Background: Very little is known about cancer stem cells in ductal carcinoma in situ (DCIS).
Results: Basal-like DCIS contain CD49f+/CD24− ALDH1+ stem-like subpopulations with enhanced migratory capacity, unique exosomal microRNA signature, and sulforaphane sensitivity.
Conclusion: We characterized basal-like DCIS stem cells and demonstrate a potential chemopreventive strategy.
Significance: DCIS stem cells are important therapeutic targets for preventing recurrence or progression to invasive disease.
Keywords: Exosomes, MicroRNA, Breast Cancer, Cancer Stem Cells, Chemoprevention, Basal-like DCIS Stem Cell, Sulforaphane
Abstract
Previously, we found that basal-like ductal carcinoma in situ (DCIS) contains cancer stem-like cells. Here, we characterize stem-like subpopulations in a model of basal-like DCIS and identify subpopulations of CD49f+/CD24− stem-like cells that possess aldehyde dehydrogenase 1 activity. We found that these cells show enhanced migration potential compared with non-stem DCIS cells. We also found that the chemopreventive agent sulforaphane can target these DCIS stem-like cells, reduce aldehyde dehydrogenase 1 (ALDH1) expression, and decrease mammosphere and progenitor colony formation. Furthermore, we characterized exosomal trafficking of microRNAs in DCIS and found that several microRNAs (miRs) including miR-140, miR-29a, and miR-21 are differentially expressed in exosomes from DCIS stem-like cells. We found that SFN treatment could reprogram DCIS stem-like cells as evidenced by significant changes in exosomal secretion more closely resembling that of non-stem cancer cells. Finally, we demonstrated that exosomal secretion of miR-140 might impact signaling in nearby breast cancer cells.
Introduction
Ductal carcinoma in situ (DCIS)3 is an early stage, non-invasive breast cancer. Therapeutic management of DCIS involves surgery, radiation, and where appropriate, hormonal therapies (1). Roughly 15% of patients with DCIS show recurrent disease following therapy (2). Identifying which patients are at greatest risk for disease recurrence and progression to invasive disease is a critical issue facing clinicians.
Similar to invasive ductal carcinoma, DCIS is a heterogeneous disease consisting of multiple molecular subtypes identified through gene expression profiling. For luminal subtype DCIS, estrogen receptor α-positive (ER+) patients benefit from adjuvant tamoxifen treatment (3, 4). However, there are no available targeted therapies for patients with basal-like DCIS malignancies.
Previous studies indicated that DCIS lesions are the precursor of most invasive breast tumors (5). We recently published evidence that cancer stem-like cells can be identified within basal-like DCIS that might drive malignant progression. Furthermore, we demonstrated that these DCIS stem-like cells possessed self-renewal properties in vitro and in vivo (6).
Studies in invasive breast tumors have identified multiple markers delineating cancer stem cells (CSCs) from non-stem breast cancer cells. The first solid tumor stem cell markers were identified in breast tumors where CD44-high/CD24-low breast cancer cells were shown to be enriched in CSCs (7, 8). These markers are sufficient for enriching for CSCs primarily in luminal type breast tumors, whereas basal-like or claudin-low breast tumors consist primarily of CD44-high breast cancer cells (9–11). It may be possible to utilize additional markers such as EpCAM+ (ESA) to further enrich for CSCs in breast tumors that are primarily CD44 high (9). Others have taken a different approach, utilizing enzymatic activity of ALDH1 (aldefluor assay) to identify stem-like cells in breast tumors, with basal-like invasive ductal carcinoma possessing higher levels of ALDH1+ cells (12). Finally, some groups have identified breast cancer stem cells by separating tumor cells that possess the highest number of drug efflux pumps by using dye exclusion assays (the side population) (13).
Many studies have examined the molecular profiles of various stem-like cells in invasive ductal carcinoma tumors. However, the molecular characteristics of DCIS stem cells are less clear. Here, we examine a model of basal-like DCIS and identify stem like subpopulations involving ALDH1+ and CD49f+/CD24− cells. We demonstrate that these cells possess enhanced migration capacity; this aggressive phenotype suggests these cells may be malignant precursor cells. We find that these cells are sensitive to the dietary chemopreventive compound SFN. Treatment with SFN reduces mammosphere formation and decreases ALDH1 expression in these DCIS stem-like cells.
Signaling within the tumor microenvironment is considered to play an important role in the progression from DCIS to invasive disease (14). One mechanism through which tumor cells signal within the tumor microenvironment is exosomal secretion (15). Exosomes are small (<100 nm) vesicles secreted by cells that contain cargo proteins and nucleic acids. It has been shown that specific miRs are secreted by tumor cell exosomes that can signal to nearby tumor, immune, or endothelial cells. We characterized unique cell-cell signaling of these DCIS stem-like cells involving exosomal trafficking of miRs, which we demonstrate might regulate stem cell activity in nearby cells.
EXPERIMENTAL PROCEDURES
Cell Culture
MCF10DCIS were grown in DMEM/F12 supplemented with 5% horse serum (Invitrogen) and 1% l-glutamine (Invitrogen). MCF-7, MDA-MB-231, and HEK-293T cells were grown in DMEM with 5% FBS (HyClone; Rockford, IL) and 1% l-glutamine. SUM102PT cells were grown in Ham's F12 with 10 ng/ml EGF, 5 μg/ml insulin, 1 μg/ml hydrocortisone, 5 mm ethanolamine, 10 mm HEPES, 5 μg/ml transferring, 10 nm of triiodothyronine, and 0.1% BSA. Cells were incubated in 5% CO2 at 37 °C. Sulforaphane (Sigma) was dissolved in EtOH.
