Abstract
Background and Purpose
Previous univariate analyses have suggested that proximal middle cerebral artery (MCA) infarcts with insular involvement have greater severity, and are more likely to progress into surrounding penumbral “tissue-at-risk”. We hypothesized that a practical, simple scoring method to assess percent insular-ribbon infarction (“PIRI-score”) would improve prediction of penumbral-loss over other common imaging biomarkers.
Methods
Of consecutive acute stroke patients from 2003–2008, forty-five with proximal-MCA-only occlusion met inclusion criteria, including available penumbral imaging. Infarct (DWI), tissue-at-risk (MR-MTT), and final infarct-volume (MR/CT) were manually segmented. DWI images were rated according to the 5-point PIRI-score (“0”=normal, “1”<25%, “2”=25–49%, “3”=50–74%, and “4”≥75% insula involvement). Percent-mismatch-loss (PML) was calculated as an outcome measure of infarct progression. ROC and multivariate analyses were performed.
Results
Mean admission DWI-infarct-volume was 30.9 (±38.8) ml, and median (IQR) PIRI-score was 3 (0.75–4). PIRI-score was significantly correlated with PML (p<0.0001). When PML was dichotomized based on its median value (30.0%), ROC-AUC (area-under-curve) was 0.89 (p=0.0001) with a 25% insula-infarction optimal-threshold. After adjusting for time-to-imaging and treatment, binary-logistic regression, including dichotomized PIRI (25% threshold), age, NIHSS-score, DWI-infarct-volume, and CTA-collateral-score as covariates, revealed that only dichotomized insula-score (p=0.03) and age (p=0.02) were independent predictors of large (68.2%) vs. small (8.1%) mismatch-loss. There was excellent inter-observer agreement for dichotomized PIRI-scoring (κ =0.91).
Conclusions
Admission insular infarction >25% is the strongest predictor of large mismatch-loss in this cohort of proximal-MCA occlusive stroke. This outcome marker may help to identify treatment-eligible patients who are in greatest need of rapid reperfusion therapy.
Keywords: Insula, Stroke, MCA, Imaging-predictor, Penumbra
INTRODUCTION
In the setting of proximal cerebral artery occlusion, a practical and reliable imaging biomarker predictive of infarct growth into surrounding hypoperfused penumbral “tissue-at-risk” is not yet established. Such a marker could help to identify patients with proximal occlusions likely to have infarct expansion absent timely reperfusion, thereby facilitating more rational assessment of possible benefits (versus risks) of reperfusion therapy.1 The need for such an imaging predictor is highlighted by the marked heterogeneity in presentation/progression associated with proximal middle cerebral artery (MCA) strokes.2, 3
Important current imaging/clinical markers of severity and outcome in proximal-MCA stroke include admission infarct volume & Alberta-stroke-program-early-CT-score ASPECTS (detected by unenhanced CT or DWI),4, 5 “malignant” CTA collateral or perfusion profile,6, 7 and NIH-stroke-scale (NIHSS) score (“clinical penumbra”).8 The role of perfusion “mismatch” imaging in MCA-stroke management, although promising, remains controversial.8–10
Previous univariate analyses and observational studies have suggested that proximal-MCA infarcts with substantial insular involvement have greater severity, and are more likely to progress into surrounding “tissue-at-risk”.11, 12 This concept has intuitive appeal, as the vascular supply to the insula not only reflects the confluence of bulk flow through the superior and (less consistently) inferior MCA divisions, but is also determined by the combined effects of anatomic variants, clot location, and collateral flow.11, 13, 14
We hypothesized that a practical, easy-to-use scoring method, based on percent-insular-ribbon-infarction at admission (the “PIRI-score”), would enhance prediction of mismatch-loss in proximal-MCA occlusive stroke patients over other currently utilized imaging biomarkers.
METHODS
Patients
We reviewed the clinical/imaging records of consecutive patients with new onset acute stroke symptoms that underwent the standardized acute stroke-imaging algorithm at our institution from April 2003-April 2008. Our institutional review board approved this study; written informed consent was not required for this HIPAA-compliant retrospective analysis of prospectively acquired routine clinical data. Forty-five patients who met the following criteria were included: (1) admission CT, CTA, MR-DWI, and perfusion imaging within 9 hours of stroke onset; (2) proximal-MCA occlusion only on CTA (“M1” branch); and (3) either CT or MR follow-up at >24 hours, but not at 3–7 days (to avoid maximal vasogenic edema); exclusion criteria were: (4) lacunar infarct (solitary DWI lesion <15 mm); and (5) CTA occlusions at any of the following: contralateral MCA, ICA, ACA, and PCA.
CT/MR acquisitions
All imaging was obtained in the emergency department for routine clinical indications according to our standardized institutional acute stroke algorithms. Non-contrast head CT (NCCT) was performed helically (LightSpeed 64; GE Healthcare, Milwaukee, WI), at 120 kV, 250 mA, and 0.7 seconds/rotation. Filtered-back projection (FBP) with standard kernel was used for image reconstruction at 5-mm-thick contiguous sections, field-of-view (FOV) 22 cm, and matrix size 512×512. CTA immediately followed NCCT, at 120 kV, 300 mA, 0.5–0.7 seconds/rotation, with 80–100 ml of nonionic contrast (Omnipaque 370; Nycomed, Roskilde, Denmark) followed by 40 ml of saline, both at 4 ml/s. Images were reconstructed at 1.25-mm thickness and 0.625-mm intervals with standard kernel FBP.
