Figure 1. Animal allocation to different treatment groups and analysis cohorts in Study 1 and Study 2.
In Study 1, the reason for the loss/removal of animals from the Efficacy cohort is described on the left side of the schematic.
In Study 2, 28 guinea pigs completed the two-dose vaccination course; three groups of eight animals received unadjuvanted HCMV gB (hgB) vaccine, hgB/AS01E and hgB/AS01B, respectively, and four animals received PBS (Figure 1B).
3.2. Immunogenicity of GPCMV gB vaccines (Study 1)
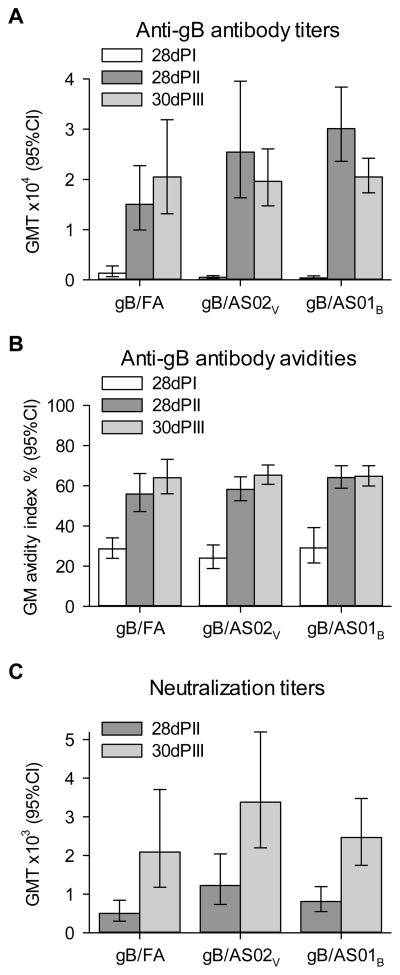
GPCMV-specific antibody and neutralizing responses were detected in all vaccinated animals. GPCMV-specific antibody GMTs were (11–75-fold) higher in each vaccine group after Doses 2 and 3 compared with after Dose 1 (Figure 2). After Dose 1, the antibody GMTs in the gB/AS01B and gB/AS02V groups were lower than that in the gB/FA group (p<0.05), whereas after Dose 2, the GMT in the gB/AS01B was higher than that in the gB/FA group (p=0.005). Antibody GMTs were not significantly different between the three vaccine groups after Dose 3, nor were the GMTs significantly different when compared with those after Dose 2.
Figure 2. Immunogenicity of gB vaccines.
Guinea pigs were immunized with three doses of either gB/FA, gB/AS02V or gB/AS01B at monthly intervals. Geometric mean (GM) (A) anti-gB antibody titers (GMTs), (B) avidity indices and (C) GPCMV neutralizing titers in the immunogenicity cohort of Study 1. Immunogenicity was measured in samples taken at (A–C) 28 days post 1st immunization (28dPI); (A–C) 28 days post 2nd immunization (28dPII); and (A and B) 30 days post 3rd immunization (30dPIII). Error bars describe 95% confidence intervals (95% CIs). GPCMV-specific antibody GMTs were (11–75-fold) higher in each vaccine group after Doses 2 and 3 compared with after Dose 1, but were not significantly different between the three vaccine groups after Dose 3. GMTs were significantly different following Dose 3 than when compared with those following Dose 2 for each group.
Geometric mean avidity indices (AIs) were 2.0–2.7-fold higher in each vaccine group after Doses 2 and 3 compared with after Dose 1, and suggested a degree of antibody affinity maturation after the second dose. The peak AIs were similar to the AI of 70% found with antibodies induced by GPCMV infection [25]. Geometric mean AIs were not significantly different between the three vaccine groups after each of the three doses.
GPCMV-neutralizing GMTs were 2.8–4.2-fold higher in each vaccine group after Dose 3 compared with after Dose 2 (Figure 2). After Dose 2, the GPCMV-neutralizing GMT in the gB/AS02V was higher than in the gB/FA group (p=0.014) whereas after Dose 3, the GMTs were not significantly different between the three vaccine groups.
3.3. Vaccine efficacy (Study 1)
Vaccine efficacy was estimated from two outcomes: protection against GPCMV-lethality in the offspring and protection against GPCMV-viremia in the dams (Tables 1 and 2).
Table 1.