Transwell Migration
Transwell migration assays were carried out using Transwell migration chambers (8-μm pore size; Costar; Cambridge, MA). Cells were grown in DMEM (0.5 × 105 cells/ml) in the upper chamber. The lower chamber contained DMEM with 10% FBS. The cells were allowed to migrate toward the 10% FBS gradient overnight. Non-migrated cells were removed with cotton swabs. The migrated cells were stained with 1% crystal violet in methanol/PBS and counted using light microscopy. Five random fields were counted per experiment.
Quantitative Real-time PCR and MicroRNA Array
qRT-PCR analysis of mRNA/miR expression was performed as described previously (16) with normalization to GAPDH mRNA or U6 snRNA (GAPDH, GAAGGTGAAGG TCGGAGTC (forward) and GAAGATG GTGATGGGATTTC (reverse)). Exosomal microRNA expression was screened for an 88-miR panel, human miFinder array (SABioscience; Flat Lake, MD).
Flow Cytometry, Immunofluorescence, and Western Blotting
Flow cytometry was performed on cells stained with CD44-APC, CD49f-APC, and CD24-PE antibodies (BD Pharmingen). Immunostaining of breast cancer cells was performed using anti-CK8/18 antibody (University of Iowa Developmental Hybridoma Studies Bank, clone Troma-I) and anti-CK14 (Covance; San Diego, CA). Western blotting was performed as described previously (16) using β-actin (Sigma) and ALDH1 (BD Pharminigen) antibodies. Immunofluorescent staining was performed using Alexa Fluor 488/555-conjugated secondary antibodies (Invitrogen) with DAPI counterstaining. Fluorescent staining was visualized using an Olympus IX81 spinning disk confocal microscope.
Mammosphere, Aldefluor Assay, and Progenitor Differentiation Assay
Single cells were obtained using cell dissociation buffer (Millipore; Billerica, MA) and 40-μm cell strainers (Fisher Scientific; Pittsburgh, PA) and counted. 20,000 cells/ml were seeded in six-well plates coated with 2% polyhema (Sigma) in DMEM/F12 containing 2% B27, 20 ng/ml EGF, 4 μg/ml insulin, and 0.4% BSA. After 7 days, spheres >100 μm were quantified by light microscopy. Aldefluor assay was performed according to manufacturer's instructions (StemCell Technologies, Durham, NC). Progenitor differentiation assays were performed by culturing 103 breast cancer cells with 5 × 104 irradiated NIH-3T3 fibroblasts in DMEM/F12 containing 2% B27, 20 ng/ml EGF, 4 μg/ml insulin, and 0.4% BSA. After 7 days, cells were visualized by light microscopy or stained with fluorescent antibodies.
Xenograft Formation
All studies were done in accordance with federal guidelines and institutional policies of University of Maryland Animal Care and Use Committee. Six-week-old nude mice (Charles River Laboratories, Wilmington, MA) were injected with DCIS cells resuspended in a 1:1 ratio of media/Matrigel at the no. 5 mammary gland nipple. Tumor size was monitored weekly using digital calipers. Paraffin embedding, sectioning, and H&E staining were performed by University of Maryland Histology Core.
Exosomal Isolation and Internalization Assay
Exosomes were isolated using the Total Exosome Isolation kit (Invitrogen). Exosomal RNA was isolated using the Total Exosome RNA and Protein Isolation kit (Invitrogen). HEK-293T cells were co-transfected with miR-140 overexpression vector and CD9-GFP vector using Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions. After 3 days, exosomes were isolated and added to MCF10DCIS or MDA-MB-231 cell medium. 24 h later, cells were washed, and RNA was harvested. All exosomal experiments were performed in exosome-free media as obtained via ultracentrifugation.
Statistical Analysis
Statistical analysis was performed by Student's t test. p values of < 0.05 (*) were considered significant. Data are presented as mean ± S.E. Data were analyzed using GraphPad Prism software (version 6.0).
RESULTS
Basal-like DCIS Lesions Contain a CD49f+/CD24− Stem-like Subpopulation Enriched in ALDH1 Activity
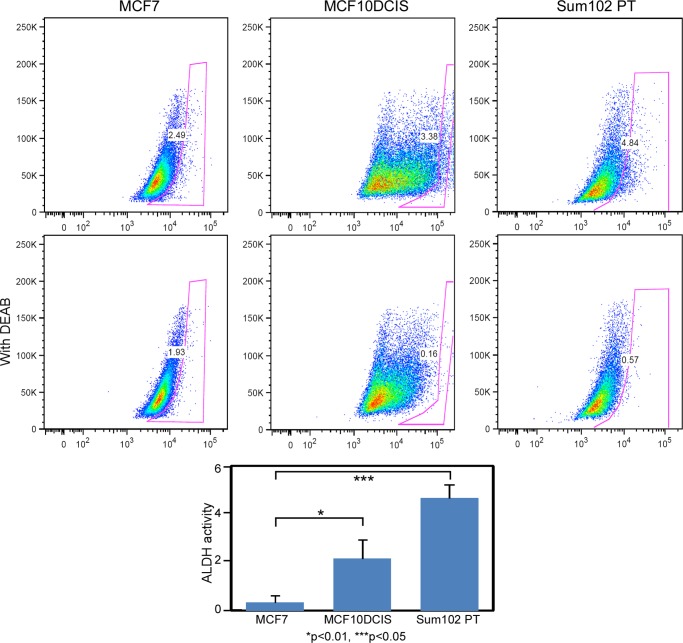
In our previous studies, we have examined the underlying biology of several models of basal-like DCIS (6). Here, we set out to better characterize the stem cell compartment in these DCIS lesions. Previous studies of invasive ductal carcinoma have revealed that ALDH1 activity is a marker of mammary stem cells and breast cancer stem cells (12). Specifically, basal-like invasive ductal carcinoma has been shown to contain a stem cell subpopulation that possesses ALDH1 activity (11). We examined ALDH1 activity levels in MCF10DCIS cells, which are clonally derived from H-Ras-transformed MCF10A cells (non-tumorigenic mammary epithelial cells) (18), and in SUM102PT cells, a breast cancer cell line derived from a patient with DCIS and concomitant invasive ductal carcinoma lesions. We performed the aldefluor fluorescent assay to measure ALDH1 activity and found that compared with MCF-7 luminal invasive carcinoma (known to show little to no ALDH1 activity), MCF10DCIS and SUM102PT cells possess a stem-like subpopulation (roughly 3–5% of cells) with high ALDH1 activity (Fig. 1).