MR exams were obtained on a 1.5-Tesla Signa whole-body scanner (GE Healthcare, Milwaukee, WI). DWI was performed using single-shot echoplanar spin-echo; high-b-value images (b=1000 seconds/mm2) were acquired in 6 different gradient directions, in addition to a single low-b-value (b=0 seconds/mm2). Imaging parameters were: TR/TE 5000/80-110 ms, FOV 22 cm, matrix 128×128 zero-filled to 256×256, and slice thickness 5-mm with 1-mm gap. Perfusion imaging was performed using dynamic susceptibility technique; serial echoplanar gradient-echo images were acquired with TR/TE 1500/40 ms, FOV 22 cm, matrix 128×128, and slice-thickness 5-mm with a 1-mm gap. Fourteen-to-16 slices were acquired every 1.5 seconds for 46-to-80 images/slice. Ten seconds after the start of image acquisition, 20 ml of gadopentetate dimeglumine 0.5 mmol/ml (Magnevist; Bayer HealthCare Pharmaceuticals) was administered at 5 ml/s using a power injector (Medrad, Warrendale, PA), followed by a 20-ml normal saline bolus.
Image analysis
Mean transit time (MTT) maps were post-processed from the MR perfusion source data using a previously validated singular value decomposition deconvolution software platform.15 MTT images were reviewed using a “rainbow” color-scale display that facilitated visual distinction of pixels values <7 seconds from those with values ≥ 7–10 seconds (the latter considered penumbral “tissue at risk”). Ischemic lesion volumes for admission infarct (DWI/ADC), tissue-at-risk (MR-MTT), and final infarct (MR-FLAIR or CT), were manually segmented using semi-automated commercially available software (Analyze 8.1; Analyze-Direct, Mayo Clinic, Rochester, Minnesota) by an MD research scientist (SK, 4-years’ experience) and edited by a board certified neuroradiologist (AK and/or MHL). The patency of the CTA collateral circulation was scored on the CTA-source images at two combined levels (sylvian fissure and convexity) according to a published 5-point rating scale (from “absent” to “exuberant”) by experienced board-certified neuroradiologists (AK, MHL).6
Percent insula ribbon infarction (PIRI) was independently rated on the admission DWI/ADC images according to a simple 5-point score, based on percent involvement in quartiles (“0”=normal,“1”<25%,“2”=25–49%,“3”=50–74%, and “4”≥75%), by two neuroradiologists, each with at least 2 years experience (RCB, LM), blinded to all correlative clinical and other imaging data except laterality. Assessment was based on visual estimation using three contiguous axial-slices depicting the longest extent of the insular ribbon; disagreements were decided by consensus.
Percent-mismatch-loss (“PML”) was calculated as an outcome measure of admission DWI/ADC infarct growth into the MTT-defined tissue-at-risk, based on published methodology11, as follows:
PML = 100 × [Final infarct – admit DWI] volumes / [admit MTT – admit DWI] volumes
Statistical analyses
Continuous variables are shown as means and standard deviations (SD), or as medians and inter-quartile ranges (IQR). Discrete variables are reported as counts (n) and percents (%). The kappa-statistic (κ) was used to determine inter-observer agreement for PIRI grading, based on the Landis and Koch guideline values.16
Spearman rank correlation and univariate linear regression were used to test the association of PIRI score with PML. PML was dichotomized at its median value, and “large” versus “small” mismatch-loss were defined as the median PML values above and below this dichotomized threshold, respectively. Receiver-operating-characteristic (ROC) curve analysis was performed to test the accuracy of PIRI for prediction of “large” mismatch loss.
In addition, a multivariate approach was performed to predict PML using a linear and a binary logistic multivariate-regression model. After adjusting for time-to-imaging and treatment assignment, the multivariate regression model of PML included PIRI-scores and the four most relevant and well established univariate predictors of infarct growth (age, admission NIHSS score, DWI-infarct volume or DWI-ASPECT score, and CTA-collateral score) 5–8, 17, 18. All these variables were “entered” in the multivariate model and additionally tested with “forward-selection” and “backward-elimination”. There was no evidence of non-linearity between PML and independent variables; in patients for whom the final infarct was larger than the “tissue-at-risk”, percent-penumbra-lost was recorded as 100%. Kolmogorov-Smirnov test confirmed the normality of model residuals (p=0.998). In the linear-regression model, PML outcome and PIRI were treated as continuous and ordinal variables, respectively. In the binary-logistic regression model, PML outcome was dichotomized at its median value, and PIRI was dichotomized at the operating point of the ROC curve. Analyses were performed using MedCalc (version: 11.5.1.0, Mariakerke, Belgium), with significance defined as p<0.05.
RESULTS
Patients Characteristics
Patient’s characteristics, both overall and stratified based on dichotomized PML at the 30% median (7.6–65.3% IQR) cutoff value, are shown in table 1.
Table 1.
