Background: The mechanisms involved in heme handling in trematodes are poorly understood.
Results: The biochemical and functional characteristics of a new family of small proteins (MF6p/FhHDM-1) secreted by Fasciola and other trematodes are reported.
Conclusion: The Fasciola MF6p/FhHDM-1 major antigen is a heme-binding protein.
Significance: Our results provide new insights into the biology of hematophagous trematodes.
Keywords: Heme, Iron, Parasite, Trafficking, Transporters
Abstract
Blood-feeding parasites have developed biochemical mechanisms to control heme intake and detoxification. Here we show that a major antigen secreted by Fasciola hepatica, previously reported as MF6p, of unknown function (gb|CCA61804.1), and as FhHDM-1, considered to be a helminth defense molecule belonging to the family of cathelicidin-like proteins (gb|ADZ24001.1), is in fact a heme-binding protein. The heme-binding nature of the MF6p/FhHDM-1 protein was revealed in two independent experiments: (i) immunopurification of the secreted protein·heme complexes with mAb MF6 and subsequent analysis by C8 reversed-phase HPLC and MS/MS spectrometry and (ii) analysis of the binding ability of the synthetic protein to hemin in vitro. By immunohistochemistry analysis, we have observed that MF6p/FhHDM-1 is produced by parenchymal cells and transported to other tissues (e.g. vitellaria and testis). Interestingly, MF6p/FhHDM-1 is absent both in the intestinal cells and in the lumen of cecum, but it can be released through the tegumental surface to the external medium, where it binds to free heme molecules regurgitated by the parasite after hemoglobin digestion. Proteins that are close analogs of the Fasciola MF6p/FhHDM-1 are present in other trematodes, including Clonorchis, Opistorchis, Paragonimus, Schistosoma, and Dicrocoelium. Using UV-visible spectroscopy and immunoprecipitation techniques, we observed that synthetic MF6p/FhHDM-1 binds to hemin with 1:1 stoichiometry and an apparent Kd of 1.14 × 10−6 m−1. We also demonstrated that formation of synthetic MF6p/FhHDM-1·hemin complexes inhibited hemin degradation by hydrogen peroxide and hemin peroxidase-like activity in vitro. Our results suggest that MF6p/FhHDM-1 may be involved in heme homeostasis in trematodes.
Introduction
Trematodes, also known as flukes, are flatworms belonging to the class Trematoda, which comprises the subclasses Aspidogastrea and Digenea. Whereas the former includes a relatively small number of species that are typically endoparasites of molluscs, fish, turtles, and decapod crustaceans (1), those of the subclass Digenea are highly abundant and are the causative agents of important human and animal diseases (2). Among these diseases, fasciolosis (also known as fascioliasis) is a food-borne trematodosis with a considerable impact on public health; it has been estimated that between 2.4 (3) and 17 million (4) people suffer from the infection in 55 countries and that 91 million others are at risk of infection (5). The disease also constitutes a serious veterinary problem because infection of livestock leads to loss of revenue through reduced meat and milk production (6, 7). Fasciolosis is caused by species of liver flukes of the genus Fasciola, F. hepatica, which is widely distributed in Europe, Africa, Asia, Oceania, and America, and F. gigantica, which is restricted to tropical areas of Southern Asia, Southeast Asia, and Africa (8).
After ingestion of trematode metacercariae, which are present on aquatic plants, by the definitive host (usually a herbivore), the parasites excyst and the newly excysted juveniles migrate first to the liver parenchyma and then to the bile ducts (9). During migration and development within the host, the diet of the juvenile flukes mainly comprises tissue cells, although some blood is also ingested; the diet of the adult fluke essentially comprises blood (10). Host erythrocytes are hemolyzed in the acidic gut lumen of flukes (11), and the released proteins are digested by proteolytic enzymes (e.g. l-cathepsins) to produce amino acids, which are used for parasite development (12). Hemoglobin constitutes 90% of the total protein in red blood cells, and its degradation also results in the release of large amounts of the iron-containing prosthetic group heme (13), which is very toxic when free in biological systems because it can enter membranes and damage cells (14). Indeed, heme strongly catalyzes the generation of reactive oxygen species, leading to the oxidation of lipids, proteins, and DNA (15, 16), and because of its lipophilic nature, it can also associate with phospholipid membranes, disrupting their physical integrity (17). To avoid such problems, blood-feeding parasites have developed diverse strategies for detoxifying excess heme derived from the catabolism of host red cells (18). One of the best known adaptations among blood feeders involves the crystallization of heme into hemozoin, an insoluble pigment that is not toxic to the parasites and can be released to the bloodstream. It has been estimated that up to 75% of heme released from hemoglobin by malaria parasites is transformed into hemozoin (13). Hemozoin formation was initially considered to be unique in malaria parasites, but it was also later described in the trematodes Schistosoma mansoni and Echinostoma trivolvis, the protist Hemoproteus columbae, and the triatomine Rhodnius prolixus (18). Other mechanisms that have been proposed involve heme compartmentalization, whereby heme is retained in the peritrophic matrix or via formation of non-crystalline aggregates that accumulate in a specialized organelle termed hemosome, as occurs in the hematophagous arthropods Aedes aegypti and Rhipicephalus (Boophilus) microplus, respectively (17–19).
On the other hand, heme is also a cofactor required by most living organisms to form heme proteins, which are involved in biochemical processes as diverse as cell respiration, oxygen binding and transport, and oxidative biotransformations (20). Most eukaryotic cells possess enzymes that enable complete de novo heme biosynthesis, but it is generally accepted that parasites that have evolved hematophagy and even free living nematodes, such as Caenorhabditis elegans, have lost this capacity (18, 21). Total or partial loss of de novo heme biosynthesis has been postulated for several species of parasites, including nematodes (e.g. Ancylostoma caninum, Hemonchus contortus, Ascaris suum, and Brugia malayi), the digenean S. mansoni, and trypanosomatids (e.g. Leishmania amazonensis, Leishmania infantum, and Trypanosoma cruzi) (18). These organisms have had to develop mechanisms for heme intake, transport, and storage while protecting their tissues from the putative toxic effects of such molecules (22). However, the molecular mechanisms involved in heme homeostasis in hematophagous parasites remain largely unknown, despite their medical importance and the fact that the molecules involved may be possible targets for developing new drugs and vaccines. Here, we demonstrate that a small major protein secreted by Fasciola hepatica and also present in other hematophagous trematodes is a member of a new family of HBPs.3 In this study, we refer to this protein as MF6p/FhHDM-1 because the same molecule has previously been annotated as MF6p, of unknown function (gb|CCA61804.1), and as FhHDM-1, a helminth defense molecule belonging to the family of cathelicidin-like proteins (gb|ADZ24001.1).
EXPERIMENTAL PROCEDURES
Ethics Statement
This study was carried out in strict accordance with the guidelines of the European Directive 2010/63/EU and the Spanish Law (RD 53/2013) on Care and Use of Laboratory Animals. The protocol was approved by the Ethics Committee of the Universidad de Santiago de Compostela and by the Xunta de Galicia (Code 15007AE/12/DIG ENF 06), Spain. The parasite samples used in this study were obtained from local abattoirs.
Parasites and Antigens
The Fasciola SAs were obtained as reported previously (23). Briefly, live adult flukes collected from bile ducts of naturally infected cows were washed, first in sterile saline solution containing antibiotics (penicillin/streptomycin) and glucose (2 g/liter) at 38 °C and then in RPMI 1640 cell culture medium supplemented with 20 mm HEPES, 0.3 g/liter l-glutamine, 2 g/liter sodium bicarbonate, and antibiotics at 38 °C under 5% CO2 in air. The flukes were then transferred to 75-cm2 tissue culture flasks and maintained in culture medium (3 ml/fluke) at 38 °C under 5% CO2 in air. After incubation for 24 h, the medium containing the SAs was removed and centrifuged at 10,000 × g for 20 min at 4 °C in the presence of protease inhibitors (SigmaFast Protease Inhibitor Tablets, Sigma-Aldrich). The supernatant was then passed through a 0.45-μm pore filter disk, concentrated in an Amicon 8050 ultrafiltration cell (Amicon, Inc., Beverly, MA) equipped with a YM10 membrane (10-kDa cut-off), dialyzed against PBS, sterilized by filtration, and stored at −80 °C until required. The protein concentration in the supernatant was determined using the Micro BCA Protein Assay Kit (Pierce).
Fresh Fasciola eggs obtained from the gall bladder of infected cattle were washed on a mesh (pore size 63 μm) with tap water. The eggs were then collected, allowed to settle, and washed four times with PBS. The egg sediment (volume 50 μl) was resuspended in 200 μl of the same buffer and sonicated for 3 min on ice with five cycles of 30-s pulses at 100 W (Branson Sonic Power Co., Danbury, CT). Finally, the supernatant containing the whole soluble egg extract was recovered by centrifugation at 13,000 × g for 15 min at 4 °C and stored at −80 °C until use. The protein concentration was measured as above. sMF6p/FhHDM-1 Fasciola protein, corresponding to the complete secreted protein (gb CCA61804.1), was obtained (≥95% pure) from GeneCust Europe (Dudelange, Luxembourg).
Production of MM3 and MF6 mAbs
Hybridoma cells secreting IgG1/κ mAbs reacting with cathepsins L1 and L2 from Fasciola (mAb MM3) or with MF6p/FhHDM-1 (mAb MF6) were obtained as described previously (24) by fusion of P3-X63-Ag8.653 myeloma cells with spleen cells from BALB/c mice hyperimmunized with O-deglycosylated (MM3) F. hepatica SAs contained in peak IV (23) or non-deglycosylated whole SAs (MF6). The secreting hybridoma cells were grown intraperitoneally in PristanTM-primed BALB/c mice, and the anti-F. hepatica IgG1/κ antibodies were purified from the ascitic fluid by affinity chromatography on a protein G column (HiTrap Protein G, GE Healthcare) according to the manufacturer's protocol.
Isolation of Fasciola SAs
Whole F. hepatica SAs (200 μl/run) were isolated by size exclusion chromatography on a Superdex 75 HR 10/30 column (Amersham Biosciences) connected to an LC system (ÄKTA Basic 10, Amersham Biosciences) with simultaneous monitoring at 280 and 402 nm. The column was calibrated with a mixture of proteins of known molecular weight (Gel Filtration LMW Calibration Kit, Amersham Biosciences). The protein concentration in each peak collected was determined as above.
Endotoxin Removal from SAs
To remove endotoxin, the F. hepatica SAs were passed several times through an Endotrap Red resin (Hyglos GmbH, Bernried, Germany), according to the manufacturer's instructions. The SAs were then isolated by size exclusion LC, as described above. Protein determinations were performed as indicated above, and the endotoxin content at each cleaning step was measured using the Pierce LAL Chromogenic Endotoxin Quantitation kit (Thermo Fisher Scientific).
Affinity Chromatography with mAb MF6
The MF6p/FhHDM-1 protein was purified from Fasciola SAs by affinity chromatography with the mAb MF6, which was conjugated on CNBr-activated Sepharose 4B (GE Healthcare) according to the manufacturer's instructions. After equilibrating the affinity column with PBS, F. hepatica SAs diluted in PBS were applied to the column, which was then washed with PBS to remove unbound proteins. Bound proteins were eluted with 0.1 m glycine-HCl, pH 2.5, and neutralized with 2 m Tris before being dialyzed against distilled water. Finally, the purified proteins were concentrated in a 3K Macrosep centrifugal concentrator (Pall Filtron Corp., Port Washington, NY) and stored in aliquots at −80 °C until use after determination of the protein content with the Micro BCA Protein Assay Kit.
A second CNBr-activated Sepharose 4B column containing immobilized mAb MF6 was used to investigate the presence of MF6p/FhHDM-1 in bile from infected cattle. Bile (300 ml), obtained in the slaughterhouse from a positive cow harboring 86 adult flukes, was filtered through a 30-μm stainless steel mesh and centrifuged at 10,000 × g for 30 min. The pellet was discarded, and the supernatant was diluted 1:1 with ethyl-acetate, vortexed vigorously, and centrifuged at 2,500 × g for 15 min. The aqueous phase was then collected, and the protease activity was inhibited with a mixture of protease inhibitors (Sigma-Aldrich), as described above. The resulting sample was diluted 1:10 with PBS, filtered through a sintered funnel (15–40 μm porosity), and passed through the affinity chromatography column at a flow rate of 5 ml/min. The column was then washed with 20 volumes of PBS, and the retained proteins were eluted with glycine-HCl, pH 2.5. The presence of proteins in the elution buffer was monitored continuously at 282 nm (0.02 absorbance units full scale).