FIGURE 1.
Basal-like DCIS cell lines possess high levels of ALDH1 activity. We examined ALDH1 activity levels in DCIS and SUM102PT cells using the aldefluor assay. The percentage of ALDH1+ cells minus the amount detected in the presence of diethylaminobenzaldehyde (DEAB) inhibitor is graphed below. Invasive MCF-7 (luminal) cell lines are used as a control as they are known to possess very little ALDH1 activity.
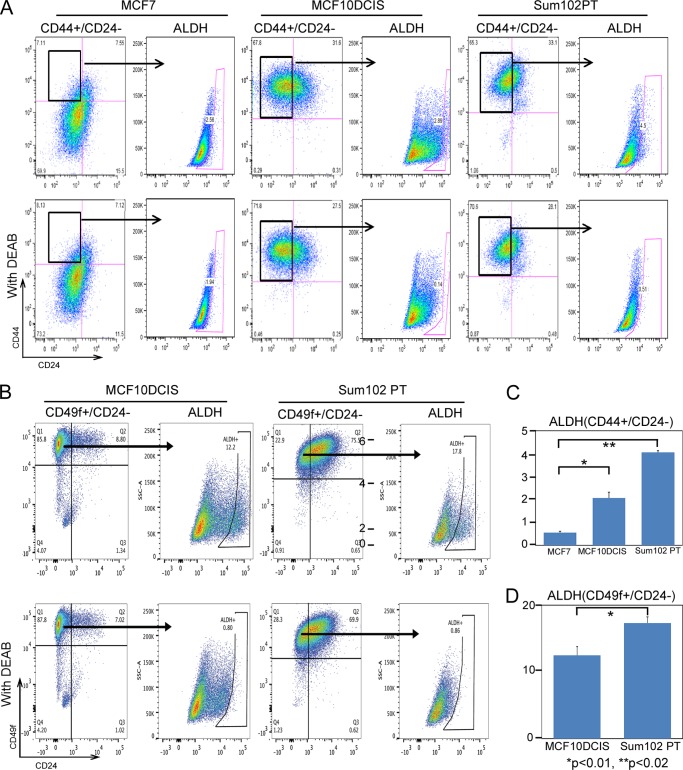
We have previously demonstrated that MCF10DCIS cells are enriched in CD44-high/CD24-low cells (6), well known markers for breast cancer stem cells. We examined whether use of these combined markers, CD44-high/CD24-low and ALDH1 activity might allow greater enrichment of highly tumorigenic cells. However, as shown in Fig. 2A, we found that the CD44-high/CD24-low subpopulation of cells failed to show an enrichment in ALDH1 activity as compared with the whole cell population (still roughly 3–5% of cells) in either MCF10DCIS cells or SUM102PT cells (Fig. 2C).
FIGURE 2.
There is no overlap of CD44-high/CD24-low and ALDH1+ cells in basal-like DCIS. A, CD44-high/CD24-low cells do not overlap with ALDH1+ cells in basal-like DCIS. Cells were stained with CD44-APC/CD24-PE antibodies and ALDH1 activity was measured using the aldefluor kit. B, CD49f-high/CD24-low cells are enriched in ALDH1+ cells. Cells were stained with CD49f-APC/CD24-PE antibodies, and ALDH1 activity was measured using the aldefluor kit. C and D, quantification of ALDH1+ cells (minus diethylaminobenzaldehyde (DEAB) control).
As there was little overlap between CD44-high/CD24-low and ALDH1+ subpopulations, we examined other potential stem cell markers that might further enrich for DCIS stem-like cells. We examined CD49f (α6 integrin) expression, a marker of mouse mammary stem cells (19), in MCF10DCIS and SUM102PT cells (Fig. 2B). We found that CD49f- high/CD24-low subpopulation of cells were enriched in ALDH1+ populations (10–18% of cells) (Fig. 2D). This result suggests that these overlapping markers might allow greater enrichment of DCIS stem-like cells for further study.