Patients Characteristics Stratified by Median Percent Mismatch Loss (PML)
| Parameter* | All Patients | “Low” Mismatch-loss |
“High” Mismatch-loss |
P |
|---|---|---|---|---|
| Subjects, n (%) | 45 | 23 (51%) | 22 (49%) | - |
| Admit PML, median(IQR) | 30.0% (7.6–65.3) | 8.1% (4.2–13.1) | 68.2% (52.1–100) | <0.0001 |
| Age (year), mean (±SD) | 72.4 (±17.1) | 68.9 (±18.8) | 76.1 (±14.5) | 0.15 |
| Gender (male), n (%) | 19 (42%) | 9 (40%) | 10 (45%) | 0.77 |
| Atrial fibrillation, n (%) | 18 (40%) | 10 (43%) | 8 (36%) | 0.76 |
| Hypertension, n (%) | 34 (75%) | 16 (70%) | 18 (81%) | 0.49 |
| Coronary artery disease, n (%) | 15 (33%) | 6 (26%) | 9 (40%) | 0.35 |
| Diabetes mellitus, n (%) | 3 (6%) | 1 (4%) | 2 (9%) | 0.61 |
| Admit NIHSS, median (IQR) | 14 (9–19) | 9 (5.25–13.75) | 17.5 (15–22) | <0.0001 |
| Laterality (right sided), n (%) | 29 (64%) | 16 (69%) | 13 (59%) | 0.54 |
| Admit DWI infarct-vol (ml), mean (±SD) | 30.9 (±38.8) | 13.1 (±25.9) | 49.6 (±41.7) | 0.0013 |
| DWI infarct-vol <100ml, n(%) | 40 (89%) | 22 (96%) | 18 (82%) | 0.15 |
| Admit DWI-ASPECT score, median (IQR) | 6 (4.75–7) | 7 (7–8) | 5 (3–6) | <0.0001 |
| Admit MTT vol (ml), mean (±SD) | 161.1 (±74.6) | 151.1 (±70.5) | 171.5 (±79.1) | 0.36 |
| Admit PIRI, median (IQR) | 3 (0.75–4) | 1 (0–1.75) | 4 (3–4) | <0.0001 |
| Admit PIRI >25%, n (%) | 26/45 (58%) | 6/23 (26%) | 20/22 (91%) | <0.0001 |
| Time to DWI (hr), mean (±SD) | 5.1 (±1.9) | 5.3 (±1.9) | 4.7 (±1.9) | 0.29 |
| Admit CTA-Collateral Score, median (IQR) | 3 (2–4) | 4 (3–5) | 2.5 (2–3) | 0.0023 |
| Treatment (IV-tPA and/or IA), n (%) | 21 (46%) | 8 (35%) | 13 (59%) | 0.14 |
| Final Infarct Volume (ml), mean (±SD) | 87.9 (±92.3) | 29.4 (±36.8) | 149.1 (±93.6) | <0.0001 |
PIRI= percent insular-ribbon infarct, NIHSS= NIH-stroke-scale score, DWI= diffusion weighted imaging, ASPECT= Alberta-stroke-program-early-CT score, MTT= mean-transit-time MRI perfusion maps, CTA= CT angiography, tPA= tissue-plasminogen-activator, IA= intra-arterial,