Vaccine efficacy evaluation with respect to pup mortality
| Group | No. litters | No. litters with dead pups | No. pups (N) | Live pups
|
Dead pups
|
% pup mortality (100 x nd/N) | Odds ratio (exact 95% CI) | VEb, % | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Mean pup weight (SD), g | CMV+ (%) | No. (nd) | Mean pup weight (SD), g | CMV+ (%) | ||||||||
| control | 14 | 9 | 57 | 20 | 107 (32) | 6 (32a) | 37 | 98 (23) | 30 (83a) | 65 | - | - | - |
|
| |||||||||||||
| gB/FA | 10 | 5 | 33 | 21 | 115 (16) | 14 (67) | 12 | 88 (17) | 10 (83) | 36 | 0.31 (0.12–0.78) | 44 | 0.014c |
|
| |||||||||||||
| gB/AS02V | 10 | 3 | 39 | 35 | 116 (12) | 16 (46) | 4 | 114 (18) | 3 (75) | 10 | 0.06 (0.02–0.21) | 84 | <0.001c |
|
| |||||||||||||
| gB/AS01B | 9 | 4 | 34 | 26 | 113 (14) | 9 (35) | 8 | 100 (24) | 6 (75) | 24 | 0.17 (0.06–0.45) | 64 | <0.001c |
One pup not included in the PCR analysis.
Vaccine efficacy estimated from odds ratios.
Significantly different from control (Generalized linear mixed model with binomial distribution followed by Student t with a Bonferroni correction for multiple comparisons, such that α=0.05/3=0.017).
No., number. N, total number of pups/group; nd, number of dead pups/group, CMV+, number of CMV-positive pups/group; CI, confidence interval; SD, standard deviation; VE, vaccine efficacy.
Table 2.
Vaccine efficacy evaluation with respect to viremia
| Group | No. dams, (N) | Viremia positive dams
|
Viremia negative dams
|
% viremia+ (100 x nv+/N) | Odds ratio (exact 95% CI) | VE, % | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (nv+) | GM, 105 CMV DNA copies/mL | Litters
|
No. | Litters
|
||||||||
| All live pups (≥1 CMV+ pup) | ≥1 dead pup (≥1 CMV+ pup) | All live pups (≥1 CMV+ pup) | ≥1 dead pup (≥1 CMV+ pup) | |||||||||
| control | 14 | 13 | 6.8 | 5 (1) | 8 (8) | 1 | 0 (0) | 1 (1) | 93 | - | - | - |
|
| ||||||||||||
| gB/FA | 10 | 7 | 0.9 | 3 (3) | 4 (4) | 3 | 2 (1) | 1 (1) | 70 | 0.180 (0.016–2.064) | 25 | 0.272 |
|
| ||||||||||||
| gB/AS02V | 10 | 3 | 1.7 | 2 (2) | 1 (1) | 7 | 5 (5) | 2 (1) | 30 | 0.033 (0.0007–0.478) | 68 | 0.002a |
|
| ||||||||||||
| gB/AS01B | 9 | 1 | 0.9 | 0 (0) | 1 (1) | 8 | 5 (4) | 3 (2) | 11 | 0.0096 (0.0002–0.235) | 88 | <0.001a |
Significantly different from control (Fisher exact test with a Bonferroni correction for multiple comparisons, such that α=0.05/3=0.017).
No., number. N, total number of dams/group; nv+, number of viremia-positive dams/group; nv−, number of viremia-negative dams/group, ≥1 CMV+ pup, at least one CMV-positive pup/litter/group; viremia+, viremia-positive; GM, geometric mean; CI, confidence interval;. VE, vaccine efficacy.
Sixty four percent (9/14) of litters born to control (GPCMV-infected) dams included dead pups (Table 1). In total, 65% (37/57) of these pups were dead. In the three vaccine groups overall, 41% (12/29) of litters included dead pups. In the gB/FA, gB/AS02V and gB/AS01B groups, 36% (12/33), 10% (4/39) and 24% (8/34) of pups were dead, giving estimates of vaccine efficacies against pup mortality as 44% (p=0.014), 84% (p<0.001) and 64% (p<0.001), respectively (Table 1).
One live pup and one dead pup (both from the control group) could not be retrieved for PCR analysis. Hence, a total of 101 live pups and 60 dead pups were evaluated for congenital GPCMV transmission (see Table 1). In control and vaccinated dams, GPCMV was detected (by PCR in the liver or spleen) in a higher proportion of dead pups (82%; 49/60) than of live pups (45%; 45/101), suggesting that congenital GPCMV transmission was associated with pup mortality.