SDS-PAGE and WB
All samples were separated in 10–20% linear gradient polyacrylamide gels, as described previously (25). The separated proteins were stained with Imperial Protein Stain (Thermo Fisher Scientific) or transferred to PVDF membranes (Immobilon-P, Millipore Ibérica SA, Madrid, Spain) in a Trans-Blot SD transfer cell (Bio-Rad) and blocked for 2 h at room temperature with PBS with 0.2% Tween 20 and 1% dry SM (PBS-T-SM). Membranes were then incubated with mAb MF6 diluted 1:10,000, or MM3 diluted 1:200 (as control), in PBS-T-SM for 1 h at room temperature on an orbital shaker. The membranes were washed three times for 5 min each with PBS-T and then incubated with HRP-conjugated rabbit anti-mouse Ig antibody (Dako Diagnósticos SA, Barcelona, Spain), diluted 1:800 in PBS-T-SM, for 1 h at room temperature. Finally, the membranes were washed again, and the bands were developed using 3,3′-diaminobenzidine (DAB) tetrahydrochloride tablets (Sigma-Aldrich), following the supplier's instructions.
HPLC and Mass Spectrometry
For protein sequence analysis, a sample of the immunopurified MF6p/FhHDM-1 (see above) was isolated by SDS-PAGE (on a 10–20% linear gradient); the band at 8 kDa was manually excised from the gel by using a Teflon punch connected to a micropipette tip. The sample was washed twice with water, shrunk for 15 min with 100% acetonitrile, and dried in a centrifugal evaporator (Savant AS160 SpeedVac, Thermo Fisher Scientific) for 30 min. The sample was then reduced with 10 mm DTT in 25 mm ammonium bicarbonate for 30 min at 56 °C and subsequently alkylated with 55 mm iodoacetamide in 25 mm ammonium bicarbonate for 15 min in the dark. Finally, samples were digested overnight with 12.5 ng/μl sequencing grade trypsin (Roche Applied Science) in 25 mm ammonium bicarbonate (pH 8.5) at 37 °C, and the resulting peptides were sequenced in a 4800 Proteomics Analyzer MALDI-TOF/TOF mass spectrometer (Applied Biosystems, Framingham, MA) under the same experimental conditions as described previously (26).
The m/z values of the undigested MF6p/FhHDM-1 immunopurified protein as well as that of the corresponding associated pigment were determined by MALDI-TOF (in the Proteomics Unit (RIAIDT) at the University of Santiago de Compostela) in an Autoflex apparatus (Bruker Daltonics, Bremen, Germany) equipped with a UV/N2 laser and an Ultraflex III TOF/TOF equipped with Smartbeam laser (Bruker Daltonics). In this case, a sample of the MF6p/FhHDM-1 immunopurified protein was dialyzed against distilled water, acidified with TFA (0.1% final concentration), and the components were isolated on a C8 RP column (5 cm × 2.1 mm; 3 μm; 300 Å; Discovery BIO Wide Pore; Sigma-Aldrich) coupled to a 600 S HPLC system equipped with a 996 diode array detector (Waters Corp., Milford, MA). Water and acetonitrile containing 0.1% TFA were used as mobile phases A and B. The chromatographic parameters were as follows: injection volume, 10 μl; mobile phase linear gradient, 10–60% B in 30 min; flow rate, 0.4 ml/min; diode array detector monitoring at λ = 210 and 402 nm. During the chromatographic process, the eluted peaks were manually collected in 0.5-ml Eppendorf tubes, dried in a Savant SpeedVac, and refrigerated until use. The target samples for MALDI-TOF MS analysis were prepared by dissolving the dried samples in distilled water containing 0.1% TFA and then diluting them 1:1 with the matrix (a saturated solution of α-cyano-4-hydroxycinnamic acid or sinapinic acid in 0.1% TFA, acetonitrile (2:1)). For analysis, 1 μl of sample/matrix solution mix was spotted on a MALDI target and left to dry at room temperature. Each mass spectrum was acquired in linear and reflectron modes, and at least 600 laser shots were accumulated. Protein Calibration Standard I (Bruker Daltonics) was used for external calibration. Acquired spectra were processed with FlexAnalysis (Bruker Daltonics) by using the Centroid algorithm from peak annotation.
To measure the exact mass of the type of pigment associated with the immunopurified MF6p/FhHDM-1 Fasciola protein, a fresh sample eluted from the immunoaffinity column in the elution buffer (glycine-HCl, pH 2.5) was mixed 1:1 with ethyl methyl ketone, vortexed, and centrifuged at 1,000 × g for 10 min (27). The reddish upper phase was then collected by aspiration, and an aliquot was used for analysis by LC-ESI-MS. The equipment consisted of a Supelco-Kromasil C8 column (5 μm, 100 Å, 250 × 3.2 mm) connected to an HPLC system (Agilent 1100) coupled to an ESI-TOF mass spectrometer (micrOTOF, Bruker Daltonics). Water and acetonitrile containing 0.1% acetic acid were used as mobile phases A and B. The mobile phase gradient was 40–100% B in 30 min, the flow rate 0.4 ml/min, the injection volume 20 μl, and the analytical column temperature was 30 °C. The ESI-TOF mass spectrometer was operated in positive mode, and the capillary voltage was −4,500 V. The scan mode was acquired between 250 and 2,000 m/z. Acquired analyses were processed with DataAnalysis (Bruker Daltonics).
The concentration of 56Fe in samples was measured in an ICP-MS (Varian 820-MS, Darmstadt, Germany), which was equipped with a Micromist glass low flow nebulizer, a Scott-type spray chamber cooled with a Peltier (3 °C) system, and a quartz torch. For measurement, a sample of 300 μl diluted to 2 ml with MilliQ water was placed directly in the instrument. External calibration standards for 56Fe, at concentrations between 0.1 and 100 μg/liter, were prepared in 1% HNO3, and 32Ge (diluted to 20 μg/liter) was used as an internal standard. The working conditions were as follows: plasma flow, 17 liters/min; auxiliary flow, 1.65 liters/min; sheath gas, 0.19 liter/min; nebulizer flow, 1.00 liter/min; torch alignment (sampling depth, 6 mm); RF power, 1.4 kW; pump rate, 5 rpm; stabilization delay, 25 s; skimmer gas source, H2; skimmer flow, 50 ml/min; scan mode sampling time, 140 s; and dwell time, 20,000 μs.
Cloning and Expression of the Gene Encoding the Recognized Protein MF6p/FhHDM-1
Primers 5-RACE (5′ ATGCGCTTCATTGTTCTTCTCTGTCTTGCTGTGGTCC 3′) and 3-RACE (5′ TTAATTTCCCGCGTATTTCTCCAAGCGATCGGTGA 3′) were used to clone the MF6p/FhHDM-1 gene coding for the recognized protein MF6p/FhHDM-1 by using information from the EST Hans30081e06.q1k, available at the Wellcome Trust Sanger Institute. The full gene was amplified using a collection of adult F. hepatica cDNA (26) as template, and the coding sequence was submitted to GenBankTM (CCA61804.1).
ELISA for LPS Binding
Polystyrene microtiter plates (Greiner Bio-One; Soria-Melguizo, Madrid, Spain) were coated with 100 μl of sMF6p/FhHDM-1 (1 μg/well in PBS) and incubated for 2 h at 37 °C. After washing, sMF6p/FhHDM-1 and control plates without sMF6p/FhHDM-1 were blocked with 1.5% casein hydrolysate in PBS, and 100 μl of FITC-labeled LPS from Escherichia coli serotype O111:B4 or O55:B5 (Sigma-Aldrich) were incubated at concentrations ranging from 3.125 to 400 ng/well in PBS or PBS containing 1% BSA for 1 h at 37 °C. The plates were then washed five times with PBS-T and incubated with HRP-conjugated rabbit anti-FITC (AbD Serotec, Oxford, UK) diluted 1:2,000 in PBS-T containing 1% of BSA. After a washing step, bound LPS was revealed by adding 100 μl/well substrate (SigmaFast o-phenylenediamine dihydrochloride, Sigma-Aldrich) and incubating for 20 min at room temperature. The reaction was then halted with 25 μl of 3 n H2SO4, and the OD was measured at 492 nm.
Immunohistochemistry
The adult F. hepatica flukes obtained in the slaughterhouse were fixed immediately in 10% buffered formalin for 24 h at room temperature and washed twice with PBS. The flukes were then dehydrated through an increasing ethanol gradient (1 h each), cleared with xylene (20 min), embedded in Histosec (Merck), cut into 5-μm-thick sections (on a Jung Supercut 2065 microtome, Leica, Nussloch, Germany), and mounted on slides. The sections were dewaxed and rehydrated through xylene and a decreasing ethanol gradient (5 min each) and finally rinsed with distilled water. The sections were then blocked for 2 h in TBS (50 mm Tris, 150 mm NaCl, pH 7.3) containing 0.2% Tween 20 and 1% dry SM (TBS-T1-SM) at room temperature, incubated with mAb MF6 (1:10,000) or MM3 (1:200) (for comparison), and washed three times with TBS-T1 for 5 min each. Thereafter, the endogenous peroxidase in the tissues was quenched by treatment with 3% hydrogen peroxide (H2O2) in TBS for 30 min at room temperature, and the sections were then washed with TBS-T1 and incubated with HRP-labeled rabbit anti-mouse Ig antibody (1:800). All antibodies were diluted in TBS-T1-SM and were incubated for 1 h at room temperature. The sections were then washed again with TBS-T1 and incubated with 0.5 mg/ml 4CN (Sigma-Aldrich) or with DAB in TBS containing 0.005% H2O2. The slides were finally washed with TBS and mounted with glass coverslips in PBS-glycerol (1:1). The slides were examined, photographed, and then immersed in 70% ethanol, to remove the coverslips, and stained with Wheatley's trichrome, as described previously (26, 28). Briefly, the slides were placed in trichrome stain for 10 min and then dipped twice in 90% ethanol containing 0.5% acetic acid, dipped twice in 90% ethanol, dehydrated through an increasing ethanol gradient and xylene (5 min each), and mounted in Entellan (Electron Microscopy Sciences, Hatfield, PA). Some sections revealed with DAB were also counterstained with Mayer's hematoxylin followed by trichrome stain. The sections were examined and photographed using an Olympus Provis AX-70 microscope equipped with a DP 70 digital camera system.
Immunoblot of Fasciola Surface Proteins
Live adult flukes were killed by direct immersion in 70% (v/v) ethanol for 5 min, washed with PBS, and placed (ventral surface downward) on a nitrocellulose membrane (Trans-Blot Transfer Medium, Bio-Rad), previously prewetted with distilled water, and deposited on a microscope slide. Another nitrocellulose membrane was placed on the dorsal surface of the parasite, and gentle pressure was exerted with a finger (protected with a glove). The membranes were washed with distilled water and blocked with PBS-T-SM for 2 h at room temperature and then incubated with mAb MF6 diluted 1:10,000 and subsequently with HRP-conjugated rabbit anti-mouse Ig antibody diluted 1:800 in PBS-T-SM for 1 h at room temperature on an orbital shaker after washing three times with PBS-T for 5 min each. The membranes were finally washed once more, and MF6p/FhHDM-1 was detected with DAB (Sigma-Aldrich).
Immunoprecipitation with mAb MF6 to Determine the sMF6p/FhHDM-1·Hemin Complex Stoichiometry
Taking into account the difficulty in removing excess heme by dialysis (27), the sMF6p/FhHDM-1·hemin complex stoichiometry was determined by immunoprecipitation with mAb MF6. Aliquots of hemin (Sigma-Aldrich), previously solubilized in DMSO at 5 mg/ml, were placed in several tubes, at a concentration of 15 μm, and incubated with increasing amounts of sMF6p/FhHDM-1 (1–30 μm) or alone (control) in TBS, pH 7.3, with 0.1% Tween 20 (TBS-T2) for 30 min at room temperature. Excess mAb MF6 immobilized onto CNBr-activated Sepharose 4B was then added to immunoprecipitate sMF6p/FhHDM-1 with bound hemin. The percentage of bound hemin was determined indirectly by measuring the absorption at 395 nm of free hemin remaining in the supernatant of the samples with sMF6p/FhHDM-1 relative to the control containing hemin alone.