Median percent mismatch loss was 30%
Continuous, ordinal and discrete variables compared with unpaired t-test, Mann-Whitney U-test and Fisher's Exact test, respectively.
45 patients who met all inclusion/exclusion criteria were studied. Mean age was 72.4 (±17.1) years. The median admission NIHSS score was 14 (9–19). 64 percent of the strokes were right-sided. Mean time-to-DWI from stroke onset was 5.1 (±1.9) hours. The mean baseline admission DWI and final infarct volumes were highly variable; 30.9 (±38.8) ml and 87.9 (±92.3) ml respectively.
Accuracy of Percent Insular Ribbon Infarction (PIRI) score for Mismatch- Loss
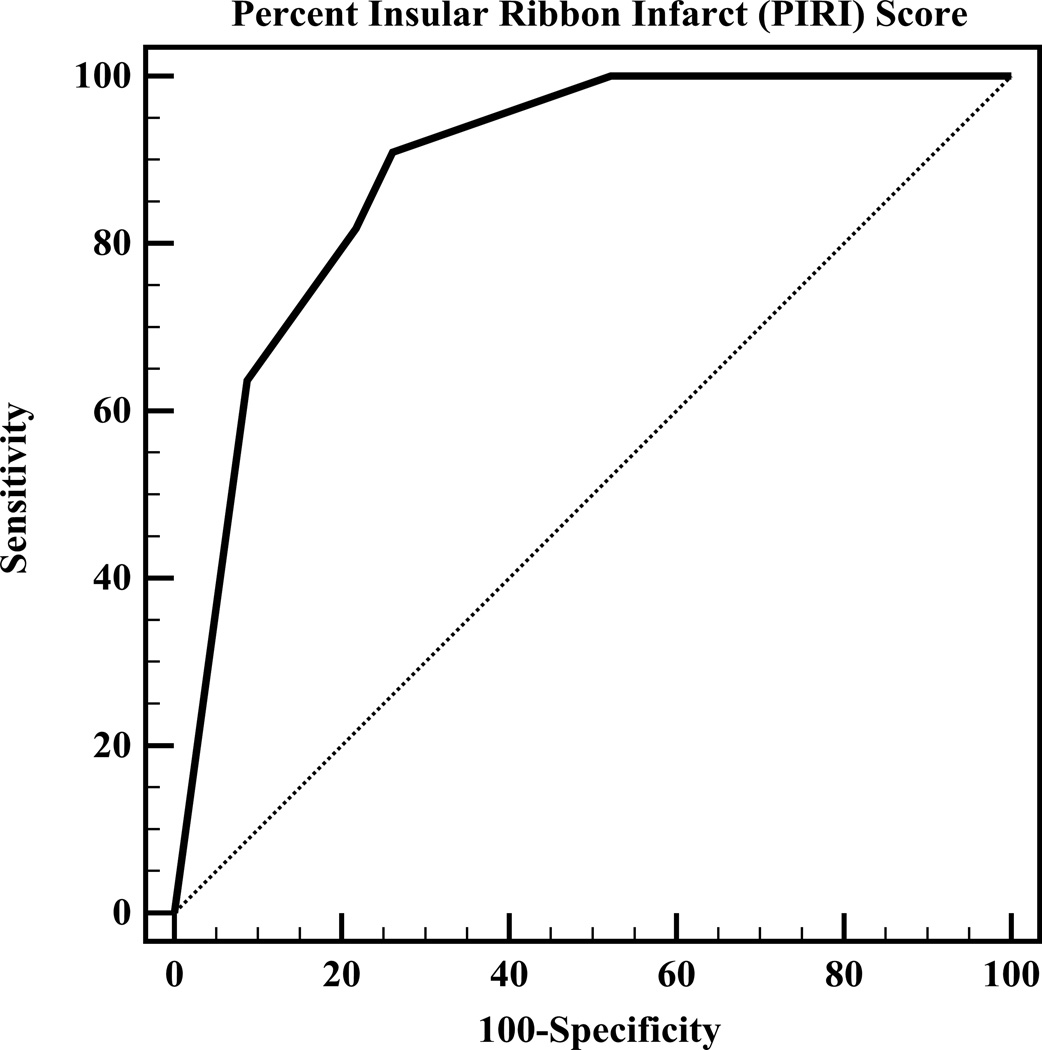
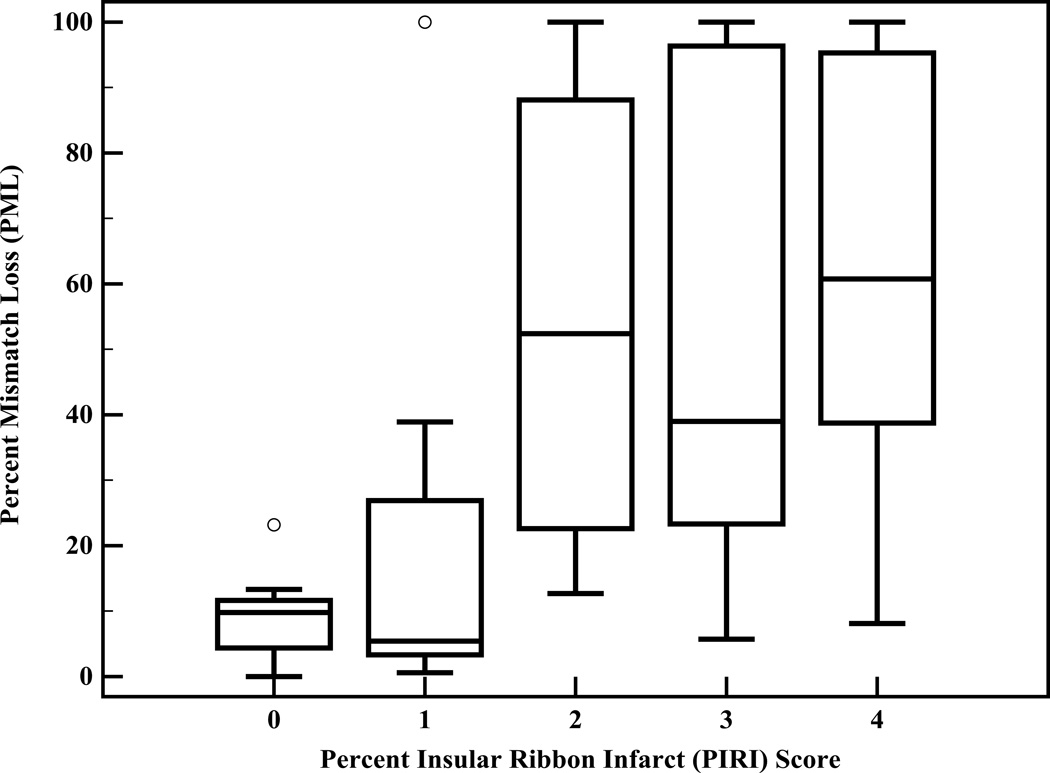
Both Spearman rank correlation and univariate linear regression analyses revealed that PIRI-score is significantly correlated with PML (p<0.0001; slope=13.8 (±2.6)). ROC curve analysis revealed that PIRI score predicts large mismatch loss with OR 3.2 (95%CI=1.8–5.8) for each step increase in PIRI-score from 0 to 4 (p<0.0001). The overall accuracy (ROC area-under-curve, Figure 1A) of the PIRI-score to predict large mismatch loss was 0.89 (95%CI=0.79–0.98). At the optimal operating point of PIRI-score “1” (<25% insula infarction), sensitivity was 91%, specificity was 74%, positive-likelihood ratio was 3.5, and negative-likelihood ratio was 0.12. PML stratified by PIRI-score is shown in Table 2. Kruskal-Wallis test with pairwise post-hoc analysis (Figure 1B) revealed that PIRI-scores of 0 and 1 were significantly different from PIRI scores of 2, 3, and 4 (p=0.0003).
Figure 1.
A, ROC-curve analysis for PIRI prediction of “large” mismatch loss shows area-under-the-curve of 0.89. The operating-point corresponded to a PIRI threshold score of “1” (<25% insula infarction at admission). B, Box-plot diagram of PML versus PIRI score.
Table 2.