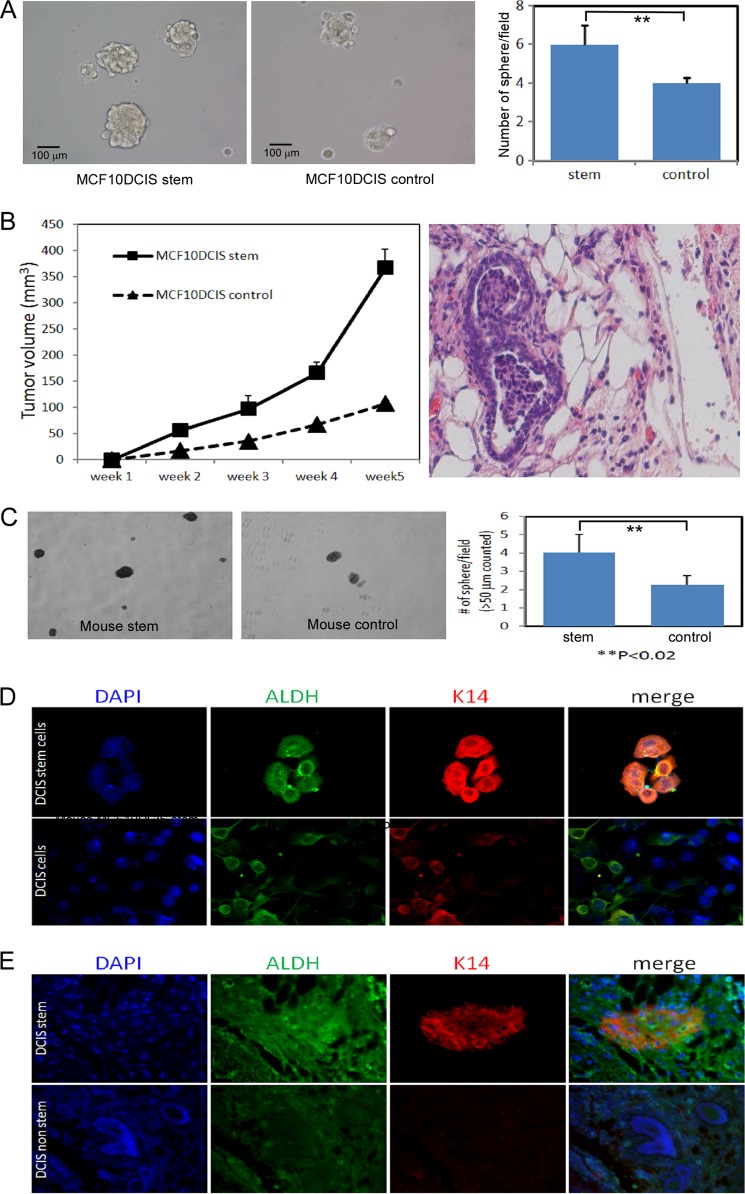
Next, we were also interested in potential stem cell activity in MCF10DCIS cells grown in attachment-free mammosphere conditions. We found that CD49f+/CD24− DCIS stem-like cells formed more mammospheres than MCF10DCIS control cells (Fig. 3A), indicating enrichment in self-renewal capability.
FIGURE 3.
DCIS stem cells can form mammospheres and are enriched in ALDH1 staining in vivo. A, mammosphere formation of CD49f+/CD24− MCF10DCIS cells and MCF10DCIS control cells. B, DCIS stem-like cells are highly tumorigenic in vivo. Nude mice were injected with CD49f+/CD24− MCF10DCIS stem-like cells or MCF10DCIS control cells, and tumor growth was monitored weekly using digital calipers (left). Only one of five mice injected with MCF10DCIS control cells showed DCIS. However, four of five mice injected with MCF10DCIS stem cells showed DCIS. H&E staining of MCF10DCIS stem cell xenograft tumor showing DCIS structure (right). C, tumor cells were harvested and dissociated and then grown in attachment free mammosphere culture. D, DCIS stem-like cells were grown as mammosphere for 7 days after which cells were harvested and allowed to attach to coverslips for 24 h before performing immunofluorescence for ALDH1 and cytokeratin 14 (K14) and counterstained for DAPI. E, tissue from xenograft tumors (DCIS stem-like cells or DCIS non-stem cells) were examined by immunofluorescence for ALDH1 and cytokeratin 14.
DCIS Stem-like Cells Are Highly Tumorigenic in Vivo
Next, we examined the tumorigenicity of these cells in vivo to further assess their stem cell properties. We performed xenograft experiments by injecting mammary glands of nude mice with CD49f+/CD24− MCF10DCIS stem-like cells. We found that MCF10DCIS stem-like cells could form fast growing tumors in nude mice (Fig. 3B, left) when compared with MCF10DCIS control cells. We observed very low tumorigenic formation in mice injected with MCF10DCIS control cells. Furthermore, we observed DCIS structures in mice injected with MCF10DCIS stem cells, which were confirmed by paraffin embedding, tissue sectioning, and H&E staining of tumors (Fig. 3B, right). Dissociated cells from the MCF10DCIS tumors demonstrated increased mammosphere formation compared with MCF10DCIS control cells, providing further evidence for the self-renewal capacity of these MCF10DCIS stem cells (Fig. 3C).
Next, we used immunofluorescence to examine ALDH1 expression in mammospheres and in tumor tissues. We found that a majority of mammospheres from MCF10DCIS stem-like cells contained ALDH1-bright cells (Fig. 3D) as compared with whole population cells, which had variable intensity ALDH1 staining. We analyzed tumor tissues of xenograft tumors from MCF10DCIS stem-like cells and from MCF10DCIS whole populations and found that tumor cells from MCF10DCIS stem-like cells had much higher expression of ALDH1 stem cell marker and intense staining of the basal lineage marker cytokerratin 14 (Fig. 3E). This corresponds with our earlier observation that CD49f+/CD24− stem-like cells were enriched in ALDH1 activity (Fig. 2, B and D).
DCIS Stem-like Cells Possess Enhanced Migratory Capacity
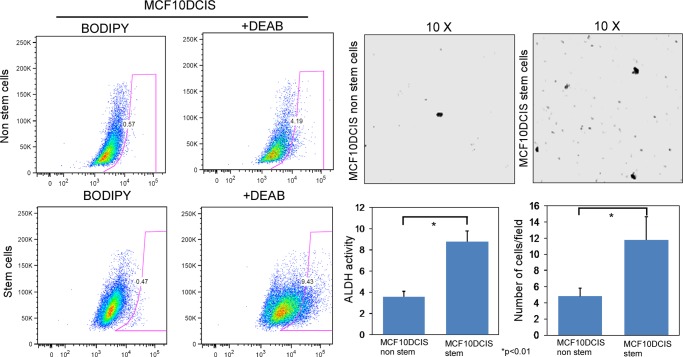
Malignant precursor cells are predicted to exist within DCIS lesions that predetermine invasive progression and recurrence. DCIS stem-like cells are a likely candidate to contain this malignant program and therefore might be an important therapeutic target for prevention of disease progression. As such, we sought to functionally characterize the invasive potential of DCIS stem-like cells. We isolated ALDH1+ and ALDH− populations from MCF10DCIS cells and performed cell invasion assays on Transwell inserts (8-μm pore) using serum as a chemoattractant. As expected, a majority of cells (ALDH−) demonstrated low migratory potential. However, ALDH1+ stem-like cells demonstrated a significantly enriched migratory capacity (Fig. 4). This suggests that these cells might be primed to become invasive.
