Background: Frequent loss of liver kinase B1 (LKB1) has been reported in human neoplasm.
Results: LKB1 depletion correlated with enhanced RTK phosphorylation in human lung cancer cell line. Ectopic LKB1 expression in deficient lung and cervical cancer cell lines abrogated a repertoire of phospho-RTKs associated with tumor development and progression.
Conclusion: LKB1 promotes protein tyrosine phosphatase activity and inhibition of RTK-activated pathways.
Significance: LKB1 protects against RTK-driven tumors.
Keywords: Epidermal Growth Factor Receptor (EGFR), Growth Factors, LKB, Lung Cancer, Receptor Tyrosine Kinase, LKB1
Abstract
Aberrant receptor tyrosine kinase phosphorylation (pRTK) has been associated with diverse pathological conditions, including human neoplasms. In lung cancer, frequent liver kinase B1 (LKB1) mutations correlate with tumor progression, but potential links with pRTK remain unknown. Heightened and sustained receptor activation was demonstrated by LKB1-deficient A549 (lung) and HeLaS3 (cervical) cancer cell lines. Depletion (siRNA) of endogenous LKB1 expression in H1792 lung cancer cells also correlated with increased pRTK. However, ectopic LKB1 expression in A549 and HeLaS3 cell lines, as well as H1975 activating-EGF receptor mutant lung cancer cell resulted in dephosphorylation of several tumor-enhancing RTKs, including EGF receptor, ErbB2, hepatocyte growth factor receptor (c-Met), EphA2, rearranged during transfection (RET), and insulin-like growth factor I receptor. Receptor abrogation correlated with attenuation of phospho-Akt and increased apoptosis. Global phosphatase inhibition by orthovanadate or depletion of protein tyrosine phosphatases (PTPs) resulted in the recovery of receptor phosphorylation. Specifically, the activity of SHP-2, PTP-1β, and PTP-PEST was enhanced by LKB1-expressing cells. Our findings provide novel insight on how LKB1 loss of expression or function promotes aberrant RTK signaling and rapid growth of cancer cells.
Introduction
Although receptor tyrosine kinases (RTKs)2 belong to different families and/or sub-families, their activation as measured by phosphorylation of intracellular tyrosine residues is typically achieved through binding with respective growth factors. Receptor dimerization, auto-phosphorylation, and subsequent recruitment of adaptor molecules with varying anchoring domains (e.g. Shc, Grb2, and Src) result in the activation of specific downstream pathways. Aberrant RTK signaling has been implicated as mediators of several pathological conditions, including human neoplasms. Hence, modulation of dysregulated receptor activation may provide interventions against RTK-driven tumors and related disorders.
Among several RTKs linked with cancer, the epidermal growth factor receptor (EGFR) represents a well studied gene and perhaps one of the first receptors strongly associated with human malignancies (1, 2). In addition to intrinsic tyrosine kinase activity of the receptor, phosphatases also play a role in regulating the phosphorylation status of EGFR. More specifically, protein tyrosine phosphatases (PTPs) are described as a dynamic and reversible process required for regulated control of tyrosine phosphorylation (3, 4). EGFR has more than five major tyrosine phosphorylation sites and has been linked to various PTPs such as PTP1β and SHP (4). Therefore, the balance between kinases and phosphatases play an important role in regulating receptor activation.
Liver kinase B1 (LKB1) is another gene that is frequently associated with human neoplasms, including lung, breast, endometrial, and cervical cancers (5–9). For example, LKB1 mutations are thought to be present in about one-third of lung cancer cases and potentially contribute to the development or progression of the disease (10–12). The gene was initially identified in patients with Peutz-Jeghers syndrome, which is associated with gastrointestinal hamartomatous polyps and increased risk of malignant growths (8, 13, 14). LKB1 displays multiple functions, mainly via associations with several targets (13, 15–18). For example, complex formation with STRAD (STE20-related adaptor) and MO25 (mouse protein-25) promotes LKB1 activity, nuclear export, and cytosolic cell distribution (19, 20). To date, LKB1 is reported to regulate 13 kinases, many of which lack clearly defined functions (21). One of the widely studied LKB1 substrate, AMP-activated protein kinase (AMPK) is regarded as a metabolic master switch (6, 22). Furthermore, the association of LKB1 with critical biological processes such as p53-mediated cell death support important roles in cell fate determination (7, 15, 17, 23). Together, these functions highlight why LKB1 alterations can severely impact malignant transformations.