Percent mismatch loss (PML) stratified by percent insular-ribbon infarction (PIRI) Score
| PIRI Score | n (%) | Median PML (IQR) |
|---|---|---|
| 0 (0% insula-infarction) | 11 (24%) | 9.8% (4.3–11.6) |
| 1 (<25% infarction) | 8 (18%) | 5.4% (3.3–26.9) |
| 2 (25–49% infarction) | 3 (7%) | 52.4% (22.6–88.1) |
| 3 (50%-74% infarction) | 7 (16%) | 39.0% (22.3–96.3) |
| 4 (≥75% infarction) | 16 (35%) | 60.7% (38.7–95.3) |
Inter-rater agreement
According to the guidelines of Landis and Koch, the strength of interobserver agreement was substantial (κ =0.77, 95%CI=0.66 to 0.93) for the entire range of PIRI ratings, and was almost perfect (κ =0.91, 95%CI=0.79–1.00) for dichotomized PIRI at the <25% insula infarction cutoff.
Predictors of mismatch-loss
Univariate and multivariate regression analysis results are shown in Table 3. Univariate linear-regression showed that admission PIRI-score, DWI-infarct volume, CTA-collateral score, and NIHSS score, were significant predictors of mismatch-loss. In the multivariate linear-regression analysis, PIRI-score was the strongest independent imaging-predictor of mismatch-loss (p=0.03). Patient age (p=0.01) and NIHSS-score (p<0.001) were significant independent clinical-predictors of mismatch-loss. In the binary-logistic regression analysis including dichotomized PIRI (25% threshold), age, NIHSS score, DWI-ASPECTS/infarct volume, and CTA-collateral-score as covariates, only dichotomized insula-score (Figures 2, 3; p=0.03) and age (p=0.02) were independent predictors of large (68.2%) vs. small (8.1%) mismatch-loss.
Table 3.
Predictors of mismatch loss in proximal-MCA occlusive stroke patients
| Admission Parameter |
R2-univariate | p-univariate | p-multivariate* Linear Model |
p-multivariate* Binary Logistic |
|---|---|---|---|---|
| Insula score† | 0.39 | <0.0001 | 0.03 | 0.03 |
| Age | 0.05 | 0.15 | 0.01 | 0.02 |
| NIHSS score | 0.52 | <0.0001 | 0.0005 | 0.17 |
| DWI-infarct volume | 0.18 | 0.004 | 0.69 | 0.36 |
| - or – | ||||
| DWI-ASPECT score | 0.26 | <0.0001 | 0.86 | 0.11 |
| CTA-collateral score | 0.28 | 0.0002 | 0.73 | 0.36 |
| Time-to-DWI imaging | 0.02 | 0.35 | 0.18 | 0.49 |
| IV-tPA or IA treatment | 0.08 | 0.06 | 0.91 | 0.98 |
| Full-regression model | - | <0.001 | <0.0001 |
PIRI= percent insular-ribbon infarction, NIHSS= NIH-stroke-scale score, DWI= diffusion weighted imaging, ASPECT= Alberta-stroke-program-early-CT score, CTA= CT angiography, tPA= tissue-plasminogen-activator, IA= intra-arterial, R= correlation coefficient.
In the multivariate analyses, the p-values for both “forward selection” and “backward elimination” data entry were similarly highly significant only for PIRI and age.
“Insula score” was ordinal PIRI for the univariate and multivariate linear-regression analyses, and dichotomized PIRI at a 25% threshold for the binary-logistic model.
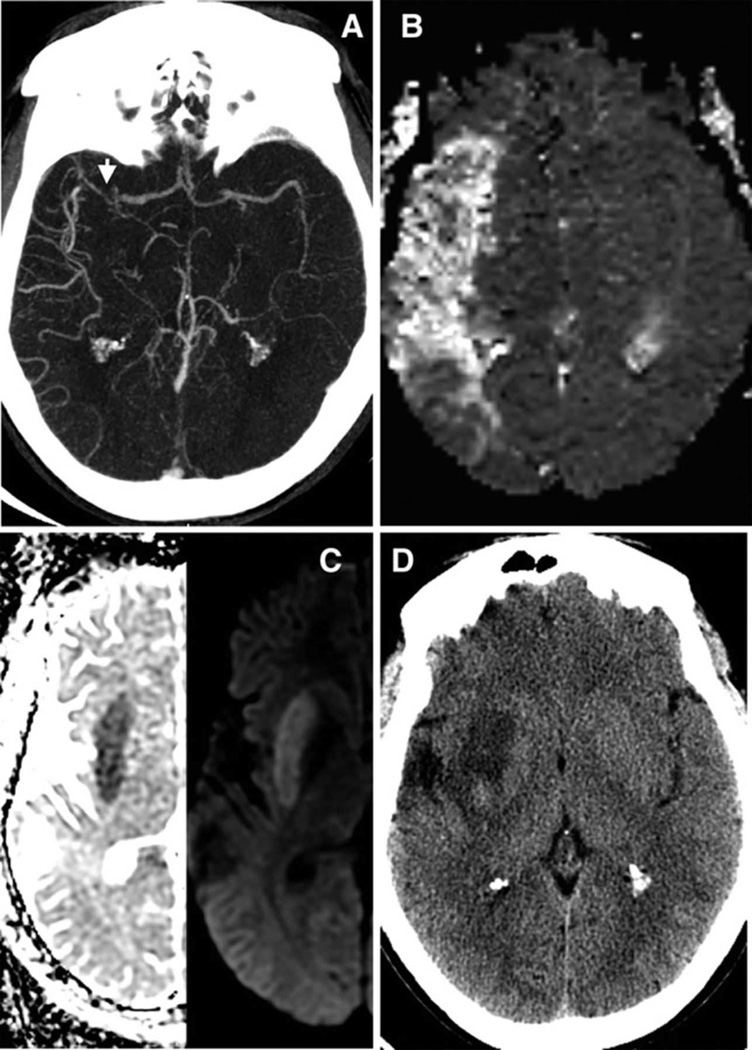
Figure 2. Example of a Low PIRI-score without significant mismatch loss.