Determination of sMF6p/FhHDM-1 Affinity for Hemin
The hemin binding affinity of sMF6p/FhHDM-1 was determined by measuring the difference UV-visible spectra for sample cuvettes containing 1 ml of a fixed concentration of hemin (3 μm) in TBS-T2, to which 2-fold dilutions of sMF6p/FhHDM-1 (starting at 12 μm) were added, relative to a reference cuvette containing 3 μm hemin alone. After incubation for 30 min to ensure complex formation, spectra were measured (300–450 nm) in a Multiskan GO UV-visible microplate spectrophotometer (Thermo Fisher Scientific) at 22 °C. Values at the wavelength of maximal difference absorbance were fitted to a one-site binding model by using Equation 1 (29, 30),
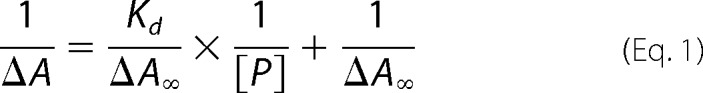 |
where ΔA and ΔA∞ are the variations in absorbance caused by the addition of the protein at a given concentration or at saturation concentration, respectively; Kd is the dissociation constant; and [P] is concentration of protein added. The Kd was obtained by plotting 1/ΔA at 398 nm against 1/[P] and calculating the slope (Kd/ΔA∞) using the linear regression function of OriginPro 8 software.
sMF6p/FhHDM-1 Binding to Other Porphyrins
CNBr-activated Sepharose 4B containing immobilized sMF6p/FhHDM-1 was used to determine the interaction between sMF6p/FhHDM-1 and PPIX, CuPPIX, CoPPIX, ZnPPIX (Cymit Quimica, Barcelona, Spain), and hemin (for reference purposes). The PPIX derivatives were dissolved in DMSO at a concentration of 5 mg/ml and diluted to a final concentration of 46 μm in TBS, pH 9, containing 0.1% Tween 20 and 10% DMSO. Each porphyrin solution (200 μl) was placed in an Eppendorf tube and incubated with a (previously determined) fixed amount of sMF6p/FhHDM-1-Sepharose beads, capable of binding ≥95% of the hemin solution. In parallel, porphyrin solutions were also incubated in a second tube with the same amount of Sepharose CL-4B beads as a control. The solutions were incubated for 30 min at room temperature under gentle stirring, and the percentage of bound porphyrin was determined by measuring the absorbance at the Soret peak of unbound porphyrin remaining in the supernatant of samples with sMF6p/FhHDM-1-Sepharose relative to the controls with non-conjugated Sepharose beads.
Hemin Degradation Mediated by H2O2
The H2O2-induced oxidation of hemin was evaluated, as described previously (31), in samples of 0.5 ml containing 23 μm hemin alone (control) or complexed with sMF6p/FhHDM-1 at protein/hemin ratios of 1:1, 2:1, and 5:1, or BSA at a ratio of 5:1, in TBS-T2. After incubation for 30 min, the reaction was initiated by the addition of excess H2O2 (890 μm) and monitored by measuring the decrease in the absorption at the Soret band at various times within 30 min, at 22 °C, in a Multiskan GO UV-visible microplate spectrophotometer (Thermo Fisher Scientific). Results were expressed as percentages of the initial absorbance, at the Soret peak, remaining in each sample measured at various times after the addition of H2O2.
Inhibition of Peroxidase-like Hemin Activity
The peroxidase-like activity of hemin alone or hemin complexed with sMF6p/FhHDM-1 was measured by monitoring the oxidation of TMB by H2O2 (32, 33). The pH of maximum activity of hemin alone with the TMB/H2O2 solution was first determined in the pH range 4.5–7.5, and it was found to occur at pH 6 (34). To obtain a final pH of 6, the peroxidase substrate solution B of a two-component system of TMB (KPL Inc., Gaithersburg, MD) was diluted 2-fold in TBS-T1 with 0.02% H2O2 and subsequently mixed with an equal volume of the TMB peroxidase substrate. Hemin (23 μm) was incubated with sMF6p/FhHDM-1 at protein/hemin molar ratios ranging from 0.5:1 to 5:1 or alone (control) in TBS-T2 for 30 min at room temperature prior to the addition of the substrate. An aliquot (20 μl) of each sample was added to the wells of a 96-well microtiter plate and mixed with 100 μl of the TMB solution. The reaction was measured by monitoring the increase in the absorbance at 650 nm in a Multiskan GO UV-visible microplate spectrophotometer (Thermo Fisher Scientific) for 30 min at 22 °C. Results were expressed as absorbance values against time or as the percentage inhibition of the peroxidase-like activity of hemin after 30 min, as a function of the protein/hemin molar ratio. The percentage inhibition was calculated using the formula, (OD2 − OD1)/OD2 × 100, where OD1 is the absorbance of the samples of hemin mixed with the protein, and OD2 is the absorbance of hemin alone.
RESULTS
Fasciola Secretes a Major HBP Recognized by mAb MF6
Fractionation of F. hepatica SAs by size exclusion chromatography on a Superdex 75 HR 10/30 column revealed four typical peaks (Fig. 1A) that could be monitored either at 280 nm (typical of proteins; blue spectrum) or at 402 nm (pigment; red spectrum). However, comparison of the blue and red spectra revealed that the pigment was mainly present in peak II (retention time 8.96 min and an apparent molecular mass ≥150 kDa), and absorption at 402 nm was 2.4 times that observed at 280 nm. In parallel with these experiments, we have also observed the following: (i) that more than 75% (as determined by maximal absorption at 402 nm) of the brownish product present in whole F. hepatica SAs was retained by mAb MF6 immobilized on a Sepharose 4B affinity chromatography column (see Fig. 1B and inset of Fig. 1A), and (ii) the absorption spectrum corresponding to the fraction not retained by mAb MF6 showed a shift from 402 to 409 nm with respect to whole SAs (see black chromatogram in the inset of Fig. 1A), suggesting that Fasciola secretes different pigment-containing proteins. Following the corresponding analysis of the compounds present in peak II by SDS-PAGE, we have observed that this fraction contains a major protein component, with a molecular mass of about 8 kDa, as well as several minor proteins that were revealed as faint bands in the 20–70-kDa range (Fig. 1C, lane 1). The molecular mass of the major protein component coincided with that obtained by WB analysis of the protein retained by mAb MF6 and subsequently eluted with glycine-HCl buffer (Fig. 1C, lane 2). Quantification of the amount of protein retained by mAb MF6 (MF6p/FhHDM-1) revealed that this is a major component among the proteins secreted because it represents 29% of the overall proteins secreted in vitro by the parasite.
FIGURE 1.
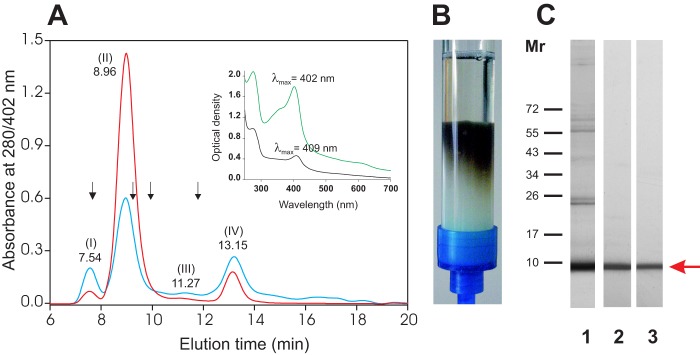
Purification of native MF6p/FhHDM-1. A, elution profiles of SAs from adult F. hepatica obtained by size exclusion chromatography (Superdex 75 HR 10/30, ÄKTA LC) and monitoring the absorbance at 280 nm (blue line) and 402 nm (red line). The elution time of the major peaks is indicated above each peak. Of the four peaks, peak II (retention time 8.96 min, molecular mass of 150 kDa) exhibited the highest absorbance at 402 nm, which is consistent with the UV-visible absorption spectra obtained before (visible maximal absorbance at 402 nm; green) and after (visible maximal absorbance at 409 nm; black) removal of MF6p/FhHDM-1 from F. hepatica SAs by affinity chromatography with mAb MF6 (inset). The column was calibrated with the following internal standards: dextran blue (void volume; Mr 2,000,000), albumin (Mr 67,000), ovalbumin (Mr 43,000), chymotrysin (Mr 25,000), and ribonuclease (Mr 13,700) (arrows). B, photograph showing the brownish product retained by mAb MF6 during purification of MF6p/FhHDM-1 from SAs by affinity chromatography. C, MF6p/FhHDM-1 was detected in an 8 kDa band (arrow) by SDS-PAGE analysis of peak II (lane 1) and WB of the eluted fraction obtained directly from the affinity chromatography (lane 2) or after ethyl methyl ketone extraction and analysis of the aqueous fraction (lane 3) with mAb MF6.
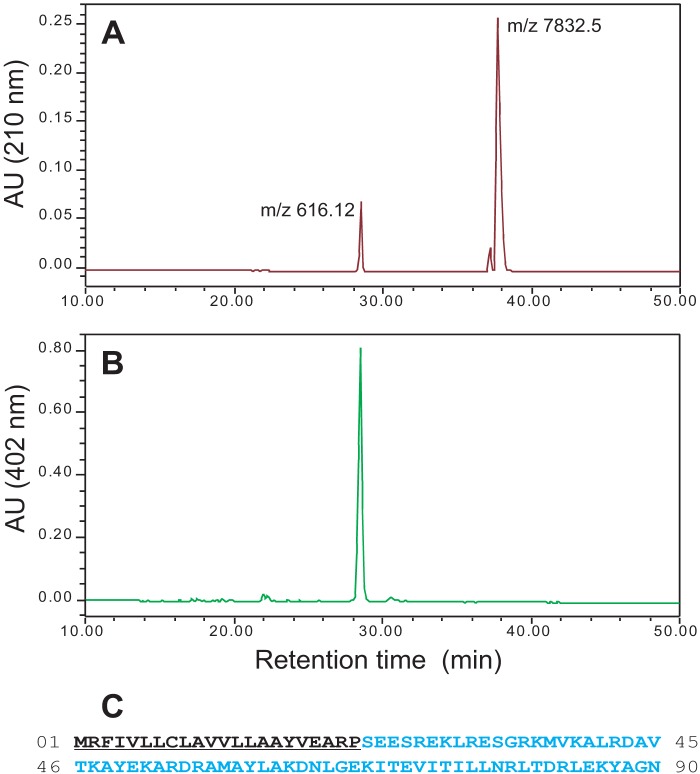
To investigate whether the colored nature of MF6p/FhHDM-1 was due to an exogenous molecule attached to the protein (e.g. a prosthetic group such as heme, as suggested by the absorption spectra shown in Fig. 1A, inset), a sample of immunoaffinity-purified MF6p/FhHDM-1 in glycine-HCl (pH 2.5) was mixed with ethyl methyl ketone (50% v/v), vortexed, and centrifuged to accelerate formation of the phases. The colored component was readily extracted by the organic solvent (λmax = 385 nm), whereas the MF6p/FhHDM-1 remained unaltered in the aqueous fraction (Fig. 1C, lane 3). In the next step, the presence of 56Fe in the extracted solution was confirmed by ICP-MS analysis, strongly suggesting that the colored component is a heme structure. To confirm this and to reveal the sequence of MF6p/FhHDM-1, an aliquot of the immunopurified MF6p/FhHDM-1 was dialyzed against distilled water and then acidified with 0.1% TFA; the components were then isolated by RP-HPLC chromatography, using a C8 column, while the absorbance was monitored simultaneously at 210 and 402 nm. The peaks were then collected, and the m/z values for each were obtained by MALDI-TOF spectrometry. RP-HPLC analysis of intact MF6p/FhHDM-1 revealed the presence of two compounds in the sample with retention times of 28.5 min (pigment absorbing at 210 and 402 nm; Fig. 2, A and B) and 38 min (absorbing at 210 but not at 402 nm, corresponding to the protein; Fig. 2A). The subsequent MALDI-TOF analysis revealed that the m/z values of the compounds were 616.12 (measured exact m/z = 616.1790, determined by ESI-TOF MS; monoisotopic mass = 616.1768 Da), which is typical of the heme B complex, and m/z 7,832.5, corresponding to the MF6p/FhHDM-1 protein.