At Day 10 post-challenge, 93% (13/14) of the control group dams had GPCMV viremia, whereas in the gB/FA, gB/AS02V and gB/AS01B groups, 70% (7/10), 30% (3/10) and 11% (1/9) of the dams had viremia (Table 2). Hence gB/AS02V and gB/AS01B efficacies against viremia were estimated at 68% (p=0.002) and 88% (p<0.001), respectively (Table 2). However, gB/FA efficacy estimate of 25% was not significantly different from control (p=0.272).
In the dams with viremia, the geometric mean concentration (GMC) of GPCMV genomes (i.e. viral load) in the combined three vaccine groups was 1.1×105 copies/mL (0.9, 1.7 and 0.9 copies/mL in the gB/FA, gB/AS02V and gB/AS01B groups, respectively; Table 2) and was 6.4-fold lower (p<0.001) than that in the control group (6.8×105 copies/mL). In the combined three vaccine groups, the GPCMV-neutralizing geometric mean titer (GMT) in the dams with viremia was 1800 but was not significantly different (p=0.125) from that (3000) in the dams without viremia (Supplementary Figure 1). No differences were identified between vaccine groups in antibody GMTs or geometric mean antibody avidities (Supplementary Figure 1).
In vaccinated dams without viremia, 78% (14/18) of litters included at least one GPCMV+ pup (Table 2) and 33% (6/18) of the litters included at least one dead pup. In vaccinated dams with viremia, all 11 litters included GPCMV+ pups (Table 2) and 55% (6/11) of the litters included dead pups. However, only 69% (9/13) of the litters from the control dams with viremia included GPCMV+ pups.
3.4. Immunogenicity of HCMV (h)gB vaccines (Study 2)
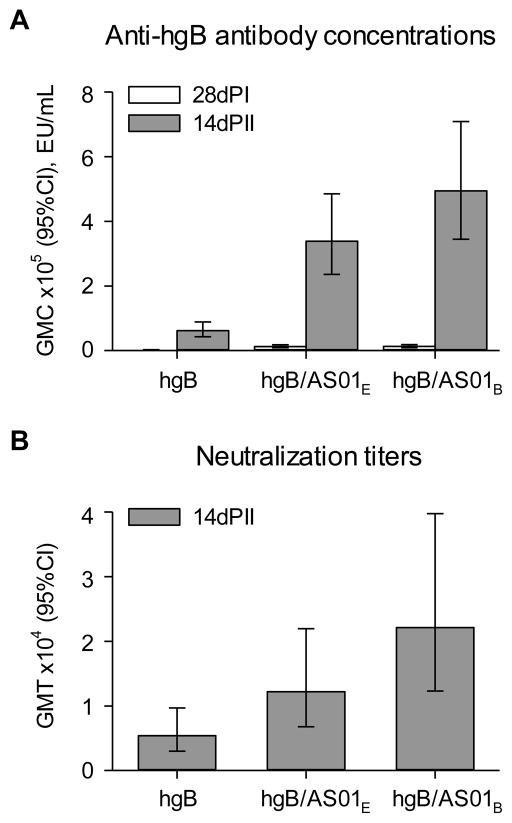
In Study 2, AS01B and AS01E (which contains half the quantities of MPL and QS-21 than AS01B) were evaluated in hgB vaccines in light of the GPCMV efficacy results and of other results of AS01-adjuvanted vaccines in clinical development [18]. HCMV (hgB)-specific responses were observed in all vaccinated animals (Figure 3). Both hgB/AS01E and hgB/AS01B induced higher human hgB-specific antibody GMCs after each of the two doses than those induced by hgB alone (p<0.001). hgB/AS01B also induced higher HCMV-neutralizing GMTs after Dose 2 than that induced by hgB alone (p<0.05). The antibody GMCs and neutralizing GMTs induced by hgB/AS01E were not significantly different from those induced by hgB/AS01B.
Figure 3. Immunogenicity of hgB vaccines.
Guinea pigs were immunized with two doses of either hgB (no adjuvant), hgB/AS01E or hgB/AS01B one month apart. Geometric mean anti-hgB antibody concentrations (GMCs) (A) and human CMV neutralizing titers (GMTs) (B) in the immunogenicity cohort of Study 2. Immunogenicity was measured in samples taken at, (A) 28 days post 1st immunization (28dPI); and (A and B) 14 days post 2nd immunization (14dPII). Error bars describe 95% confidence intervals (95% CIs).