34 year-old man presenting five hours after right hemispheric stroke onset with admission NIHSS score of 11. Axial images show: (A) acute proximal MCA occlusion with high collateral flow (versus contralateral) on CTA (arrow), (B) with a large MR-MTT hypoperfused lesion. (C) However normal insula on admission ADC/DWI (PIRI score 0,) and (D) only minimal (13%) tissue-at-risk infarcted on 48-hour follow-up CT despite lack of treatment with IV-tPA or IAT.
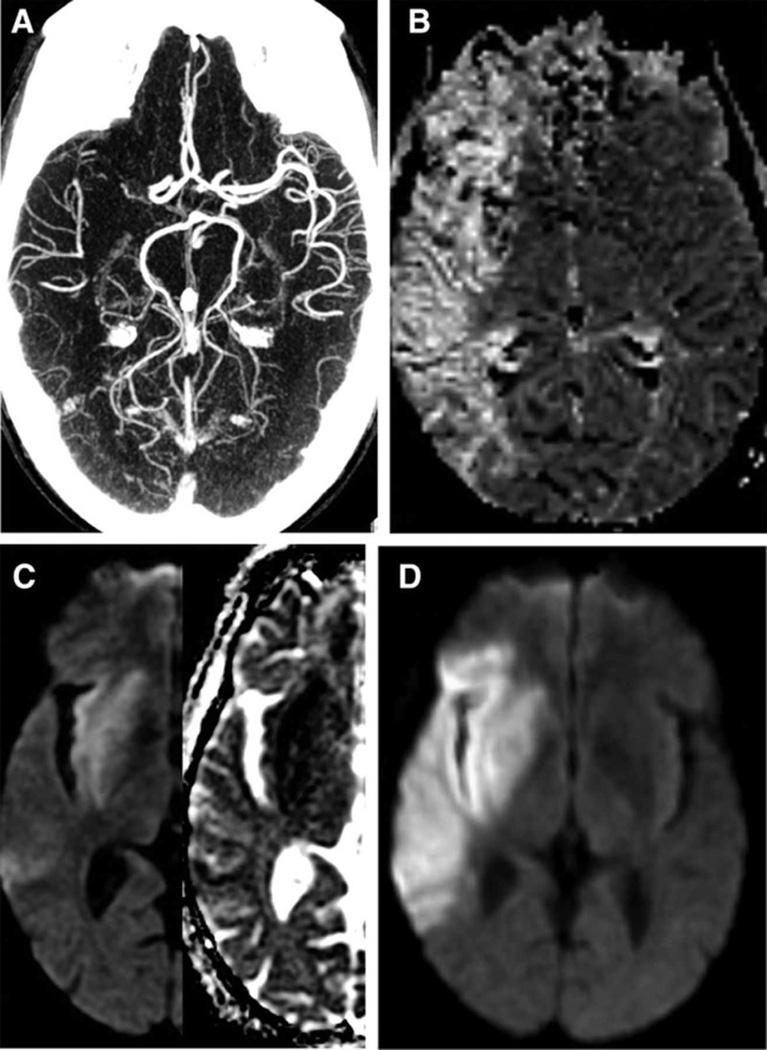
Figure 3. Example of a High PIRI-score with significant mismatch loss.
51 year-old man imaged five hours after right hemispheric stroke onset with admission NIHSS score of 13. Axial images show: (A) acute proximal MCA occlusion with low collateral flow (versus contralateral) on CTA, (B) with a large MR-MTT hypoperfused lesion. (C) However there is complete insula infarction on admission ADC/DWI (PIRI score 4) and (D) marked (60%) progressive infarction of tissue-at-risk on 33-hour follow-up DWI despite treatment with IV-tPA at outside hospital one hour and forty minutes after stroke onset.
DISCUSSION
We have shown that >25% admission insular infarction - determined using a simple, practical, highly reproducible visual assessment - is a stronger predictor of large mismatch-loss in this cohort of proximal-MCA occlusive stroke patients than NIHSS score, DWI ASPECT score, DWI infarct volume, or CTA-collateral score. In our binary logistic regression analysis, only age and PIRI-score>1 were independent predictors of large mismatch-loss.