FIGURE 4.
DCIS stem cells possess enhanced migratory capacity. We isolated CD49f+/CD24− stem-like cells, which were enriched in ALDH1 activity, from MCF10DCIS cells, and we performed transwell migration assays. After 24 h of migrating toward a chemoattractant (10% FBS), we stained migrated cells with 1% crystal violet and visualized with light microscopy. Five random fields were counted (n = 3).
Characterization of DCIS Progenitor Cells
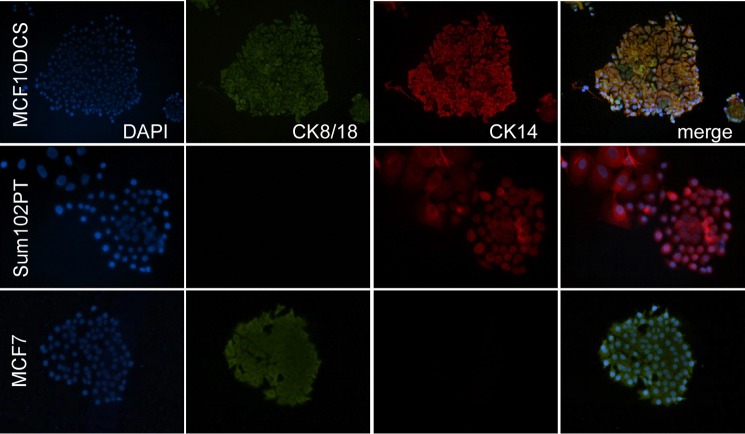
Stem cells are defined by their potential for self-renewal and multi-lineage differentiation. Mimicking normal tissue hierarchy, cancer stem cells possess aberrant self-renewal and a capacity to differentiate into non-stem cancer cells that make up the bulk of the tumor. To examine the differentiation capacity of our DCIS model, we performed co-culture colony formation assays that promote progenitor proliferation and differentiation. We grew MCF10DCIS and SUM102PT cells in clonal conditions atop a feeder layer of irradiated NIH-3T3 fibroblasts. We examined colony formation by morphologic analysis and the expression of lineage-associated cytokeratins denoting basal (cytokeratin 14) or luminal (cytokeratin 8/18) lineage (20). As expected, MCF-7 control cells formed only CK18+/CK14− luminal colonies with visible luminal epithelial morphology and cell-cell contacts (Fig. 5). SUM102PT cells, previously characterized as basal-like, were found to form only CK14+/CK18− basal-like colonies with fewer visible cell-cell junctions. Finally, MCF10DCIS cells demonstrated strong CK14+ staining with weak CK18 staining resembling cells of primarily basal lineage. Their morphology, however, more closely resembled that of luminal lineage MCF-7 breast cancer cells. These results suggest that MCF10DCIS cells may possess mixed lineages of progenitor cells or could contain bipotent progenitors.
FIGURE 5.
Progenitor colony formation of DCIS cell models. Immunofluorescent staining for luminal (cytokeratin 8/18 (CK8/18)) and myoepithelial/basal (cytokeratin 14 (CK14)) lineage-associated markers. 1,000 DCIS cells were co-cultured with 50,000 irradiated NIH-3T3 fibroblasts. Staining was performed using Alexa Fluor 488/555 conjugated secondary antibodies. DAPI counterstaining was used to visualize cell nuclei.
Targeting DCIS Stem-like Cells with Chemopreventive Agent Sulforaphane
We previously demonstrated that treatment with the isothiocyanate SFN could inhibit DCIS stem-like cells in vitro and in vivo (6). Because our current studies further characterized these basal-like DCIS contain CD49f+/CD24− ALDH1+ stem-like subpopulations, we next decided to test whether treatment with SFN can impact DCIS stem-like characteristics, including progenitor proliferation, migratory capacity, and unique exosomal microRNA signature.
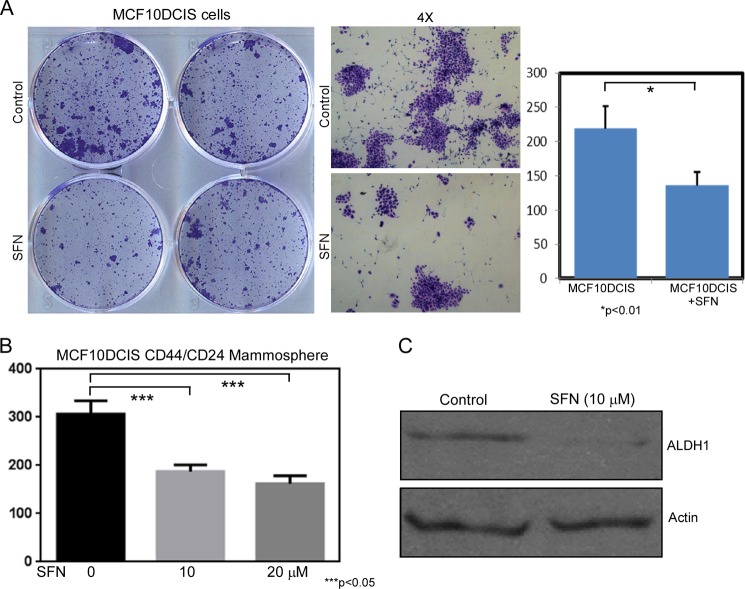
Here, we examined the impact of the SFN on colony formation of MCF10DCIS progenitor cells. We observed a decrease in colony formation upon SFN treatment possibly due to decreased progenitor proliferation (Fig. 6A). Next, we examined the impact of SFN on self-renewal of DCIS stem-like cells in mammosphere culture conditions. We observed a decrease in mammosphere formation upon SFN treatment indicating that SFN can inhibit the self-renewal of DCIS stem-like cells (Fig. 6B). Finally, we observed a decrease in ALDH1 expression following SFN treatment via Western blotting, indicating that SFN treatment could block stem cell signaling in DCIS stem-like cells (Fig. 6C).