To assess potential involvement of LKB1 on receptor-mediated signaling in human tumors, we analyzed the phosphorylation profiles of RTKs in four human cancer cell lines, namely A549, H1792, H1975, and HeLaS3. We further measured the expression and activity of PTPs in the presence or absence of LKB1. Here, we report that LKB1 promotes the activity of PTPs, instigating rapid abrogation of a repertoire of phospho-RTKs in cancer cells.
EXPERIMENTAL PROCEDURES
Cell Lines
A549, H1792, and H1975 (lung) and HeLaS3 (cervical) cancer cell lines were purchased from the American Type Culture Collection (Manassas, VA) and maintained at 37 °C in a humidified atmosphere of 5% CO2 in an open-air incubator with full medium consisting of DMEM or RPMI 1640 (Sigma) supplemented with 10% (v/v) heat-inactivated FBS, 2 mm glutamine, 100 units/ml penicillin, and 0.1 mg/ml streptomycin.
Cell Transfection
Wild-type or mutant LKB1 constructs (D194A and S428A) were generated as described previously (24). D194A is a kinase-dead LKB1 mutant, whereas S428A possess a point mutation in the C-terminal phosphorylation domain. Plasmids encoding EGFP-fused SL-26 were kindly provided by Lily Dong (University of Texas Health Sciences Center, San Antonio, TX). SL-26 is a constitutively nuclear localized LKB1 mutant originally identified in Peutz-Jeghers syndrome patients. Transient cell transfection of LKB1 constructs was undertaken using the Lipofectamine 2000 reagent from Invitrogen.
Reagents and Inhibitors
Receptor tyrosine kinase inhibitor RPI-1 (1,3-dihydro-5,6-dimethoxy-3-[(4-hydroxyphenyl)methylene]-H-indol-2-one) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Sodium orthovanadate was from Sigma. Growth factors were purchased from Promega (Madison, WI) or R&D Systems (Minneapolis, MN) respectively. For growth factor stimulation, cells were serum-starved overnight prior to stimulation at the indicated time intervals with specific or mixture of growth factors (EGF, HGF, EphrinA1, VEGF, FGF2, PDGF, and insulin). For apoptosis, cells were treated with 2 μm staurosporine (Sigma; catalog no. S-4400) or RPI-1 for 4 h. Cell death detection (TUNEL) kit was purchased from Roche and used according to the manufacturer's instructions. Human phospho-RTK array kit (ARY001) was purchased from R&D Systems and used according to the manufacturer's instructions.
Protein Tyrosine Phosphatase Activity Assays
The SensoLyte pNPP protein phosphatase assay kit was purchased from AnaSpec (Fremont, CA). Absorbance was measured at 405 nm using Tecan infinite M1000. Immunoprecipitation and phosphatase activity assay was undertaken in LacZ- or LKB1-transfected cells according to Sorenson et al. (3). The PTP assay kit was purchased from Millipore (Temecula, CA) and used according to the manufacturer's instructions.
Western Blots and Antibodies
Samples were typically run in 8% SDS-PAGE gels and transferred onto nitrocellulose from Bio-Rad. Membranes were probed with specific antibodies according to the manufacturer's instructions. Primary antibodies were LKB1, pY20, and GAPDH anti-mouse monoclonal antibody from Santa Cruz Biotechnology; anti-rabbit phospho- and total polyclonal antibodies against RTKs, SHP-1, SHP-2, PTP-1β and PTP-PEST were from Cell Signaling (Boston, MA).
Rh-EGF Labeling
Cell surface availability of EGF receptors was measured using rhodamine-labeled EGF (Rh-EGF, Molecular Probes). Monolayer of serum-starved LacZ- or LKB1-transfected cells was placed on ice for 10 min. Addition of Rh-EGF was added at indicated concentrations for 30 min. The reactions were stopped by removal of Rh-EGF and washing with cold PBS (2×). Cells were fixed with 1% paraformaldehyde, and Rh-EGF staining was detected by flow cytometry. For EGF receptor recycling, monolayers of serum-starved LacZ- or LKB1-transfected cells were stimulated with unlabeled EGF (100 ng/ul) for 30 min. Excess EGF was removed and washed with cold PBS. Fresh serum-free DMEM medium was added to the cells on ice. Cells were placed back at 37 °C for indicated times. Cells were subsequently placed on ice (10 min) followed by incubation with Rh-EGF for 30 min. The reactions were stopped by removal of Rh-EGF and cold PBS washes (2×). Cells were fixed in 1% paraformaldehyde, and Rh-EGF staining was detected by flow cytometry (LSRII analyzer).