4. Discussion
In Study 1, three-dose administration of gB/AS01B or gB/AS02V before conception protected against guinea pig pup mortality and maternal viremia in response to GPCMV challenge during pregnancy, and gB/FA protected against pup mortality alone. The three vaccines were similarly immunogenic, all inducing relatively high avidity gB-specific antibody responses after two doses and GPCMV neutralization responses after three doses. Although less frequently observed in the vaccine groups than in the control group, viremia was detected in animals with gB-specific antibody responses. Other studies of gB vaccines in guinea pigs have suggested that high neutralization responses are associated with protection [16;17]. This association was also suggested but not clearly identified in this study, perhaps because the sample sizes were too small. Vaccine-induced cellular immunity may have also contributed to the protective vaccine efficacies [26] but this parameter was not assessed.
Congenital GPCMV infection appeared to be associated with pup mortality, although approximately one-half of the infected pups were alive at birth. Moreover, congenital GPCMV infections occurred in 78% of the vaccinated dams that did not develop detectable viremia. The assessment of viremia in dams at only one time point (Day 10 post-inoculation) is one possible limitation of this study. Nevertheless, immune responses in some of the vaccinated dams may not have been sufficient to clear the relatively high load of injected virus in time to prevent a transmission event from occurring. To what extent neutralizing antibodies were involved in this response is difficult to judge because neutralization of GPCMV maternal-fetal transmission may be more appropriately assessed with epithelial- or endothelial-cell based assays in addition to the fibroblast-based assay [27;28].
The demonstration that protection can be achieved with gB/AS01B and gB/AS02v, extends the previous observation that immunostimulatory adjuvants are required in a gB and AS02v in subunit vaccine formulation [17]. Moreover, the use of AS01B, AS01E clinical trials of candidate malaria, hepatitis B and HIV vaccines has revealed that both antigen-specific humoral and cell-mediated immune responses are induced [29–32], and in the case of the candidate malaria vaccine, both types of response are associated with protection [31;33]. However, the evaluation of protective efficacy against HCMV is not feasible in the guinea pig challenge model, given the inability of HCMV to infect these animals. Immunogenicity was evaluated in the guinea pig though, and both HCMV-derived gB vaccines were immunogenic. Vaccines formulated with either AS01B or AS01E were more immunogenic than the unadjuvanted vaccine, supporting the combined use of HCMV-derived gB antigen and AS01 in clinical development. Moreover, hgB/AS01E was not significantly less immunogenic than hgB/AS01B suggesting that AS01E, with its lower quantities of immunostimulants, could be a more suitable formulation in humans. Indeed, a clinical trial with the candidate gB/AS01E subunit vaccine is now underway in healthy volunteers.
Supplementary Material
Guinea pigs were immunized with three doses of either gB/FA, gB/AS02V or gB/AS01B at monthly intervals, mated and experimentally challenged with CMV. Geometric mean (GM) (A) anti-gB antibody titers (GMTs), (B) avidity indices and (C) GPCMV neutralizing titers 30 days post 3rd immunization in dams with and without viremia in the Efficacy cohort of Study 1. Error bars describe 95% confidence intervals (95% CIs).
Highlights.
Cytomegalovirus (CMV) infection in pregnant women can cause birth defects.
Three gB antigen vaccines were evaluated in a pregnant-guinea pig CMV-challenge model.
Two vaccines also contained clinically-relevant Adjuvant Systems AS01 and AS02.
gB/AS01 and gB/AS02 offered protection against viremia in dams and mortality in pups.
Efficacy and immunogenicity results support clinical testing of gB/AS01 and gB/AS02.
Acknowledgments
Frédéric Renaud (GSK Vaccines), Gaël de Lannoy (GSK Vaccines) and Georges Carletti (STRATEGIESTAT) performed the statistical analyses. Matthew Morgan (MG Science Communications) provided science and writing advice in the manuscript’s development. Pascal Cadot (XPE Pharma) provided editorial advice. Ulrike Krause (GSK Vaccines) provided editorial advice and coordinated the manuscript’s development. Technical support for hgB vaccine evaluations was provided by Christine Charlier (GSK Vaccines). Technical support with animal dissections was provided by Katherine L. Schleiss (University of Minnesota Medical School), supported by a summer research fellowship funded by the American Legion and Auxiliary Heart Research Foundation fund.