FIGURE 6.
SFN treatment targets DCIS stem-like cells. A, SFN treatment (10 μm) reduces progenitor colony formation. Colonies were stained with crystal violet, and colonies were quantified by light microscopy. B, SFN treatment reduced mammosphere formation of DCIS stem-like cells. Isolated CD44-high/CD24-low stem-like cells were culture in mammosphere conditions with or without SFN treatment (0, 10, 20 μm), and after 7 days, spheres > 100 μm were quantified by light microscopy. C, SFN treatment reduces ALDH1 expression. Western blotting was used to quantify ALDH1 expression following SFN treatment (10 μm). β-Actin was used as a loading control.
Characterization of DCIS Exosomal Trafficking
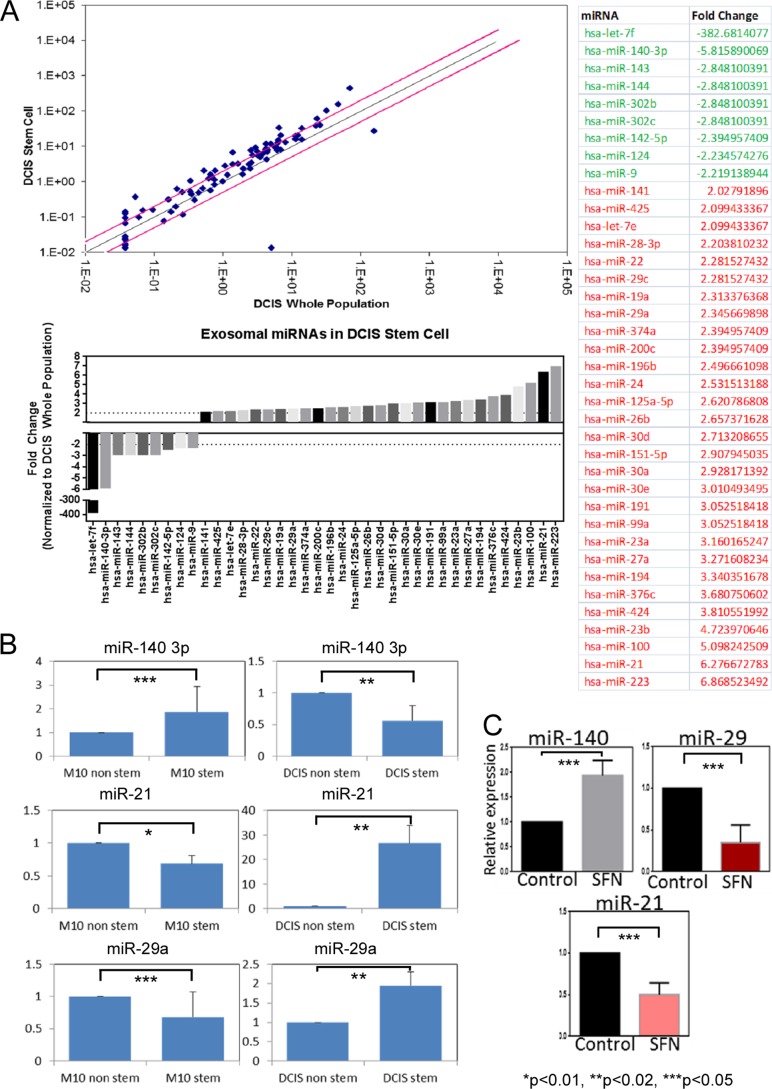
We next examined how DCIS stem-like cells might signal between other cancer cells and/or tumor microenvironment to influence disease progression. Previous studies have found that invasive breast cancers secrete tumor-derived exosomes, small membrane bound vesicles of endosomal origin that contain proteins and RNAs that can provide active protumor signals to recipient cells within the tumor and surrounding stroma (15). We first isolated exosomes from MCF10DCIS stem-like cells and MCF10DCIS whole cell populations and then extracted exosomal RNA content. We performed array-based analysis of miR species present in the exosomes from MCF10DCIS stem-like cells. Compared with exosomes isolated from DCIS whole-cell populations, exosomes from DCIS stem-like cells contained altered miR content (Fig. 7A). miR array analysis revealed differential expression of 38 miRs (nine down-regulated and 29 up-regulated) in DCIS stem-like cell exosomes, among them miR-140 was found to be down-regulated in DCIS stem-like exosomes. This matches what we previously observed regarding cellular expression of miR-140 in DCIS stem-like cells (where miR-140 is down-regulated compared with non-stem like cells).
FIGURE 7.
Differential expression of exosomal miRs in DCIS stem-like cells. A, miR qRT-PCR array, comparing exosomal expression of 88 miRs in MCF10DCIS stem-like cell exosomes (CD44-high/CD-24 low) and DCIS whole cell population exosomes. Results that met a 2-fold cutoff are listed in the table. B, follow-up qRT-PCR validation of array results for miR-140–3p, miR-21, and miR-29a. Results are normalized to U6 snRNA. M10, MCF10A; DCIS, MCF10DCIS. C, qRT-PCR examining miR-140, miR-21, and mi-29a levels following 10 μm SFN treatment.