FIGURE 2.
Identification and characterization of native MF6p/FhHDM-1. A and B, the immunopurified MF6p/FhHDM-1 complexes were separated by RP-HPLC while monitoring the absorbance at 210 and 402 nm, and the m/z values of the peaks were determined by MALDI-TOF MS to be 616.12, suggestive of a heme B group, and 7,832.5, corresponding to MF6p/FhHDM-1. AU, absorbance units. C, the MS/MS sequencing of the tryptic peptide encoding the amino acid residues 63–86 enabled identification of two ESTs, one of which was used to clone the entire cDNA sequence coding for MF6p/FhHDM-1. The full-length amino acid sequence of MF6p/FhHDM-1 deduced from the cloned cDNA sequence (CCA61804.1) is shown. The predicted N-terminal signal peptide is underlined.
Cloning and Sequencing of MF6p/FhHDM-1
In a subsequent experiment to determine the nature of the MF6p/FhHDM-1 protein, an aliquot of MF6p/FhHDM-1 was purified by immunoaffinity, isolated by SDS-PAGE, and stained with Coomassie Blue. The corresponding band was isolated from the gel, sliced, and submitted to in-gel digestion with trypsin, and the peptides thus obtained were used for MS/MS sequencing. This procedure allowed us to identify two peptides with m/z = 1,941.09, compatible with the sequence DN(I/L)GE(K/Q)(I/L)TEV(I/L)T(I/L)(I/L)(I/L)NR, and a longer peptide with m/z = 2,796.6, compatible with the sequence DN(I/L)GE(K/Q)(I/L)TEV(I/L)T(I/L)(I/L)(I/L)NR(I/L)TDR(I/L)E(K/Q). After a detailed search of several combinations of the latter sequence, using BLAST tools, we were unable to identify any known Fasciola protein. However, when we used these sequences to carry out a search using the EST database collection of pathogens at the Wellcome Trust Sanger Institute as a target, we identified two ESTs containing DNA readings coding for the above sequences (HAN3008-1e06.p1k and HAN3008-1e06.q1k). We then used the sequence of HAN3008-1e06.p1k to design 5′ and 3′ primers to clone the MF6p/FhHDM-1 protein by RT-PCR, using Fasciola cDNA as a template. The 90-amino acid full-length sequence of the cloned protein (Fig. 2C) was recorded at GenBankTM under the name MF6p and accession number CCA61804.1. Using available Expasy proteomic bioinformatics tools, we obtained a predicted molecular mass = 10,271.10 Da and a theoretical pI = 9.56 for this protein. This predicted molecular mass was higher than the molecular mass = 7,832.5 Da value obtained by MS for the mature protein, which revealed the existence of a signal peptide of 22 residues (Fig. 2C, underlined) and a secreted mature protein containing 68 residues (theoretical molecular mass = 7,828.0 Da), not containing Cys, Gln, His, Phe, Pro, or Trp residues (Fig. 2C).
Homologous Sequences of MF6p/FhHDM-1 Are Present in Other Trematodes
After a more recent search for protein homologs of our annotated MF6p (gb CCA61804.1), using the BLASTp bioinformatics tool at NCBI, we observed that the MF6p sequence was identical to the Fasciola protein FhHDM-1 (gb|ADZ24001.1), reported in parallel by Robinson et al. (35) as belonging to the family of Host Defense Molecules (HDM) related to cathelicidins (36). Consequently, we decided to combine both names and to refer to the protein as MF6p/FhHDM-1. In addition, the BLASTp tool also revealed the existence of other homologous sequences in trematodes with identity higher than 45% to the MF6p/FhHDM-1 protein and the same 90-aa full length (68-aa protein length for the mature protein; Fig. 3A). These included the proteins gb|AMM55183.1 from Clonorchis sinensis (57% identity), gb|ES416124 from Opistorchis viverrini (47% identity), gb|AT007125 from Paragonimus westermani (53% identity), and gb|JZ330559 from Dicrocoelium dendriticum (46% identity) (37). Members of the genus Schistosoma express a 91-aa length MF6p/FhHDM-1 protein homolog (Fig. 3A), although in this case, the sequence identity with Fasciola decreased to 37%, and the maximal homology was concentrated in the C-terminal region of the molecule. Apart from the MF6p/FhHDM-1 homologs, some of these trematodes also express other proteins with fragment sequences of outstanding homology with MF6p/FhHDM-1 but of very different length (Fig. 3B).
FIGURE 3.

MF6p/FhHDM-1 homologs from other trematodes. Amino acid sequences of MF6p/FhHDM-1 homologs were identified in the NCBI database by using the BLASTp tool. A, aligned sequences of the same or similar length and high homology. B, aligned sequences with proteins of different length and high homology, mainly in the C-terminal region of MF6p/FhHDM-1. Amino acid residues in common with MF6p/FhHDM-1 are shaded in green, and residues shared among trematodes other than F. hepatica are shaded in gray or blue. Fh, F. hepatica; Cs, C. sinensis; Ov, O. viverrini; Dd, D. dendriticum; Pw, P. westermani; Sj, S. japonicum; Sm, S. mansoni.
The MF6p/FhHDM-1 Protein Is Produced and Distributed by Parenchymal Cells and Can Be Released to the External Medium through the Tegumental Surface
Fasciola species are hermaphrodite leaf-shaped organisms with an oral sucker around the mouth and a ventral sucker that is used for locomotion and attachment to the host. These organisms also have a blind intestinal system (two extensively branched ceca), a reproductive system (comprising ovary, uterus, testis and other related organs), a primitive excretory system (comprising flame cells and collecting tubules draining to a longitudinal excretory canal or bladder, which opens in an excretory pore situated at the hind end), and a central and peripheral nervous system (38). Like other trematodes, Fasciola species lack a specialized circulatory system, and the space between different organs is occupied by parenchymal cells. The surface of the parasite (tegument) consists of an anucleate syncytium covered with glycocalix and connected by cytoplasmic extensions with cell bodies that are located beneath the basal lamina and muscle cells (39).
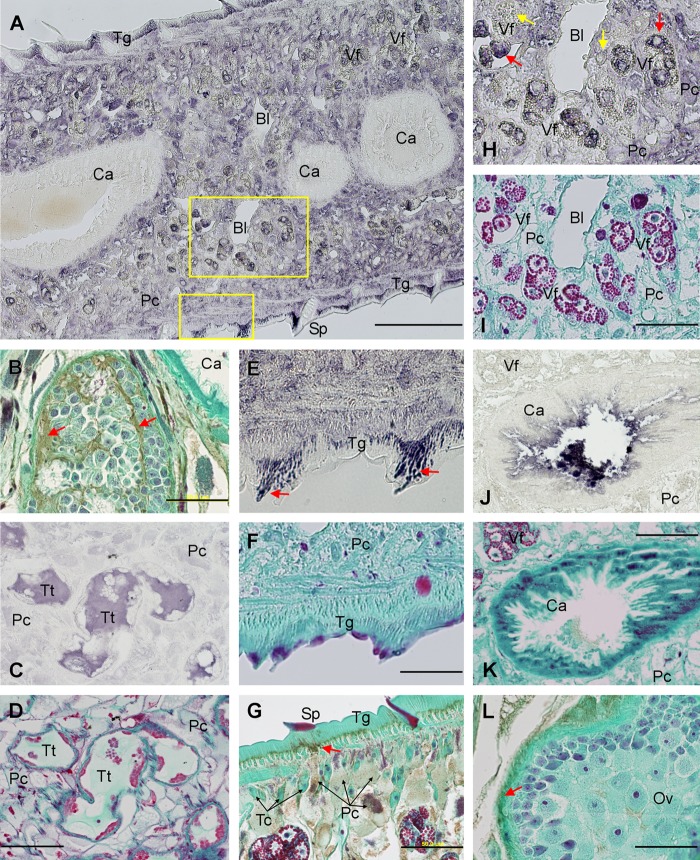
To obtain information about the physiological function of MF6p/FhHDM-1, we carried out an immunohistochemical study, using mAb MF6 to recognize the protein in Fasciola tissues. For better identification of the Fasciola structures, we also stained all slides with Wheatley's trichrome stain (28). For comparative purposes, we stained sections with mAb MM3, which stains l-cathepsins from the L1 and L2 clades (26). We show some representative images obtained after staining sagittal sections, cross-sections, and frontal sections of the parasite in Fig. 4. Overall, we observed that MF6p/FhHDM-1 is present at three main locations (see also Table 1): (i) the cytoplasm of parenchymal cells (apparently Pc1 and Pc2 cells, according to the classification of Pankao et al. (40)) from any location, although the cells lining the cecum and below the tegument were the most intensely stained (see Fig. 4A); (ii) the sustentacular tissue and the extracellular matrix material present in the testis (Fig. 4B) as well as the fluid present in testis tubules (Fig. 4, C and D); and (iii) the tegument, where strongly stained and orientated granules accumulated to form a dense layer along the tegument, which is located below the external membrane and above the basal membrane although tending to fuse and concentrate around the spines prior to secretion (Fig. 4, E and F). The presence of MF6p/FhHDM-1 in the cytoplasm of parenchymal cells and secretion of this protein toward the tegument can be observed in more detail in Fig. 4G (see arrow). In addition to the above structures, variable staining (weak to strong) was also observed in the cytoplasm of maturing vitelline cells (Fig. 4, H and I), in the uterine fluid content, and, sporadically, in some developing eggs. However, and in contrast to l-cathepsins recognized by mAb MM3 (Fig. 4, J and K), MF6p/FhHDM-1 was not present in either the intestinal cells or the cecum content (Fig. 4, A and B). The ovary also tested negative (Fig. 4L).
FIGURE 4.
Immunolocalization of MF6p/FhHDM-1 in histological sections of adult F. hepatica by mAb MF6. A, overview of a cross-section showing positive staining in parenchymal cells (Pc), vitelline follicles (Vf), and tegument (Tg), mainly around the spines (Sp). The cecum (Ca) and bladder (Bl) were not stained. B, sagittal section showing positive staining of the sustentacular tissue and extracellular matrix material present in testis (greenish brown, red arrows). C, frontal section indicating the presence of MF6p/FhHDM-1 in the content of testis tubules (Tt). E, high magnification of the boxed area in A showing accumulation of strong stained granules in tegument (Tg) mainly around spines (arrows). G, sagittal section showing positive staining in the cytoplasm of parenchymal cells (Pc), with emphasis on those present among the tegumental cell bodies (Tc) and beneath the tegument toward where the protein is secreted (greenish brown, red arrow). H, high magnification of the boxed area in A detailing strong positive staining of parenchymal cells (Pc) and maturing (red arrows) but not immature (yellow arrows) cells in the vitelline follicles (Vf). J, cross-section immunostained with mAb MM3 for comparison, showing the presence of cathepsin L1 proteinase only in cecum (Ca). L, sagittal section showing the absence of staining inside the ovary but some staining in the outer wall of the ovary (red arrow). Positive reaction with mAbs MF6 or MM3 in sections A, C, E, H, and J was revealed with 4CN. Sections D, F, I, and K were stained with Wheatley's trichrome after decoloring the 4CN stain of the corresponding sections. Positive reaction with mAb MF6 in sections B, G, and L was revealed with DAB followed by Mayer's hematoxylin and Wheatley's trichrome. Scale bars, 500 μm (A), 100 μm (C–F and H–K), and 50 μm (B, G, and L).
TABLE 1.
Summarized results of the immunohistochemical study performed in F. hepatica adults to investigate the location of the protein MF6p/FhHDM-1 recognized by mAb MF6
| Structures | Staininga | Structures | Staininga |
|---|---|---|---|
| Cecum | − | Parenchyma | +++ |
| Cecum lumen | − | Tegument | +++ |
| Eggsb | − | Tegumental cell bodies | + (speckled/ peripheral) |
| Excretory channel | − | Testis | ++ |
| Glycocalix | − | Testis tubules content | ++ |
| Mehlis' gland | ± | Uterus content | + |
| Musculature | − | Vitelline follicles | Variable (± to +++) |
| Ovary | − | Yolk reservoir | Variable (− to ±) |
a −, no stain; ±, faint stain; +, ++, and +++, increasing intensities of positive stain.
b The content of a small proportion of eggs may test positive.