Funding Source
This work was supported by National Institute of Health grants AI-65289 and HD38416-01, and by a grant from GSK Biologicals, SA. The costs associated with the development of the manuscript, including scientific writing assistance and statistical advice, were also covered by GSK Biologicals SA.
ABBREVIATIONS
- HCMV
human cytomegalovirus
- GPCMV
guinea pig cytomegalovirus
- gB
glycoprotein B antigen
- hgB
human CMV-derived gB antigen
Footnotes
Contributors
Mark R. Schleiss and Marc Van Damme developed and designed the study. Mark R. Schleiss, K. Yeon Choi, Jodi Anderson and Janine Gessner acquired the data. Mark R. Schleiss performed and supervised the analysis with assistance from K. Yeon Choi. Mark R. Schleiss, Martine Wettendorff, Sally Mossman and Marc Van Damme were involved in the interpretation of the data. Mark R. Schleiss wrote the initial draft of the manuscript. All authors were involved in the further drafting of the manuscript or revising it critically for important intellectual content. All authors approved the manuscript before it was submitted by the corresponding author. All authors had full access to the data and had final responsibility to submit for publication.
Conflict Of Interest
All authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and declared that the following interests are relevant to the submitted work. Martine Wettendorff, Sally Mossman and Marc Van Damme are employees of the GlaxoSmithKline (GSK) group of companies; Martine Wettendorff and Sally Mossman report ownership of shares or options to shares in GSK.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark R. Schleiss, Email: schleiss@umn.edu.
K. Yeon Choi, Email: kchoi@tamhsc.edu.
Jodi Anderson, Email: kaise024@umn.edu.
Janine Gessner Mash, Email: jmash@natera.com.
Martine Wettendorff, Email: MARTINE.WETTENDORFF@GSK.COM.
Sally Mossman, Email: SALLY.P.MOSSMAN@GSK.COM.
Marc Van Damme, Email: MARC.VAN-DAMME@GSK.COM.
References
- 1.Demmler GJ. Congenital cytomegalovirus infection and disease. Semin Pediatr Infect Dis. 1999;10:195–200. [PubMed] [Google Scholar]
- 2.Bale JF, Miner L, Petheram SJ. Congenital Cytomegalovirus Infection. Curr Treat Options Neurol. 2002;4:225–30. doi: 10.1007/s11940-002-0039-8. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine (US), Committee to study priorities for vaccine development. Vaccines for the 21st century: A tool for decisionmaking. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 4.Schleiss MR, Heineman TC. Progress toward an elusive goal: current status of cytomegalovirus vaccines. Expert Rev Vaccines. 2005;4:381–406. doi: 10.1586/14760584.4.3.381. [DOI] [PubMed] [Google Scholar]
- 5.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191–9. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein DI, Reap EA, Katen K, Watson A, Smith K, Norberg P, et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2009;28:484–93. doi: 10.1016/j.vaccine.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet. 2011;377:1256–63. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bia FJ, Griffith BP, Fong CK, Hsiung GD. Cytomegaloviral infections in the guinea pig: experimental models for human disease. Rev Infect Dis. 1983;5:177–95. doi: 10.1093/clinids/5.2.177. [DOI] [PubMed] [Google Scholar]
- 9.Schleiss MR. Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns. ILAR J. 2006;47:65–72. doi: 10.1093/ilar.47.1.65. [DOI] [PubMed] [Google Scholar]
- 10.Griffith BP, McCormick SR, Fong CK, Lavallee JT, Lucia HL, Goff E. The placenta as a site of cytomegalovirus infection in guinea pigs. J Virol. 1985;55:402–9. doi: 10.1128/jvi.55.2.402-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schleiss MR. Congenital cytomegalovirus infection: molecular mechanisms mediating viral pathogenesis. Infect Disord Drug Targets. 2011;11:449–65. doi: 10.2174/187152611797636721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter AM. Animal models of human placentation--a review. Placenta. 2007;28 (Suppl A):S41–S47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol. 1994;102:122–34. doi: 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]
- 14.Mess A. The Guinea pig placenta: model of placental growth dynamics. Placenta. 2007;28:812–5. doi: 10.1016/j.placenta.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui Y, Kosugi I, Kawasaki H. Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev Med Virol. 2005;15:327–45. doi: 10.1002/rmv.475. [DOI] [PubMed] [Google Scholar]