Confocal Microscopy
Cells were fixed in 4% paraformaldehyde followed by permeabilization with either 0.1% Triton X-100 or 0.2% saponin. Immunofluorescence experiments were performed using the following antibodies: Alexa Fluor 555 or Alexa Fluor 647 conjugated anti-mouse IgG for LKB1 (1:100), Alexa Fluor 555 conjugated anti-rabbit IgG for EGFR or EGF (1:100), Alexa Fluor 488 conjugated anti-mouse IgG for pY1068-EGFR (1:100). All secondary Alexa Fluor® antibodies (Invitrogen) were used at a 1:400 dilution. Cells were mounted using SureMount© with DAPI. Imaging was carried out using a Zeiss LSM 510/710 confocal microscope with a 63× objective. pY1068 fluorescent intensity was measured using the LSM Zeiss 510 imaging software profile function. Briefly, a selected region of interest (profile) was determined in the cytoplasmic area defined by light field images and the exclusion of DAPI. The average peak fluorescence intensities were computed within the area of the cell. A total of 7–10 cells were counted for each group. Laser intensity, detector gain, and pinhole remained constant during data acquisition.
Statistical Analysis
Statistical analysis was done using Student's t test, one-way analysis of variance, or two-way analysis of variance. p values of <0.05 were considered significant. Significance for each figure is noted as follows: #, p < 0.05; *, p < 0.01; **, p < 0.001, and ***, p < 0.0001. All analysis was done using GraphPad Prism software.
RESULTS
LKB1 Abrogates RTK Activation in Human Cancer Cell Lines
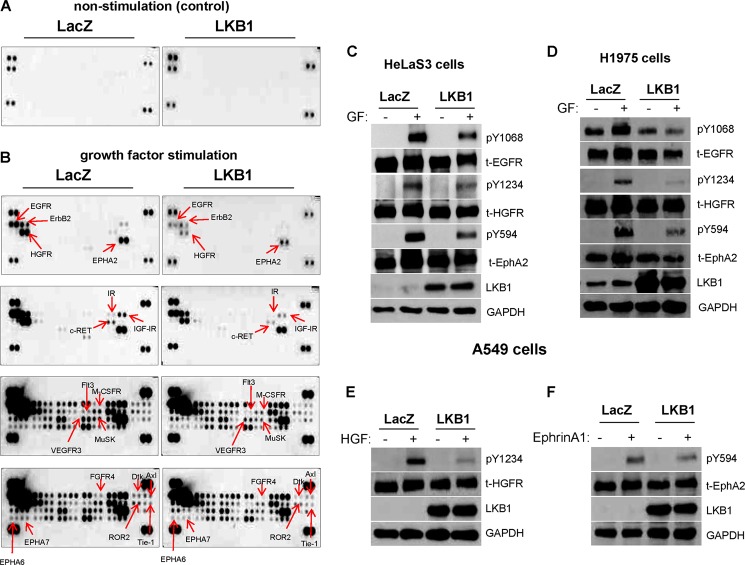
Tyrosine kinase phosphorylation serves as readout for receptor activation and mediates trans-membrane signal transduction pathways within cells. For measurement of phospho-RTK profiles, we selected LKB1-deficient A549 lung cancer cells due to the frequent inactivation of LKB1 in the disease. Empty-vector (LacZ) or LKB1-transfected cells were serum-starved and stimulated with a mixture of exogenous growth factors prior to incubation on phospho-RTK array membranes. Under basal, non-stimulatory conditions, RTK phosphorylation was undetected in both LacZ- and LKB1-expressing cells, as would be expected (Fig. 1A). However, growth factor stimulation resulted in phosphorylation of a subset of RTKs, including EGFR, ErbB2 (HER2), HGFR (c-Met), EphA2, RET, and insulin-like growth factor I receptor but was dramatically blocked upon LKB1 expression (Fig. 1B). The repertoire of phospho-RTKs across different receptor families that are attenuated by LKB1 is listed in Table 1. LKB1 expression in HeLaS3 cervical cancer cells (another LKB1-deficient cell), exhibited similar attenuation of multiple RTK phosphorylation as observed previously in A549 lung cancer cells (Fig. 1C). We next overexpressed LKB1 in H1975 lung adenocarcinoma cells, which possess activating EGFR mutations (a primary point exon 21 mutation, and secondary mutation ascribed to drug resistance). Importantly, phospho-RTK attenuation by LKB1 was demonstrated in a cell model possessing activating EGFR mutations (Fig. 1D). Finally, cell stimulation with specific growth factors was employed and confirmed further the inhibition of selected RTKs by LKB1, including HGFR and EphA2 (Fig. 1, E and F). Taken together, these data reveal the varying activation states of a repertoire of RTKs within cancer cells and demonstrate a global, but differential attenuation by LKB1.