Because the epitope recognized by mAb MF6 on MF6p/FhHDM-1 might be masked or altered due to the action the tissue fixative, we carried out experiments to confirm that MF6p/FhHDM-1 is present in the tegumental surface but absent from the digestive tract. We blotted the antigens present in the dorsal and ventral surface of the parasite on a nitrocellulose membrane and subsequently detected MF6p/FhHDM-1 with mAb MF6. The blots show that MF6p/FhHDM-1 was present along the entire dorsal and ventral surface of the parasite, but no stain was observed at the positions of the oral sucker or the genital pore (Fig. 5, A–C). In addition, the presence of MF6p/FhHDM-1 in mature eggs was confirmed by WB analysis with mAb MF6 of a whole soluble extract obtained from washed mature eggs collected from the gall bladder of parasitized cows (see Fig. 5D).
FIGURE 5.
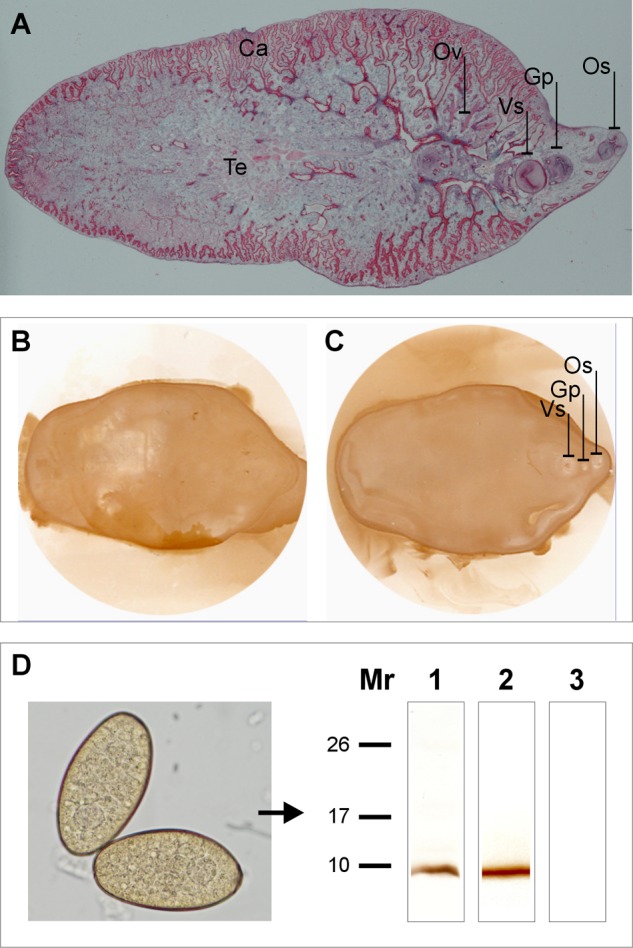
MF6p/FhHDM-1 is secreted through the tegument and is present in F. hepatica eggs. A, overall view of a frontal section of an F. hepatica adult stained with Wheatley's trichrome. Dorsal (B) and ventral (C) imprints of tegumental antigens from freshly killed adult flukes were revealed with mAb MF6. Strong staining of the whole tegument surface and no staining of the genital pore (Gp) or oral sucker (Os) was observed. Ca, cecum; Ov, ovary; Te, testis; Vs, ventral sucker. D, WB of soluble antigens from F. hepatica mature eggs (photograph) revealed with mAb MF6 (lane 1), showing the presence of MF6p/FhHDM-1. F. hepatica SAs were used as a positive control (lane 2). The negative control was revealed with mAb MM3 (lane 3).
The presence of large amounts of MF6p/FhHDM-1 in SAs from in vitro cultured parasites led us to investigate whether the Fasciola MF6p/FhHDM-1 is also released in vivo. To this end, we attempted to identify the protein in bile from a heavily infected cow (86 Fasciola adults in biliary ducts, confirmed in the slaughterhouse) by using an affinity chromatography column with immobilized mAb MF6, as described under “Experimental Procedures.” Despite the large amount of bile sampled (300 ml) and predilution of the sample to minimize interference from biliary salts, unexpectedly, we did not observe any protein peak after elution of the retained fraction.
The sMF6p/FhHDM-1 Protein Binds Hemin with 1:1 Stoichiometry
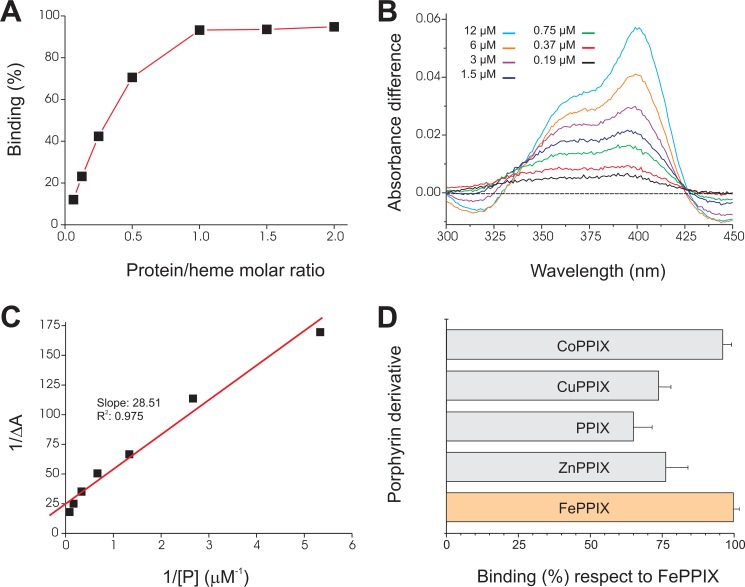
The small size of the MF6p/FhHDM-1 protein, the absence of some typical residues related to heme binding in its sequence, and the ability of the protein to form complexes of high molecular weight (as shown in Fig. 1A and in previous studies (35)) raise questions as to the stoichiometry with which the protein binds heme. To address this question, we measured the protein to heme molar ratios in three different samples: native protein·heme complexes present in peak II (Fig. 1A), native protein·heme complexes obtained after immunopurification by affinity chromatography with mAb MF6, and protein·heme complexes obtained by in vitro incubation of a solution of hemin (the oxidized form of heme) with increasing concentrations of sMF6p/FhHDM-1 and then immunoprecipitated with an excess of Sepharose 4B beads containing immobilized mAb MF6. For the latter, we included Tween 20 (0.1%) in the dilution buffer because inclusion of low concentrations of non-ionic buffers is reported to be the mildest procedure for rendering hemin monomeric and soluble in aqueous buffers (41). The protein/heme molar ratios of the native complexes (peak II and immunopurified samples) were obtained by measuring protein and 56Fe content. The values ranged between 1:0.5 and 1:0.6 but increased up to 1:1 when hemin was incubated in vitro with increasing amounts of the synthetic protein (Fig. 6A).
FIGURE 6.
Binding of hemin and other porphyrin derivatives to sMF6p/FhHDM-1. A, hemin (15 μm) was incubated with increasing amounts of sMF6p/FhHDM-1 (1–30 μm) in different tubes, and the resultant complexes were immunoprecipitated with mAb MF6-Sepharose beads. The percentage of bound hemin was determined indirectly by measuring the absorbance (395 nm) of free hemin remaining in the supernatant with respect to a control with hemin alone and was plotted against the protein/heme molar ratio. B, difference absorbance spectra (ΔA, 300–450 nm) for hemin (3 μm) incubated with increasing concentrations of sMF6p/FhHDM-1 (0.19–12 μm) versus hemin alone. C, plot of 1/ΔA at 398 nm against the inverse of protein concentration (1/[P]) fitted by linear regression to calculate the dissociation constant (Kd). D, several metalloporphyrins and PPIX solutions (46 μm) were incubated with the same amount of sMF6p/FhHDM-1-Sepharose beads or with non-conjugated Sepharose beads (as controls), and binding was determined as above. The figure shows the percentage of binding ± S.D. (error bars) for the different porphyrin derivatives relative to hemin (100% binding).
Once we demonstrated that the sMF6p/FhHDM-1 protein binds hemin with 1:1 stoichiometry, we calculated the Kd of the sMF6p/FhHDM-1·hemin complexes by measuring the difference absorbance spectra from 2-fold dilutions of the protein while maintaining constant the concentration of hemin. The difference absorbance spectra (ΔA) obtained for each protein concentration ([P]) after formation of protein·hemin complexes are shown in Fig. 6B. The Kd as obtained from the slope of plotting 1/ΔA versus 1/[P] (Fig. 6C) was estimated to be 1.14 × 10−6 m−1 for sMF6p/FhHDM-1.
In order to investigate the relevance of the PPIX moiety or the metal ion for binding to hemin, we tested the binding of sMF6p/FhHDM-1 immobilized on Sepharose 4B beads to PPIX and several metalloporphyrins. The sMF6p/FhHDM-1 bound CoPPIX to a similar extent as hemin (96 ± 3% compared with 100 ± 2%); however, the corresponding values for ZnPPIX, CuPPIX, and PPIX were lower (76 ± 10, 74 ± 6, and 65 ± 8%, respectively) (Fig. 6D).
It has recently been reported that as well as being able to bind to heme, the MF6p/FhHDM-1 protein is able to bind to bacterial LPS and that this binding can prevent an LPS-induced inflammatory response (35). Because LPS is not expected a priori to be a target for an HBP, we tested whether the reported binding is a universal phenomenon or limited only to a particular LPS and whether this phenomenon is specific. To investigate these aspects, we tested the binding of two FITC-labeled LPSs (serotypes O111:B4 and O55:B5) to sMF6p/FhHDM-1. Serotype O111:B4 is more reactive to sMF6p/FhHDM-1 than O55:B5, although the interaction seems to be nonspecific because both LPSs reacted almost with the same intensity with a casein hydrolysate used as blocking agent (Fig. 7A). Moreover, we have observed that binding of LPS to sMF6p/FhHDM-1 only occurred at relatively high concentrations of LPS and that the binding was reversed when BSA was added during the incubation process. In a second experiment, we passed whole Fasciola SAs through an affinity column to remove the endotoxin content, which was efficiently removed after four cleaning steps, with a 2 log reduction at each cleaning step (see Fig. 7B, inset). The elution profiles (Fig. 7B) at 280 nm (blue line) and 402 nm (red line) of endotoxin-free whole SAs subjected to size exclusion chromatography on a Superdex 75 HR 10/30 column matched the elution profiles of the sample before being passed through the endotoxin affinity column (Fig. 1A), indicating that LPS was not bound to the MF6p/FhHDM-1·heme complexes.
FIGURE 7.
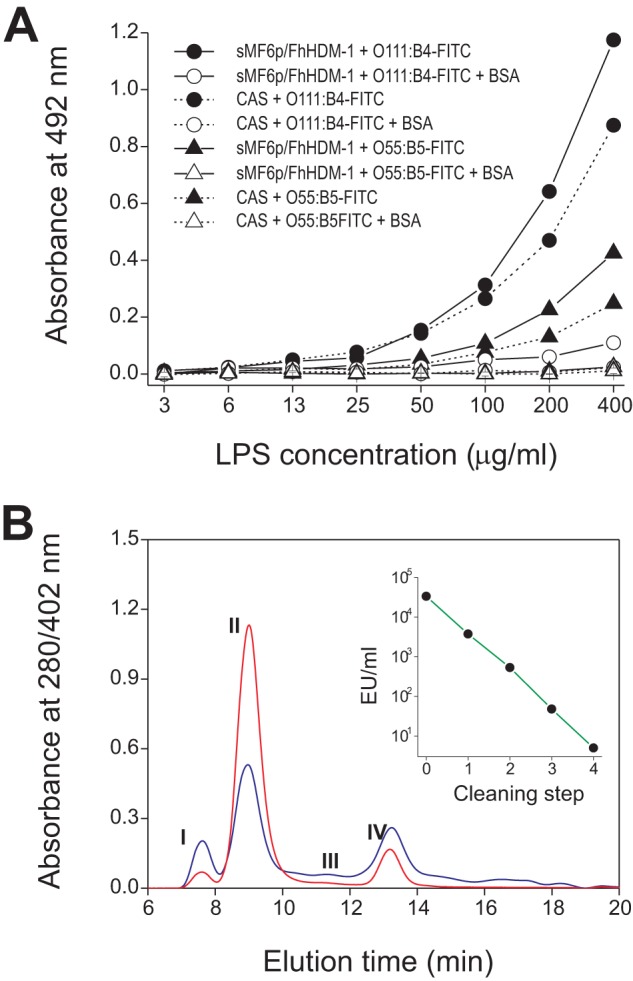
Nature of the interaction between LPS and F. hepatica SAs. A, FITC-labeled LPS binding to sMF6p/FhHDM-1 (1 μg/well; solid lines) and control wells blocked with sodium caseinate (CAS; dashed lines). LPS from two E. coli serotypes, O111:B4 (filled and unfilled circles) and O55:B5 (filled and unfilled triangles), was incubated at increasing concentrations ranging from 3.125 to 400 ng/well in the absence (filled circles and triangles) or presence (unfilled circles and triangles) of 1% BSA. B, size exclusion chromatography (Superdex 75 HR 10/30, ÄKTA LC) profiles of SAs from adult F. hepatica after removal of endotoxin on an endotoxin affinity column (Endotrap Red resin). Endotoxin (EU/ml) was removed after four cleaning steps (inset). Absorbance was monitored at 280 nm (blue spectra) and 402 nm (red spectra). The resultant peaks (numbers) match those obtained with SAs before endotoxin elimination (see Fig. 1A).