The significant predictive value of our PIRI-scoring system is in keeping with the results of earlier observational studies and univariate analyses, which suggest that proximal MCA infarcts with substantial insular involvement have greater severity, and are more likely to progress into surrounding penumbral “tissue-at-risk”.11, 12 One notable prior study dichotomized patients according to the presence or absence of admission insula infarct, also using mismatch-loss as an outcome measure.11 In their univariate-only regression analysis, Ay et al showed that patients with insula involvement had greater mismatch loss than those without insula involvement. In another study which dichotomized patients according to “major” (≥2/3) versus “minor” (<2/3) insula involvement, Fink et al showed a significant difference between groups for admission NIHSS score, presence of ≥1/3 MCA territory infarction, and lenticulostriate territory involvement.12
Our results expand on these findings by: (1) applying this idea to a homogeneous population of MCA M1 occlusion; (2) determining the optimal percent insula infarction threshold to distinguish small versus large percent mismatch-loss through ROC curve analysis; and (3) directly comparing the characteristics of our practical, simple, PIRI-scoring method to those of other important clinical and imaging biomarkers of stroke outcome shown in table 1. Specifically, a highly reproducible 25% threshold for percent insula infarction at presentation divided patients into highly distinct groups with regard to admission NIHSS score, admission and final infarct size, quality of CTA collateral circulation, and – most importantly - lesion growth. Moreover, percent insula infarction, with age, was the strongest of these potential predictors of mismatch loss in our binary logistic regression model. The addition of the "insula score" provides a visual marker for the potential "benefit of treatment" – which NIHSS score alone cannot - in patients not otherwise excluded from treatment based on time or admission infarct volume “safety” (i.e., hemorrhagic risk) criteria. It should be noted that these findings apply to patients who present with small infarcts, as the proportion of large (>100 ml) infarcts was small in this study. However, this is the group of patients of most clinical interest because they are eligible for reperfusion therapies, as the presence of extensive infarction is an established exclusion criterion. Therefore, the insula score may help to further stratify patients with small infarcts who are likely to undergo significant infarct extension and in whom rapid treatment would be expected to yield the greatest benefit.
These results make intuitive sense when the extent of admission insula infarction is viewed as a biomarker for the combined effects of both: (i) occlusion, and (ii) pial collateral flow; on blood supply to the global MCA vascular territory. This role of insula percent-infarction at presentation as an early predictor of subsequent infarct growth is supported by the unique vascular anatomy of this region. In an autopsy study of 27 patients, the superior MCA division supplied the entire insula in 51%; no insular cortex was supplied entirely by the inferior MCA division. In approximately 90%, the rolandic artery arose from the same superior division branch that supplied the central insular sulcus.14, 19
Insula involvement is a well-established “early ischemic sign” (EIS) of MCA infarct, whether detected by loss of gray-white matter differentiation on unenhanced CT or restricted diffusion on MR-DWI.11, 14, 20, 21 Functional studies have suggested that insular ribbon has the highest ischemic vulnerability of all the cortical and deep gray matter structures in the brain.12, 22 The greater clinical severity of insular versus non-insular strokes may also, in part, be attributed to a number of familiar and recently recognized important insula functions, including speech and language, volitional swallowing, autonomic (vagal) modulation, cardiovascular regulation, vestibular system activity, and possibly immune modulation.12, 14, 23, 24 Ischemic insular dysfunction might, directly or indirectly, be associated with stroke complications such as intracranial hemorrhage, cerebral vasoconstriction, increased blood-brain-barrier permeability, cardiac arrhythmia, hyperglycemia, or even hospital-acquired-pneumonia.14, 25–27
Our substantial interobserver agreement is supported by structural MRI studies showing high reproducibility for anatomic localization of the insula.14 The insula has easily and reliably recognizable features for visual inspection and rating due to its characteristic location adjacent to the frontal/temporal opercula at the brain surface and medial to the sylvian fissure, connecting the limbic system to the neocortex.11, 14, 20
Potential limitations of our study include the relatively small number of consecutive first-ever stroke patients, which was a consequence of our strict inclusion criteria; isolated proximal-MCA occlusions with admission CTA, DWI, and MR perfusion & follow-up imaging obtained within the specified time-window. This limits the power of performing subgroup analyses stratified by stroke laterality, time-to-imaging, or treatment assignment. Despite this limitation, however, we were able to adjust for time-to-imaging and treatment in our multivariate analysis, mitigating the effects of these factors to suggest that insula-score alone is more predictive of infarct growth than DWI-ASPECTS, DWI-volume, or CTA-collateral score in this population with small admission infarcts. Future analyses should also adjust for reperfusion status and timing of reperfusion, which are critical determinants of infarct growth into the penumbra. Moreover, we did not have a sufficient number of patients with reliable 60 or 90-day modified Rankin scores (mRS) to correlate the PIRI-score with clinical outcome. Because we used admission MR-MTT “at-risk” mismatch lesion volume as an outcome marker to help quantify the degree of infarct growth, we did not additionally study the correlation of other admission MR perfusion metrics with mismatch loss in our analyses.
SUMMARY
Admission insular infarction >25%, with age, is the strongest predictor of large mismatch-loss in this cohort of proximal-MCA occlusive stroke, and can be simply and reliably determined by visual inspection. Future studies of IV-lytic and intra-arterial treated patients with known recanalization status could help to determine if this observation has added value in patient selection for novel and/or late time-window acute stroke therapies.
ACKNOWLEDGMENTS
None
SOURCE OF FUNDING
Shervin Kamalian received training support from Harvard Catalyst (by National Institutes of Health (NIH) Award 8UL1TR000170-05). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the NIH.
Footnotes
DISCLOSURES
Shervin K: GE-Healthcare research support. AK, RCB, LTM, SP, AMF, Shahmir K and KLF: no disclosures. AJY: research support from Penumbra-Inc. and Remedy Pharmaceuticals-Inc. MHL: GE-Healthcare research support, GE-Healthcare stock (<$15K) and Millennium-Pharmaceuticals consultant.