FIGURE 1.
LKB1 abrogates RTK activation in human cancer cell lines. LKB1-deficient lung cancer cell line (A549) was transfected with LacZ or LKB1 vectors, serum-starved, and stimulated with a mixture of growth factors prior to phospho-RTK screen. A, under basal, non-stimulatory conditions, RTK phosphorylation was undetected in both LacZ- and LKB1-expressing cells, as expected. B, however, upon growth factor stimulation, LKB1-expressing cells showed decreased phosphorylation of multiple RTKs compared with LacZ cells. C, LKB1-mediated phospho-RTK attenuation was observed in HeLaS3, another LKB1-deficient cancer cell line. D, in H1975 lung cancer cells that possess activating EGFR mutations, LKB1 overexpression blocked RTK activation compared with LacZ cells. E and F, confirmation of pY1234-HGFR and pY594-EphA2 dephosphorylation by LKB1 following stimulation with specific growth factor for the receptors. Cells were stimulated with 50 ng/ml of HGF or ephrin A1, respectively for 5 min.
TABLE 1.
Comparison of RTK phosphorylation profiles in LKB1 versus LacZ-transfected A549 lung cancer cell line
Shown is a comparison of RTK phosphorylation profiles in the LKB1- or LacZ-transfected A549 lung cancer cell line. LKB1 mediates the dephosphorylation of a repertoire of RTKs across different receptor families. R, receptor.
| Receptor family | Receptor tyrosine kinases attenuated by LKB1 |
|---|---|
| EGFR | EGFR, ErbB2 (HER2) |
| HGFR | HGFR (c-Met) |
| EphR | EphA2, EphA6, EphA7 |
| Insulin receptor | IGF-IR, Insulin R |
| VEGFR | VEGF R3 |
| RET | c-RET |
| FGFR | FGF R4 |
| ROR | ROR2 |
| Axl | Axl, Dtk |
| MuSK | MuSK |
| PDGFR | Flt-3, MCSF-R |
| Tie | Tie-1 |
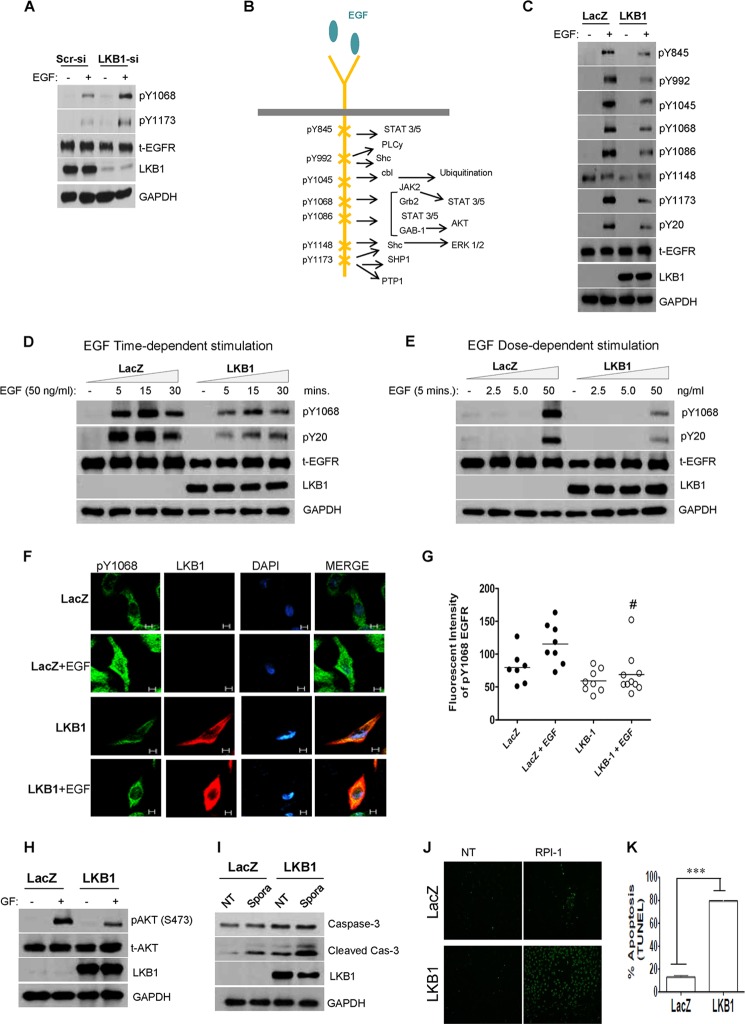
LKB1 Modulates Receptor Activation in a Dose- and Time-dependent Manner
Based on well established functions and links to lung cancer, the EGFR was selected for further investigations. LKB1 depletion (siRNA) in H1792 cells upon EGF stimulation correlated with increased phosphorylation at canonical Tyr-1068 and Tyr-1173 residues (Fig. 2A). Phosphorylation status of major EGFR tyrosine residues described previously by Sordella et al. (25) were subsequently assessed in A549 cells (Fig. 2B). Following 5 min of EGF stimulation, LKB1-expressing cells exhibited decreased phosphorylation across major EGFR tyrosine residues as compared with LKB1-null cells (Fig. 2C). Levels of total EGFR protein remained unchanged under basal or EGF stimulation, excluding receptor degradation or transcriptional regulation. Subsequent time-dependent stimulation demonstrated phosphorylation at Tyr-1068, and global phosphotyrosine-containing proteins (pTyr-20) within 5 min, which peaked at 15 min and declined by 30 min (Fig. 2D). Although similar activation patterns were exhibited across these time intervals, a stronger level was evident in LKB1-null cells. Dose-dependent stimulation revealed marked receptor activation with 50 ng/ml EGF in LKB1-deficient cells (Fig. 2E). Receptor activation in this cell model has been achieved with lower EGF concentrations (∼10 to 25 ng/ml EGF; data not shown). The dephosphorylation of Tyr-1068 by LKB1-expressing cells was further confirmed by confocal microscopy (Fig. 2, F and G).