The sMF6p/FhHDM-1 Protein Is Able to Prevent Hemin Peroxidation and to Inhibit the Peroxidase-like Activity of Hemin
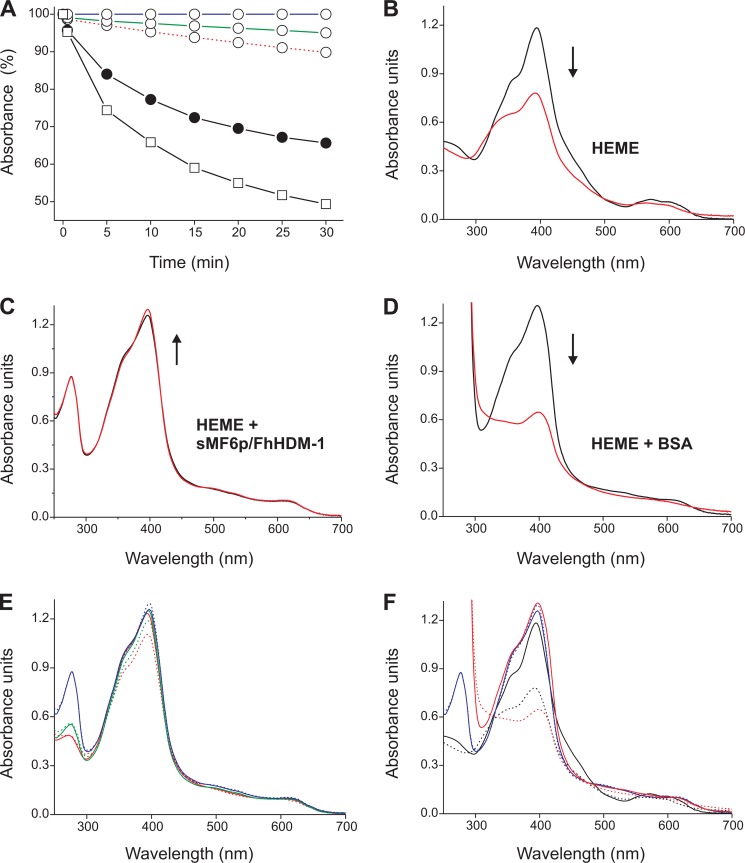
To investigate whether the MF6p/FhHDM-1 protein can interfere with the oxidative properties of free heme, we tested the ability of sMF6p/FhHDM-1 to prevent hemin oxidation by H2O2 as well as its ability to inhibit the peroxidase-like activity of hemin. To evaluate the inhibition of hemin peroxidation, we recorded the changes in hemin absorption in TBS-T2 buffer after the addition of H2O2 to the test tube in the absence or in the presence of sMF6p/FhHDM-1 or BSA. The kinetics of hemin oxidation by H2O2 measured at the Soret peak of each sample for 30 min are shown in Fig. 8A. The addition of H2O2 to hemin alone resulted in a rapid decrease of the peak absorbance at 395 nm, indicating oxidation and partial degradation of the molecule (42), whereas prior addition of the sMF6p/FhHDM-1 protein to hemin prevented such an effect (Fig. 8A). Our data also showed that the degree of inhibition increased with the protein/hemin molar ratio because the percentage of the initial absorbance remaining at 30 min of reaction increased from 89.8% (1:1 molar ratio) to 95.0% (2:1 molar ratio) and 100% (5:1 molar ratio), as determined by measuring the absorbance at 398 nm. The addition of BSA (5:1 molar ratio) to hemin increased the oxidative activity of H2O2 because the percentage of the initial absorbance at 398 nm decreased by 16.4% with respect to hemin alone after 30 min of reaction. However, the enhancing effect of BSA on hemin peroxidation was prevented by omitting 0.1% Tween 20 from the TBS buffer (data not shown).
FIGURE 8.
sMF6p/FhHDM-1 protects hemin from degradation by H2O2. A, hemin (23 μm) was incubated with sMF6p/FhHDM-1 (unfilled circles) at protein/hemin molar ratios of 5:1 (solid blue line), 2:1 (solid green line), and 1:1 (dashed red line); BSA at a molar ratio of 5:1 (unfilled squares); or without protein (filled circles), and changes in the absorption spectra (250–700 nm) were monitored before (t = 0) and at several times within 30 min after the addition of 890 μm H2O2. The percentage of the initial absorbance at the Soret peak remaining in each sample after the addition of H2O2 was plotted against time. B–D, absorption spectra at t = 0 (black line) and t = 30 (red line) of hemin alone (B), with sMF6p/FhHDM-1 (C), and with BSA (D) at the 5:1 protein/hemin ratio. The arrows indicate the direction of the change in the absorbance at the Soret band. E and F, comparison of the absorption spectra at t = 0 (solid lines) and t = 30 (dashed lines) for hemin with sMF6p/FhHDM-1 at 5:1 (blue lines), 2:1 (green lines), and 1:1 (red lines) protein/hemin ratios (E) and of hemin alone (black lines) with sMF6p/FhHDM-1 (blue lines) and BSA (red lines) at a 5:1 ratio (F).
Changes in hemin absorption in the range of 250–700 nm in the presence or absence of the above proteins are shown in Fig. 8, B–F. Except when the sMF6p/FhHDM-1 protein was included in the sample (Fig. 8C), the addition of H2O2 to hemin caused important changes in hemin absorption, mainly in the 325–450 nm range, corresponding to the hemin Soret band. Under the experimental conditions, interaction of sMF6p/FhHDM-1 or BSA with hemin provokes a small shift at the maximal absorption peak of hemin from 395 to 398 nm (Fig. 8, B–F).
The data on the inhibition of the peroxidase-like activity of hemin by sMF6p/FhHDM-1 at a 5:1 protein/hemin molar ratio are shown in Fig. 9A. Like hemin peroxidation, sMF6p/FhHDM-1 was able to inhibit the peroxidase-like activity of hemin by ∼80%, measured after 30 min of reaction. Our data also show that although inhibition of the peroxidase-like activity of hemin increased when more sMF6p/FhHDM-1 was added to the sample (Fig. 9B), the increase tended to be less prominent for protein to hemin molar ratios higher than 1:1 (the inhibition only increased by 18.5% in the range between 1:1 and 5:1).
FIGURE 9.
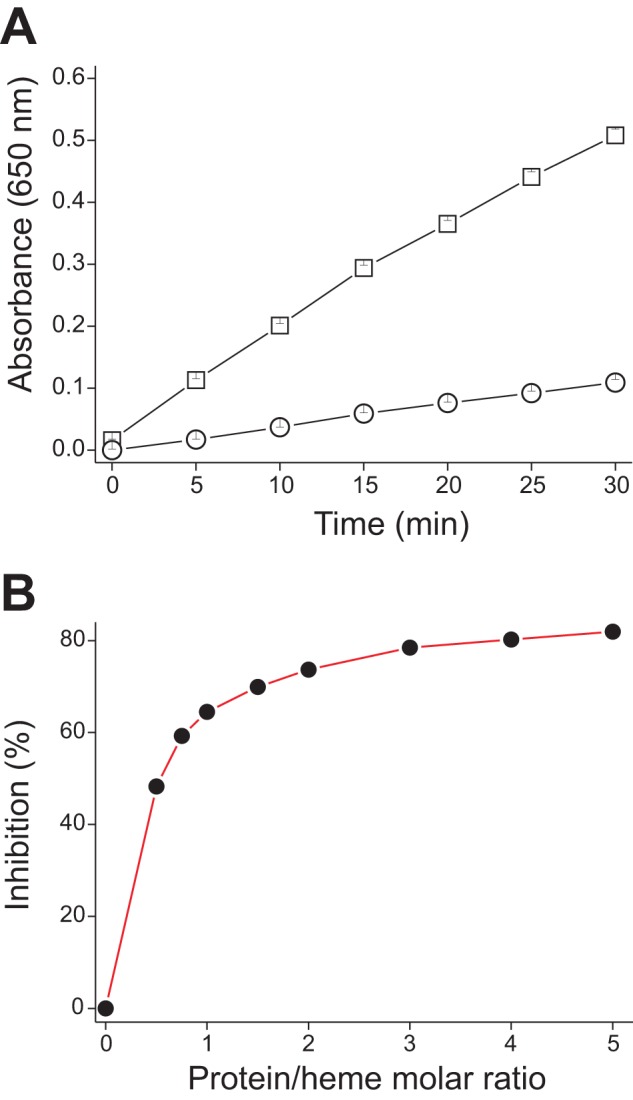
sMF6p/FhHDM-1 inhibits hemin peroxidase-like activity. Hemin alone (23 μm) and hemin with sMF6p/FhHDM-1 (at increasing protein/hemin molar ratios) were mixed with 100 μl of a commercial solution of TMB (1 mm TMB, 3 mm H2O2) adjusted to pH 6; TMB oxidation was monitored at 650 nm for 30 min. A, the absorbance at 650 nm of hemin alone (squares) and hemin with sMF6p/FhHDM-1 (at a 5:1 ratio; circles) was plotted against time. B, inhibition percentage of TMB oxidation by hemin as a function of the protein/hemin molar ratio.
DISCUSSION
Here we demonstrate for the first time that the protein MF6p/FhHDM-1 secreted by Fasciola is a member of a novel family of HBPs. Moreover, because other flukes, such as O. viverrini, C. sinensis, P. westermani, and D. dendriticum also express close homologs of MF6p/FhHDM-1, expression of this protein seems to be evolutionarily conserved. The heme-binding nature of MF6p/FhHDM-1 was clearly indicated by the following: (i) the native MF6p/FhHDM-1 remained bound to heme in Fasciola secretions, and (ii) sMF6p/FhHDM-1 was able to bind and interact with hemin in vitro. The heme-binding nature of MF6p/FhHDM-1 was anticipated by McGonigle and Dalton (43), who partially sequenced the protein; however, these authors assumed that the protein was the parasite hemoglobin, which was later identified as a different molecule by Dewilde et al. (44).
As for most hematophagous trematodes, the mechanisms involved in heme uptake and detoxification in Fasciola have never been investigated. Nevertheless, it is well known that during the intraluminal digestion of host red blood cells in the intestinal cecum by Fasciola proteases, a large excess of potentially toxic heme molecules are released from hemoglobin and must be detoxified (17, 45). Thus, in the absence of hemozoin formation, which does occur in Schistosoma (18), it is assumed that Fasciola releases most heme and other waste molecules directly to the biliary tract during the regurgitation process (11). In concordance with this means of heme detoxification, during in vitro culture of F. hepatica adults, a large amount of a brownish fluid, presumably containing heme, is released cyclically by the parasites to the medium through the oral sucker, which probably constitutes the main route of elimination. However, there are no available data indicating how Fasciola or many other eukaryote parasites that cannot biosynthesize heme (21) acquire, transport, and protect themselves against the deleterious effect of this important molecule, which is essential for synthesizing heme proteins in both the adult and offspring (eggs).