- 16.Schleiss MR, Bourne N, Bernstein DI. Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J Infect Dis. 2003;188:1868–74. doi: 10.1086/379839. [DOI] [PubMed] [Google Scholar]
- 17.Schleiss MR, Bourne N, Stroup G, Bravo FJ, Jensen NJ, Bernstein DI. Protection against congenital cytomegalovirus infection and disease in guinea pigs, conferred by a purified recombinant glycoprotein B vaccine. J Infect Dis. 2004;189:1374–81. doi: 10.1086/382751. [DOI] [PubMed] [Google Scholar]
- 18.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing Adjuvant Systems. Expert Rev Vaccines. 2011;10:471–86. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 19.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 20.Surquin M, Tielemans C, Nortier J, Jadoul M, Peeters P, Ryba M, et al. Anti-HBs antibody persistence following primary vaccination with an investigational AS02(v)-adjuvanted hepatitis B vaccine in patients with renal insufficiency. Hum Vaccin. 2011;7:913–8. doi: 10.4161/hv.7.9.16225. [DOI] [PubMed] [Google Scholar]
- 21.Schleiss MR, Jensen NJ. Cloning and expression of the guinea pig cytomegalovirus glycoprotein B (gB) in a recombinant baculovirus: utility for vaccine studies for the prevention of experimental infection. J Virol Methods. 2003;108:59–65. doi: 10.1016/s0166-0934(02)00258-6. [DOI] [PubMed] [Google Scholar]
- 22.Lazzarotto T, Varani S, Spezzacatena P, Gabrielli L, Pradelli P, Guerra B, et al. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol. 2000;13:137–41. doi: 10.1089/vim.2000.13.137. [DOI] [PubMed] [Google Scholar]
- 23.Gupta CK, Leszczynski J, Gupta RK, Siber GR. An enzyme immunoassay based micro-neutralization test for titration of antibodies to human cytomegalovirus (CMV) and its correlation with direct ELISA measuring CMV IgG antibodies. Biologicals. 1996;24:41–9. doi: 10.1006/biol.1996.0004. [DOI] [PubMed] [Google Scholar]
- 24.Britt WJ, Vugler L, Butfiloski EJ, Stephens EB. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J Virol. 1990;64:1079–85. doi: 10.1128/jvi.64.3.1079-1085.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleiss MR. Animal models of congenital cytomegalovirus infection: an overview of progress in the characterization of guinea pig cytomegalovirus (GPCMV) J Clin Virol. 2002;25 (Suppl 2):S37–S49. doi: 10.1016/s1386-6532(02)00100-2. [DOI] [PubMed] [Google Scholar]
- 26.Elkington R, Shoukry NH, Walker S, Crough T, Fazou C, Kaur A, et al. Cross-reactive recognition of human and primate cytomegalovirus sequences by human CD4 cytotoxic T lymphocytes specific for glycoprotein B and H. Eur J Immunol. 2004;34:3216–26. doi: 10.1002/eji.200425203. [DOI] [PubMed] [Google Scholar]
- 27.Cui X, Meza BP, Adler SP, McVoy MA. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine. 2008;26:5760–6. doi: 10.1016/j.vaccine.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol. 2008;89:853–65. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- 29.Vandepapelière P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008;26:1375–86. doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 30.Leroux-Roels I, Koutsoukos M, Clement F, Steyaert S, Janssens M, Bourguignon P, et al. Strong and persistent CD4+ T-cell response in healthy adults immunized with a candidate HIV-1 vaccine containing gp120, Nef and Tat antigens formulated in three Adjuvant Systems. Vaccine. 2010;28:7016–24. doi: 10.1016/j.vaccine.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS, S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200:337–46. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 32.Olotu A, Moris P, Mwacharo J, Vekemans J, Kimani D, Janssens M, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria. PLoS One. 2011;6:e25786. doi: 10.1371/journal.pone.0025786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reece WH, Pinder M, Gothard PK, Milligan P, Bojang K, Doherty T, et al. A CD4+ T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10:406–10. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Guinea pigs were immunized with three doses of either gB/FA, gB/AS02V or gB/AS01B at monthly intervals, mated and experimentally challenged with CMV. Geometric mean (GM) (A) anti-gB antibody titers (GMTs), (B) avidity indices and (C) GPCMV neutralizing titers 30 days post 3rd immunization in dams with and without viremia in the Efficacy cohort of Study 1. Error bars describe 95% confidence intervals (95% CIs).