REFERENCES
- 1.Yoo AJ, Leslie-Mazwi TM, Jovin TG. Future directions in IAT: better studies, better selection, better timing and better techniques. J Neurointerv Surg. 2013;5(Suppl 1):i1–i6. doi: 10.1136/neurintsurg-2013-010741. [DOI] [PubMed] [Google Scholar]
- 2.Maas MB, Furie KL, Lev MH, Ay H, Singhal AB, Greer DM, et al. National Institutes of Health Stroke Scale score is poorly predictive of proximal occlusion in acute cerebral ischemia. Stroke. 2009;40:2988–2993. doi: 10.1161/STROKEAHA.109.555664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemmling A, Kamalian S, Iqbal S, Lev MH. Thrombus Location Matters: Infarct Growth in Acute Proximal Middle Cerebral Artery Occlusion. ASNR 2009 Annual Meeting. P69. 306. [Google Scholar]
- 4.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nezu T, Koga M, Kimura K, Shiokawa Y, Nakagawara J, Furui E, et al. Pretreatment ASPECTS on DWI predicts 3-month outcome following rt-PA: SAMURAI rt-PA Registry. Neurology. 2010;75:555–561. doi: 10.1212/WNL.0b013e3181eccf78. [DOI] [PubMed] [Google Scholar]
- 6.Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41:2316–2322. doi: 10.1161/STROKEAHA.110.592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souza LC, Yoo AJ, Chaudhry ZA, Payabvash S, Kemmling A, Schaefer PW, et al. Malignant CTA collateral profile is highly specific for large admission DWI infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol. 2012;33:1331–1336. doi: 10.3174/ajnr.A2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo AJ, Barak ER, Copen WA, Kamalian S, Gharai LR, Pervez MA, et al. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke. 2010;41:1728–1735. doi: 10.1161/STROKEAHA.110.582874. [DOI] [PubMed] [Google Scholar]
- 9.Warach S, Al-Rawi Y, Furlan AJ, Fiebach JB, Wintermark M, Lindsten A, et al. Refinement of the magnetic resonance diffusion-perfusion mismatch concept for thrombolytic patient selection: insights from the desmoteplase in acute stroke trials. Stroke. 2012;43:2313–2318. doi: 10.1161/STROKEAHA.111.642348. [DOI] [PubMed] [Google Scholar]
- 10.Lev MH. Perfusion imaging of acute stroke: its role in current and future clinical practice. Radiology. 2013;266:22–27. doi: 10.1148/radiol.12121355. [DOI] [PubMed] [Google Scholar]
- 11.Ay H, Arsava EM, Koroshetz WJ, Sorensen AG. Middle cerebral artery infarcts encompassing the insula are more prone to growth. Stroke. 2008;39:373–378. doi: 10.1161/STROKEAHA.107.499095. [DOI] [PubMed] [Google Scholar]
- 12.Fink JN, Selim MH, Kumar S, Voetsch B, Fong WC, Caplan LR. Insular cortex infarction in acute middle cerebral artery territory stroke: predictor of stroke severity and vascular lesion. Arch Neurol. 2005;62:1081–1085. doi: 10.1001/archneur.62.7.1081. [DOI] [PubMed] [Google Scholar]
- 13.Ture U, Yasargil MG, Al-Mefty O, Yasargil DC. Arteries of the insula. J Neurosurg. 2000;92:676–687. doi: 10.3171/jns.2000.92.4.0676. [DOI] [PubMed] [Google Scholar]
- 14.Naidich TP, Kang E, Fatterpekar GM, Delman BN, Gultekin SH, Wolfe D, et al. The insula: anatomic study and MR imaging display at 1.5 T. AJNR Am J Neuroradiol. 2004;25:222–232. [PMC free article] [PubMed] [Google Scholar]
- 15.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 17.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 18.Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC. Stroke Prognostication using Age and NIH Stroke Scale: SPAN-100. Neurology. 2012 [Google Scholar]
- 19.Varnavas GG, Grand W. The insular cortex: morphological and vascular anatomic characteristics. Neurosurgery. 1999;44:127–136. doi: 10.1097/00006123-199901000-00079. discussion 136-128. [DOI] [PubMed] [Google Scholar]
- 20.Truwit CL, Barkovich AJ, Gean-Marton A, Hibri N, Norman D. Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology. 1990;176:801–806. doi: 10.1148/radiology.176.3.2389039. [DOI] [PubMed] [Google Scholar]
- 21.Moulin T, Cattin F, Crepin-Leblond T, Tatu L, Chavot D, Piotin M, et al. Early CT signs in acute middle cerebral artery infarction: predictive value for subsequent infarct locations and outcome. Neurology. 1996;47:366–375. doi: 10.1212/wnl.47.2.366. [DOI] [PubMed] [Google Scholar]
- 22.Payabvash S, Souza LC, Wang Y, Schaefer PW, Furie KL, Halpern EF, et al. Regional ischemic vulnerability of the brain to hypoperfusion: the need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke. 2011;42:1255–1260. doi: 10.1161/STROKEAHA.110.600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oppenheimer S. The anatomy and physiology of cortical mechanisms of cardiac control. Stroke. 1993;24:I3–I5. [PubMed] [Google Scholar]
- 24.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 25.Natelson BH, Chang Q. Sudden death. A neurocardiologic phenomenon. Neurol Clin. 1993;11:293–308. [PubMed] [Google Scholar]
- 26.Raichle ME, Hartman BK, Eichling JO, Sharpe LG. Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc Natl Acad Sci U S A. 1975;72:3726–3730. doi: 10.1073/pnas.72.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemmling A, Pervez MA, Lev MH, Masrur S, Payabvash S, Schwamm LH. Hospital acquired pneumonia is linked to peri-insular right hemispheric and infratentorial acute stroke. International Stroke Conference. 2010 P501. 154. [Google Scholar]