In this study, we have shown that large amounts of MF6p/FhHDM-1 accumulate in parenchymal cells. This location, together with the fact that MF6p/FhHDM-1 was not present in either the cecum wall or the cecum lumen, suggests that the protein may play a key role as a secondary heme transporter in vivo by providing different organs with heme molecules. In this context, it should be considered that parenchymal cells are thought to be responsible for transporting proteins and other substances throughout the body of the fluke by cell-to-cell contact (10). The presence of HBPs with a similar function has been reported in other hematophagous organisms: the HBP from the insect Rhodnius prolixus (RHBP) was associated with delivery of heme from the hemolymph to developing oocytes (18, 46); the heme lipoprotein (HeLp) from the tick R. microplus has been postulated to play a role in the transport and sequestration of heme once translocated from the digestive cells to the hemolymph (22); and the HRG-3 from the nematode C. elegans, a small heme chaperone of only 45 residues, has been reported to be synthesized by intestinal cells and, once complexed to heme, transports it to extraintestinal tissues, including gonads and uterus, through the interstitial fluid (47).
In addition to parenchymal tissue, two organs also seem to be relevant targets for MF6p/FhHDM-1: (i) the vitellaria, where maturing vitelline cells can reasonably accumulate MF6p/FhHDM-1·heme complexes and then provide the future embryo with heme once the mature cells migrate to the yolk reservoir and to the uterus, and (ii) the testis, in which the MF6p/FhHDM-1·heme complexes are probably required to satisfy the high heme demand associated with the high rate of cell division to produce spermatogonia, whereas the MF6p/FhHDM-1 protein may act as an antioxidant (see below) by preventing the toxic effects of free heme generated from normal catabolism of heme proteins. Because spermatozoa are largely devoid of cytoplasm, and consequently of the corresponding cytoplasm enzymatic activities (48), they are expected to be more susceptible than other cell types to oxidative injury. Therefore, at least in mammals, the seminal fluid is an important source of antioxidants (49). The presence of MF6p/FhHDM-1 in the fluid of testis tubules and in other locations, such as vitelline cells, may also explain why mature eggs tested positive in WB for this protein.
Because simple binding of heme to a protein may be not sufficient to prevent heme-induced formation of reactive oxygen species (16, 50), which, in turn may lead to non-enzymatic degradation of heme (42), we investigated the effect of sMF6p/FhHDM-1·hemin interaction on hemin reactivity. Interestingly, sMF6p/FhHDM-1 completely prevented peroxidative destruction of hemin by H2O2, which supports its possible function in preserving heme integrity against potential oxidative species in the environment and enabling its use as a source of heme for other heme proteins. In this respect, we have also observed that sMF6p/FhHDM-1·hemin complexes are able to restore HRP apoenzyme activity more efficiently than hemin alone.4 The possibility of a function as a source of heme is also supported by the observation that sMF6p/FhHDM-1 binds hemin with medium affinity (Kd = 1.14 × 10−6 m−1), although the value depends on the redox state of iron and the characteristics of the medium, among other factors. A similar interaction has been reported for other proteins, such as alkyl hydroperoxide reductase (AhpC), belonging to the heme auxotroph Streptococcus agalactiae (42), and HeLp of R. microplus (51), which also inhibit peroxidation of hemin. In addition, sMF6p/FhHDM-1 was also able to inhibit in vitro hemin reactivity to a large extent, which may be important for protecting the cellular environment from hemin toxicity. In support of this hypothesis, inhibition of hemin degradation and peroxidative activity are both also exhibited by hemopexin. This protein, which is present in vertebrates, has been attributed an antioxidant role against free heme in plasma, and similarly, HeLp and RHBP are able to prevent lipid peroxidation or free radical formation by hemin (16, 51).
Our results show that MF6p/FhHDM-1 accumulates in the tegument and is released in large amounts through the tegumental surface to the external medium, where it binds to heme molecules that are being regurgitated by the parasite. This suggests a role for this protein in heme detoxification in addition to its function as a secondary internal heme transporter. However, this phenomenon probably does not occur in vivo for the following reasons: (i) the regurgitated heme molecules are continuously removed by bile fluid; (ii) the protein was never detected in bile or feces (not shown); and (iii) the 1:1 stoichiometry observed for protein·heme complexes would not be energetically favorable for the parasite. Therefore, it appears that the large amounts of MF6p/FhHDM-1 that are released by the parasite when cultured in vitro are probably due to a reaction of parasites to the stress caused by changing from the relatively anaerobic environment of biliary ducts to the aerobic conditions of in vitro culture.
The MF6p/FhHDM-1 protein differs from other HBPs in its small size (the mature protein contains only 68 aa) and because it does not include in its sequence any of the three residues most often involved in heme binding pockets (i.e. cysteine, histidine, and phenylalanine (52)). Therefore, the two methionines and three tyrosines present in the MF6p/FhHDM-1 protein sequence are the residues most likely to be implicated in this function. However, because no similar protein has been described among the 788 non-redundant proteins contained in the Heme Protein Database (53), future structural studies are required to establish which residues are involved in binding the heme molecule.
With respect to the ligand, sMF6p/FhHDM-1 was able to bind to ferrous heme (data not shown) and to other porphyrin derivatives (including PPIX) as well as to hemin. These data indicated that binding to the protein mainly occurs via interaction with the protoporphyrin ring, although the nature of the metal ion also seems to be important for the affinity of such binding.
Since the partial (ID: ADZ24001.1) and complete (ID: CCA61804.1) MF6p/FhHDM-1 cDNA encoding sequences were independently submitted to GenBankTM by Robinson et al. and by our group, two studies dealing with the structure and putative functions of this protein have been published (35, 36). In these studies, the authors demonstrated that MF6p/FhHDM-1 adopts a predominantly α-helical structure in solution, with a conserved amphipathic helix in the C-terminal region, which seems to be structurally related to the cathelicidin family of cationic antimicrobial peptides, such as the human hCAP18/LL-37 (54, 55). Robinson et al. (35, 36) also proposed that MF6p/FhHDM-1 is able to modulate innate cell responses in two possible ways: (i) by neutralizing LPS to prevent activation of macrophages and the release of anti-inflammatory mediators via LPS-inducing TLR-4 activation (35) and (ii) by suppressing antigen processing and presentation in macrophages via inhibition of lysosomal vacuolar ATPase activity (36). Our findings are consistent with the possibility that the Fasciola MF6p/FhHDM-1 protein may have a second role in promoting suppression of the host immune response after being secreted; indeed, there is indirect evidence that proteins obtained from the tegument (56) or from parasite secretions (57, 58), in which MF6p/FhHDM-1 is present, may display such activity. However, in light of our findings, the proposal that LPS is a primary ligand for MF6p/FhHDM-1 (35) seems unlikely, for the following reasons: (i) MF6p/FhHDM-1 is present in different parasite organs in which LPS is not present; (ii) the interaction between LPS and sMF6p/FhHDM-1 is weak, as also occurred to a similar extent with a casein hydrolysate, and such binding also seems to differ, depending on the LPS serotype; (iii) binding of LPS to sMF6p/FhHDM-1 was totally inhibited by serum albumin in ELISA; and (iv) unlike heme, which remained tightly bound, the LPS present in the native MF6p/FhHDM-1 fraction (Fig. 7B) was totally removed on an LPS-specific binding column. Therefore, it appears that the reported interaction between MF6p/FhHDM-1 and LPS is probably an epiphenomenon caused by the cationic nature of the protein, as also reported in other studies with cationic peptides (59).
In summary, in this study, we have demonstrated that MF6p/FhHDM-1 is a member of a new family of small HBPs, with a probable role in heme homeostasis in Fasciola species and related trematodes. In particular, our results suggest that MF6p/FhHDM-1 may act like a heme chaperone with direct participation, as a secondary transporter, in important physiological processes for the parasite, such as heme trafficking and storage. Given the expected importance of MF6p/FhHDM-1 for parasite survival and its widespread presence among trematodes, this protein also arises as a possible target for the development of new fasciolicide molecules.
Acknowledgments
We thank Dr. Adela Valero (Universidad de Valencia, Spain) and Dr. Francisco Bolás (Universidad Complutense de Madrid, Spain) for critical reading of the manuscript. We also are indebted to Dr. Perfecto Paseiro (Dept. of Química Analítica, Nutrición y Bromatología), Dr. Joaquín Espinosa (Dept. of Fisiología), Dr. Sergio Casas and Dr. Angeles Sánchez (Departamento de Química Inorgánica), Dr. Ricardo Riguera and Dr. Xerardo García-Mera (Departamento de Química Orgánica), Dr. Luis Sánchez (Departamento de Bioquímica y Biología Molecular), and Dr. Elvira Ares, Dr. Fernanda Romarís, and Dr. Esperanza Paniagua (Departamento de Microbiología y Parasitología) from the Universidad de Santiago de Compostela (Spain) for helping with some specialized analysis and data interpretation. We are also grateful for technical assistance and advice from Lola Gutiérrez (Genomics and Proteomics Center, Universidad Complutense de Madrid), Verónica Piñeiro (Unidad de Analisis Elemental, RIAIDT, Universidad de Santiago de Compostela), and Gonzalo Hermelo (Dept. Química Analítica, Nutrición y Bromatología, Universidad de Santiago de Compostela).
This work was supported in part by Ministerio de Ciencia e Innovación, Spain, Grants AGL2010-22290-C02 and AGL2011-30563-C03; Xunta de Galicia, Spain, Grant CN 2012/155; and the European Fund for Regional Development (FEDER).
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) CCA61804.1
V. Martínez-Sernández, M. Mezo, M. González-Warleta, M. J. Perteguer, L. Muiño, E. Guitián, T. Gárate, and F. M. Ubeira, unpublished results.
- HBP
- heme-binding protein
- SA
- secretory antigen
- sMF6p/FhHDM-1
- synthetic MF6p/FhHDM-1
- WB
- Western blot
- SM
- skimmed milk
- DAB
- 3,3′-diaminobenzidine
- ICP
- inductively coupled plasma
- EST
- expressed sequence tag
- 4CN
- 4-chloro-1-naphthol
- PPIX
- protoporphyrin IX
- CuPPIX
- copper(II)-PPIX
- CoPPIX
- cobalt(III)-PPIX
- ZnPPIX
- zinc(II)-PPIX)
- TMB
- 3,3′,5,5′-tetramethylbenzidine
- ESI
- electrospray ionization.