We also tested whether expression of exosomal miRs from DCIS stem cells reflected a stem cell program in common with that of parental non-tumorigenic MCF10A stem cells. We isolated exosomal miRs from MCF10A stem cells and MCF10DCIS stem cells and compared expression of candidate miRs identified by our array. Interestingly, we observed an inverse trend regarding exosomal miR expression in normal stem cells compared with DCIS stem cells (Fig. 7B). MCF10A stem cells secreted higher levels of miR-140 than MCF10A non-stem cells, whereas MCF10DCIS stem cells secreted lower levels of miR-140 than MCF10DCIS non-stem cells. A dramatic inverse trend was also observed for miR-21 and miR-29 in which DCIS stem-like cells secreted higher levels of these miRs, whereas normal stem cells secreted lower levels of these miRs. This extracellular signaling may serve as a differentiation signal that is missing in DCIS stem cells.
We further examined whether SFN treatment might impact secretion of candidate miRs from DCIS stem-like cells. We found that sulforaphane treatment resulted in increased exosomal miR-140 and decreased exosomal miR-21 and miR-29 (Fig. 7C). This provides further evidence that SFN can inhibit DCIS stem cell signaling which may promote differentiation in recipient cells.
Exosomal Secretion and Internalization of miR-140
Finally, we examined what impact these altered exosomal miRs might have on breast cancer cell signaling. As we have previously reported on its significance in stem cell signaling in DCIS lesions, we decided to focus on miR-140 exosomal secretion (6). We constructed an artificial system to study miR-140 secretion and internalization. We overexpressed GFP-tagged CD9, a protein marker of exosomes (21), along with miR-140 expression vector in HEK293T cells cultured in exosome free media (obtained via ultracentrifugation). We isolated exosomes containing miR-140. We verified successful isolation of exosomes from cell culture medium using Western blot for CD9 (data not shown). We treated MDA-MB-231 (invasive basal-like breast cancer cells) and MCF10DCIS cells with purified exosomes and examined uptake of exosomal miR-140 using qRT-PCR. We found that exosomal transfer of miR-140 into breast cancer cells resulted in a small but detectable increase in cellular miR-140 levels in both cell lines (Fig. 8). This suggests that exosomal secretion of miR-140 has the potential to impact miR-140 signaling in nearby tumor and stromal cells. Furthermore, this suggests that in addition to directly targeting DCIS stem cells, SFN treatment may be able to also block stem cell signaling in nearby cells by increasing exosomal miR-140 secretion in the tumor microenvironment.
FIGURE 8.
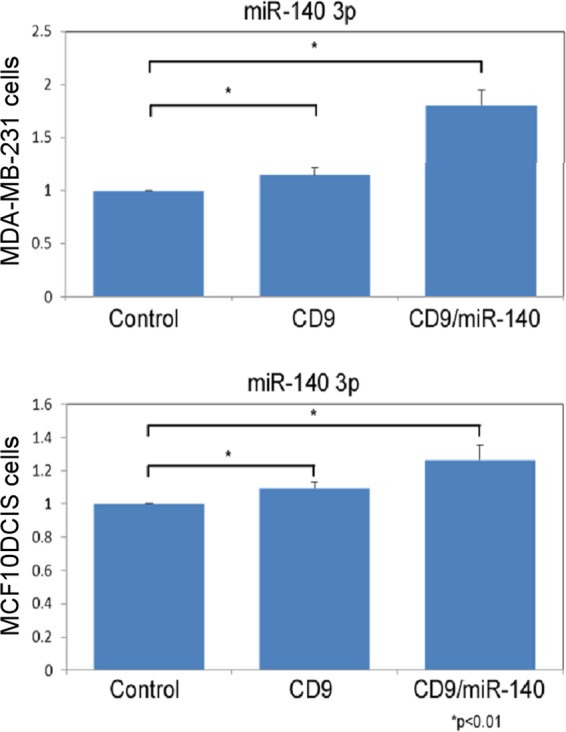
Artificial model of exosomal miR-140 secretion and internalization. HEK-293T cells were transfected with miR-140 and the exosomal marker CD9-GFP. Exosomes were harvested from the media following 3 days. MDA-MB-231 and MCF10DCIS cells were treated with purified exosomes, and after 24 h, cells were harvested for RNA. miR-140 levels were examined by qRT-PCR, normalizing to U6 snRNA.
DISCUSSION
Here, we have shown that basal-like DCIS contain subpopulations of cells with stem-like markers CD49f+/CD44+/CD24−. Furthermore, we found that these cells are enriched in ALDH1+ activity. These DCIS stem-like cells possess enhanced mobility relative to non-stem like DCIS cells. This enhanced migratory phenotype further supports that stem-like cells are the malignant precursors in DCIS lesions responsible for disease recurrence and disease progression.
Next, we also demonstrated that the dietary compound SFN can target these DCIS stem like cells. We show that SFN is able to decrease ALDH1 expression in DCIS stem-like cells and that this corresponds with decreased mammosphere and colony formation capacity of DCIS stem-like cells. This provides further support for the chemopreventive potential for the dietary compound SFN.
Finally, we identified unique exosomal secretion of miRs by DCIS stem-like cells. We found that SFN was able to dramatically alter exosomal levels of miR-21, miR-140, and miR-29a in culture of DCIS stem-like cells. Previously, we have shown that miR-140 plays an important role in regulating stemness in DCIS stem-like cells by targeting SOX9 and ALDH1 (6). It is possible that exosomal miR-140 may then regulate stem cell signaling in recipient cells. A recent study has shown that in lung cancer, miR-21 and miR-29a are frequently up-regulated in tumor-derived exosomes (22). This study found that these miRs actually signaled through nearby immune cells via interaction with toll-like receptors to up-regulate secretion of TNFα and IL-6 secretion, which promotes tumor invasion and metastases. IL-6 is known to promote invasion and metastasis in breast cancer cells (17), and it is possible that these exosomal miRs may also promote DCIS progression by targeting nearby immune cells in breast cancer.