REFERENCES
- 1. Mehlhorn H. (2008) Encyclopedia of Parasitology (Mehlhorn H., ed) p. 137, Springer, Berlin [Google Scholar]
- 2. Toledo R., Bernal M. D., Marcilla A. (2011) Proteomics of foodborne trematodes. J. Proteomics 74, 1485–1503 [DOI] [PubMed] [Google Scholar]
- 3. Rim H. J., Farag H. F., Sornmani S., Cross J. H. (1994) Food-borne trematodes: ignored or emerging? Parasitol. Today 10, 207–209 [Google Scholar]
- 4. Hopkins D. R. (1992) Homing in on helminths. Am. J. Trop. Med. Hyg. 46, 626–634 [DOI] [PubMed] [Google Scholar]
- 5. Keiser J., Utzinger J. (2005) Emerging foodborne trematodiasis. Emerg. Infect. Dis. 11, 1507–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torgerson P., Claxton J. (1999) Epidemiology and control. in Fasciolosis (Dalton J. P., ed) pp. 113–149, CABI Publishing, Wallingford, UK [Google Scholar]
- 7. Mezo M., González-Warleta M., Castro-Hermida J. A., Muiño L., Ubeira F. M. (2011) Association between anti-F. hepatica antibody levels in milk and production losses in dairy cows. Vet. Parasitol. 180, 237–242 [DOI] [PubMed] [Google Scholar]
- 8. Mas-Coma S., Valero M. A., Bargues M. D. (2009) Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 69, 41–146 [DOI] [PubMed] [Google Scholar]
- 9. Andrews S. J. (1999) The life cycle of Fasciola hepatica. in Fasciolosis (Dalton J. P., ed) pp. 1–29, CABI Publishing, Wallingford, UK [Google Scholar]
- 10. Fairweather I., Threadgold L. T., Hanna R. E. B. (1999) Development of Fasciola hepatica in the mammalian host. in Fasciolosis (Dalton J. P., ed) pp. 47–111, CABI Publishing, Wallingford, UK [Google Scholar]
- 11. Halton D. W. (1997) Nutritional adaptations to parasitism within the platyhelminthes. Int. J. Parasitol. 27, 693–704 [DOI] [PubMed] [Google Scholar]
- 12. Tielens A. G. M. (1999) Metabolism. in Fasciolosis (Dalton J. P., ed) pp. 277–305, CABI Publishing, Wallingford, UK [Google Scholar]
- 13. Jani D., Nagarkatti R., Beatty W., Angel R., Slebodnick C., Andersen J., Kumar S., Rathore D. (2008) HDP, a novel heme detoxification protein from the malaria parasite. PLoS Pathog. 4, e1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balla G., Vercellotti G. M., Muller-Eberhard U., Eaton J., Jacob H. S. (1991) Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab. Invest. 64, 648–655 [PubMed] [Google Scholar]
- 15. Aft R. L., Mueller G. C. (1984) Hemin-mediated oxidative degradation of proteins. J. Biol. Chem. 259, 301–305 [PubMed] [Google Scholar]
- 16. Graça-Souza A. V., Maya-Monteiro C., Paiva-Silva G. O., Braz G. R., Paes M. C., Sorgine M. H., Oliveira M. F., Oliveira P. L. (2006) Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 36, 322–335 [DOI] [PubMed] [Google Scholar]
- 17. Stiebler R., Soares J. B., Timm B. L., Silva J. R., Mury F. B., Dansa-Petretski M., Oliveira M. F. (2011) On the mechanisms involved in biological heme crystallization. J. Bioenerg. Biomembr. 43, 93–99 [DOI] [PubMed] [Google Scholar]
- 18. Toh S. Q., Glanfield A., Gobert G. N., Jones M. K. (2010) Heme and blood-feeding parasites. Friends or foes? Parasit. Vectors 3, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lara F. A., Lins U., Bechara G. H., Oliveira P. L. (2005) Tracing heme in a living cell. Hemoglobin degradation and heme traffic in digest cells of the cattle tick Boophilus microplus. J. Exp. Biol. 208, 3093–3101 [DOI] [PubMed] [Google Scholar]
- 20. Wilks A. (2002) Analysis of heme and hemoproteins. in Heme, Chlorophyll, and Bilins: Methods and Protocols (Smith A. G., Witty M., eds) pp. 157–184, Humana Press, Totowa, NJ [Google Scholar]
- 21. Rao A. U., Carta L. K., Lesuisse E., Hamza I. (2005) Lack of heme synthesis in a free-living eukaryote. Proc. Natl. Acad. Sci. U.S.A. 102, 4270–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamza I., Dailey H. A. (2012) One ring to rule them all. Trafficking of heme and heme synthesis intermediates in the metazoans. Biochim. Biophys. Acta 1823, 1617–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mezo M., González-Warleta M., Ubeira F. M. (2003) Optimized serodiagnosis of sheep fascioliasis by Fast-D protein liquid chromatography fractionation of Fasciola hepatica excretory-secretory antigens. J. Parasitol. 89, 843–849 [DOI] [PubMed] [Google Scholar]
- 24. Mezo M., González-Warleta M., Carro C., Ubeira F. M. (2004) An ultrasensitive capture ELISA for detection of Fasciola hepatica coproantigens in sheep and cattle using a new monoclonal antibody (MM3). J. Parasitol. 90, 845–852 [DOI] [PubMed] [Google Scholar]
- 25. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 26. Muiño L., Perteguer M. J., Gárate T., Martínez-Sernández V., Beltrán A., Romarís F., Mezo M., González-Warleta M., Ubeira F. M. (2011) Molecular and immunological characterization of Fasciola antigens recognized by the MM3 monoclonal antibody. Mol. Biochem. Parasitol. 179, 80–90 [DOI] [PubMed] [Google Scholar]
- 27. Fabian M., Solomaha E., Olson J. S., Maresso A. W. (2009) Heme transfer to the bacterial cell envelope occurs via a secreted hemophore in the Gram-positive pathogen Bacillus anthracis. J. Biol. Chem. 284, 32138–32146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanmartín M. L., Iglesias R., Santamarina M. T., Leiro J., Ubeira F. M. (1991) Anatomical location of phosphorylcholine and other antigens on encysted Trichinella using immunohistochemistry followed by Wheatley's trichrome stain. Parasitol. Res. 77, 301–306 [DOI] [PubMed] [Google Scholar]
- 29. Kumar S., Das S. K., Dey S., Maity P., Guha M., Choubey V., Panda G., Bandyopadhyay U. (2008) Antiplasmodial activity of [(aryl)arylsulfanylmethyl]pyridine. Antimicrob. Agents Chemother. 52, 705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahmad E., Rabbani G., Zaidi N., Singh S., Rehan M., Khan M. M., Rahman S. K., Quadri Z., Shadab M., Ashraf M. T., Subbarao N., Bhat R., Khan R. H. (2011) Stereo-selectivity of human serum albumin to enantiomeric and isoelectronic pollutants dissected by spectroscopy, calorimetry and bioinformatics. PLoS One 6, e26186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grinberg L. N., O'Brien P. J., Hrkal Z. (1999) The effects of heme-binding proteins on the peroxidative and catalatic activities of hemin. Free Radic. Biol. Med. 27, 214–219 [DOI] [PubMed] [Google Scholar]
- 32. Atamna H., Boyle K. (2006) Amyloid-β peptide binds with heme to form a peroxidase. Relationship to the cytopathologies of Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 103, 3381–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee K. S., Raymond L. D., Schoen B., Raymond G. J., Kett L., Moore R. A., Johnson L. M., Taubner L., Speare J. O., Onwubiko H. A., Baron G. S., Caughey W. S., Caughey B. (2007) Hemin interactions and alterations of the subcellular localization of prion protein. J. Biol. Chem. 282, 36525–36533 [DOI] [PubMed] [Google Scholar]
- 34. Li B., Du Y., Li T., Dong S. (2009) Investigation of 3,3′,5,5′-tetramethylbenzidine as colorimetric substrate for a peroxidatic DNAzyme. Anal. Chim. Acta 651, 234–240 [DOI] [PubMed] [Google Scholar]
- 35. Robinson M. W., Donnelly S., Hutchinson A. T., To J., Taylor N. L., Norton R. S., Perugini M. A., Dalton J. P. (2011) A family of helminth molecules that modulate innate cell responses via molecular mimicry of host antimicrobial peptides. PLoS Pathog. 7, e1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinson M. W., Alvarado R., To J., Hutchinson A. T., Dowdell S. N., Lund M., Turnbull L., Whitchurch C. B., O'Brien B. A., Dalton J. P., Donnelly S. (2012) A helminth cathelicidin-like protein suppresses antigen processing and presentation in macrophages via inhibition of lysosomal vATPase. FASEB J. 26, 4614–4627 [DOI] [PubMed] [Google Scholar]
- 37. Martínez-Ibeas A. M., Perteguer M. J., González-Lanza C., Gárate T., Manga-González M. Y. (2013) Analysis of an expressed sequence tag library from Dicrocoelium dentriticum. Exp. Parasitol. 135, 287–296 [DOI] [PubMed] [Google Scholar]
- 38. Kotpal R. L. (2012) Modern Text Book of Zoology Invertebrates, pp. 335–351, Rastogi Publications, New Delhi [Google Scholar]
- 39. Kumar V. (1999) Trematode Infections and Diseases of Man and Animals, pp. 19–49, Kluver Academic Publishers, Dordrecht [Google Scholar]
- 40. Pankao V., Sirisriro A., Grams R., Vichasri-Grams S., Meepool A., Kangwanrangsan N., Wanichanon C., Ardseungneon P., Viyanant V., Upatham E. S., Sobhon P. (2006) Classification of the parenchymal cells in Fasciola gigantica based on ultrastructure and their expression of fatty acid binding proteins (FABPs). Vet. Parasitol. 142, 281–292 [DOI] [PubMed] [Google Scholar]
- 41. Travascio P., Li Y., Sen D. (1998) DNA-enhanced peroxidase activity of a DNA-aptamer-hemin complex. Chem. Biol. 5, 505–517 [DOI] [PubMed] [Google Scholar]
- 42. Lechardeur D., Fernandez A., Robert B., Gaudu P., Trieu-Cuot P., Lamberet G., Gruss A. (2010) The 2-Cys peroxiredoxin alkyl hydroperoxide reductase C binds heme and participates in its intracellular availability in Streptococcus agalactiae. J. Biol. Chem. 285, 16032–16041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McGonigle S., Dalton J. P. (1995) Isolation of Fasciola hepatica haemoglobin. Parasitology 111, 209–215 [DOI] [PubMed] [Google Scholar]
- 44. Dewilde S., Ioanitescu A. I., Kiger L., Gilany K., Marden M. C., Van Doorslaer S., Vercruysse J., Pesce A., Nardini M., Bolognesi M., Moens L. (2008) The hemoglobins of the trematodes Fasciola hepatica and Paramphistomum epiclitum. A molecular biological, physico-chemical, kinetic, and vaccination study. Protein Sci. 17, 1653–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lowther J., Robinson M. W., Donnelly S. M., Xu W., Stack C. M., Matthews J. M., Dalton J. P. (2009) The importance of pH in regulating the function of the Fasciola hepatica cathepsin L1 cysteine protease. PLoS Negl. Trop. Dis. 3, e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oliveira P. L., Kawooya J. K., Ribeiro J. M., Meyer T., Poorman R., Alves E. W., Walker F. A., Machado E. A., Nussenzveig R. H., Padovan G. J. (1995) A heme-binding protein from hemolymph and oocytes of the blood-sucking insect, Rhodnius prolixus. Isolation and characterization. J. Biol. Chem. 270, 10897–10901 [DOI] [PubMed] [Google Scholar]
- 47. Chen C., Samuel T. K., Sinclair J., Dailey H. A., Hamza I. (2011) An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in. C. elegans. Cell 145, 720–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drevet J. R. (2006) The antioxidant glutathione peroxidase family and spermatozoa. A complex story. Mol. Cell Endocrinol. 250, 70–79 [DOI] [PubMed] [Google Scholar]
- 49. Udipi S., Ghugre P., Gokhale C. (2012) Iron, oxidative stress and health. in Oxidative Stress: Molecular Mechanisms and Biological Effects (Lushchak V. I., Semchyshyn H. M., eds) pp. 73–108, InTech, Open Access, Rijeka, Croatia [Google Scholar]
- 50. Logullo C., Moraes J., Dansa-Petretski M., Vaz I. S., Masuda A., Sorgine M. H., Braz G. R., Masuda H., Oliveira P. L. (2002) Binding and storage of heme by vitellin from the cattle tick, Boophilus microplus. Insect Biochem. Mol. Biol. 32, 1805–1811 [DOI] [PubMed] [Google Scholar]
- 51. Maya-Monteiro C. M., Alves L. R., Pinhal N., Abdalla D. S., Oliveira P. L. (2004) HeLp, a heme-transporting lipoprotein with an antioxidant role. Insect Biochem. Mol. Biol. 34, 81–88 [DOI] [PubMed] [Google Scholar]
- 52. Li T., Bonkovsky H. L., Guo J. T. (2011) Structural analysis of heme proteins. Implications for design and prediction. BMC Struct. Biol. 11, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reedy C. J., Elvekrog M. M., Gibney B. R. (2008) Development of a heme protein structure-electrochemical function database. Nucleic Acids Res. 36, D307–D313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dürr U. H., Sudheendra U. S., Ramamoorthy A. (2006) LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 1758, 1408–1425 [DOI] [PubMed] [Google Scholar]
- 55. Hazlett L., Wu M. (2011) Defensins in innate immunity. Cell Tissue Res. 343, 175–188 [DOI] [PubMed] [Google Scholar]
- 56. Hamilton C. M., Dowling D. J., Loscher C. E., Morphew R. M., Brophy P. M., O'Neill S. M. (2009) The Fasciola hepatica tegumental antigen suppresses dendritic cell maturation and function. Infect. Immun. 77, 2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cervi L., Serradell M. C., Guasconi L., Masih D. T. (2009) New insights into the modulation of immune response by Fasciola hepatica excretory-secretory products. Curr. Immunol. Rev. 5, 277–284 [Google Scholar]
- 58. Dowling D. J., Hamilton C. M., Donnelly S., La Course J., Brophy P. M., Dalton J., O'Neill S. M. (2010) Major secretory antigens of the helminth Fasciola hepatica activate a suppressive dendritic cell phenotype that attenuates Th17 cells but fails to activate Th2 immune responses. Infect. Immun. 78, 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Giuliani A., Pirri G., Rinaldi A. C. (2010) Antimicrobial peptides. The LPS connection. Methods Mol. Biol. 618, 137–154 [DOI] [PubMed] [Google Scholar]