This work was supported, in whole or in part, by grants from American Cancer Society and NCI, National Institutes of Health R01 CA163820 and R01 CA157779 (to Q. Z.).
- DCIS
- ductal carcinoma in situ
- qRT-PCR
- quantitative RT-PCR
- miR
- microRNA
- CSC
- cancer stem cell.
REFERENCES
- 1. Leonard G. D., Swain S. M. (2004) Ductal Carcinoma in situ, Complexities and Challenges. J. Natl. Cancer Inst. 96, 906–920 [DOI] [PubMed] [Google Scholar]
- 2. Fowble B., Hanlon A. L., Fein D. A., Hoffman J. P., Sigurdson E. R., Patchefsky A., Kessler H. (1997) Results of conservative surgery and radiation for mammographically detected ductal carcinoma in situ (DCIS). Int. J. Radiat. Oncol. Biol. Phys. 38, 949–957 [DOI] [PubMed] [Google Scholar]
- 3. Fisher B., Land S., Mamounas E., Dignam J., Fisher E. R., Wolmark N. (2001) Prevention of invasive breast cancer in women with ductal carcinoma in situ: An update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin. Oncol. 28, 400–418 [DOI] [PubMed] [Google Scholar]
- 4. Houghton J. (2003) Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomized controlled trial. Lancet. 362, 95–102 [DOI] [PubMed] [Google Scholar]
- 5. Espina V., Mariani B. D., Gallagher R. I., Tran K., Banks S., Wiedemann J., Huryk H., Mueller C., Adamo L., Deng J., Petricoin E. F., Pastore L., Zaman S., Menezes G., Mize J., Johal J., Edmiston K., Liotta L. A. (2010) Malignant Precursor Cells Pre-Exist in Human Breast DCIS and Require Autophagy for Survival. PLoS One 5, e10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Q., Yao Y., Eades G., Liu Z., Zhang Y., Zhou Q. (2013) Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene, 10.1038/onc.2013.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Hajj M., Wicha M. S., Benito-Hernandez A., Morrison S. J., Clarke M. F. (2003) Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 100, 3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu S., Clouthier S. G., Wicha M. S. (2012) Role of microRNAs in the Regulation of Breast Cancer Stem Cells. J. Mammary Gland Biol. Neoplasia 17, 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fillmore C. M., Kuperwasser C. (2008) Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Honeth G., Bendahl P. O., Ringnér M., Saal L. H., Gruvberger-Saal S. K., Lövgren K., Grabau D., Fernö M., Borg A., Hegardt C. (2008) The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 10, R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ricardo S., Vieira A. F., Gerhard R., Leitão D., Pinto R., Cameselle-Teijeiro J. F., Milanezi F., Schmitt F., Paredes J. (2011) Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 64, 937–946 [DOI] [PubMed] [Google Scholar]
- 12. Charafe-Jauffret E., Ginestier C., Iovino F., Wicinski J., Cervera N., Finetti P., Hur M. H., Diebel M. E., Monville F., Dutcher J., Brown M., Viens P., Xerri L., Bertucci F., Stassi G., Dontu G., Birnbaum D., Wicha M. S. (2009) Breast Cancer Cell Lines Contain Functional Cancer Stem Cells with Metastatic Capacity and a Distinct Molecular Signature. Cancer Res. 69, 1302–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakanishi T., Chumsri S., Khakpour N., Brodie A. H., Leyland-Jones B., Hamburger A. W., Ross D. D., Burger A. M. (2010) Side-population cells in luminal-type breast cancer have tumour-initiating cell properties, and are regulated by HER2 expression and signaling. Br. J. Cancer 102, 815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schnitt S. (2009) The transition from ductal carcinoma in situ to invasive breast cancer: the other side of the coin. Breast Cancer Res. 11, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kahlert C., Kalluri R. (2013) Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 91, 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eades G., Yao Y., Yang M., Zhang Y., Chumsri S., Zhou Q. (2011) miR-200a Regulates SIRT1 Expression and Epithelial to Mesenchymal Transition (EMT)-like Transformation in Mammary Epithelial Cells. J. Biol. Chem. 286, 25992–26002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walter M., Liang S., Ghosh S., Hornsby P. J., Li R. (2009) Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene 28, 2745–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller F. R., Santner S. J., Tait L., Dawson P. J. (2000) MCF10DCIS.com Xenograft Model of Human Comedo Ductal Carcinoma In Situ. J. Natl. Cancer Inst. 92, 1185a–1186a [DOI] [PubMed] [Google Scholar]
- 19. Stingl J., Eirew P., Ricketson I., Shackleton M., Vaillant F., Choi D., Li H. I., Eaves C. J. (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439, 993–997 [DOI] [PubMed] [Google Scholar]
- 20. Abd El-Rehim D. M., Pinder S. E., Paish C. E., Bell J., Blamey R. W., Robertson J. F., Nicholson R. I., Ellis I. O. (2004) Expression of luminal and basal cytokeratins in human breast carcinoma. J. Pathol. 203, 661–671 [DOI] [PubMed] [Google Scholar]
- 21. Bang C., Thum T. (2012) Exosomes: New players in cell-cell communication. Int. J. Biochem. Cell Biol. 44, 2060–2064 [DOI] [PubMed] [Google Scholar]
- 22. Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G. J., Zanesi N., Crawford M., Ozer G. H., Wernicke D., Alder H., Caligiuri M. A., Nana-Sinkam P., Perrotti D., Croce C. M. (2012) MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 109, E2110–E2116 [DOI] [PMC free article] [PubMed] [Google Scholar]