FIGURE 2.
LKB1 expression attenuates EGFR phosphorylation, blocking receptor-activated pathways. A, LKB1 depletion (siRNA) in H1792 lung cancer cells correlates with increased activation at canonical pY 1068 and pY1173 -EGFR upon EGF stimulation. Scr-si, scrambled siRNA. B, schematic representation of tyrosine kinase residues located on the C terminus of EGFR (adapted from Sordella et al. (25)). C, LKB1-expressing A549 cells stimulated with 50 ng/ml of EGF for 5 min show decreased EGFR phosphorylation at multiple tyrosine sites as determined by Western blot. Total EGFR expression was unaffected by LKB1 treatment. D, Time-dependent stimulation with 50 ng/ml of EGF demonstrated similar kinetic responses; however, LKB1 expressing cells displayed decreased phosphorylation of canonical pY1068 and global phosphotyrosine-containing proteins (pY20). E, EGF dose-dependent stimulation of A549 cells resulted in an optimal stimulatory dose of 50 ng/ml. At this concentration, pY1068 and pY20 phosphorylation was inhibited by LKB1 expression. F and G, representative images from confocal microscopy for LKB1 (red), pY1068-EGFR (green), and nucleus (DAPI, blue). After stimulation with 50 ng/ml of EGF, LKB1-expressing cells display reduced fluorescent intensity (one-way analysis of variance; #, p < 0.05) compared with LacZ cells (scale bar, 5 μm). H–K, negative receptor regulation by LKB1 correlates with enhanced apoptosis (H) growth factor (GF) stimulation of LKB1 cells resulted in decreased pAKT (Ser-473) compared with LKB1-null cells. I, increased cleaved caspase-3 expression was evident in LKB1-positive cells compared with LacZ cells. Straurosporin (Spora) treatment (an inducer of apoptosis) enhanced further the expression of cleaved caspase-3 in LKB1 cells. J and K, pharmacological RTK inhibitior, RPI-1 demonstrated enhanced apoptosis of LKB1-positive cells detected by TUNEL (TdT-mediated dUTP nick end labeling) (Student's t test; ***, p < 0.0001. NT, no treatment.
LKB1-mediated Receptor Attenuation Impacts Downstream RTK-activated Targets and Enhances Apoptosis
Impact of negative receptor attenuation was investigated by assessing apoptosis and apoptosis-related targets. The activation of AKT was undetected under basal conditions in empty-vector or LKB1-positive cells; however, upon growth factor stimulation, decreased AKT phosphorylation was evident in LKB1 cells compared with LacZ cells (Fig. 2H).
Furthermore, LKB1 cells exhibited increased cleaved caspase-3 expression, which was further amplified in response to staurosporine treatment (Fig. 2I). Enhanced apoptosis by LKB1 was confirmed by TUNEL assay following treatment with receptor tyrosine kinase inhibitor, RPI-1 (Fig. 2, J and K). Together, these data support a role for LKB1 in phospho-RTK attenuation, thereby leading to inhibition of RTK-activated pathways of cancer cells.
Total Receptor Protein Is Not Impacted by LKB1 Expression
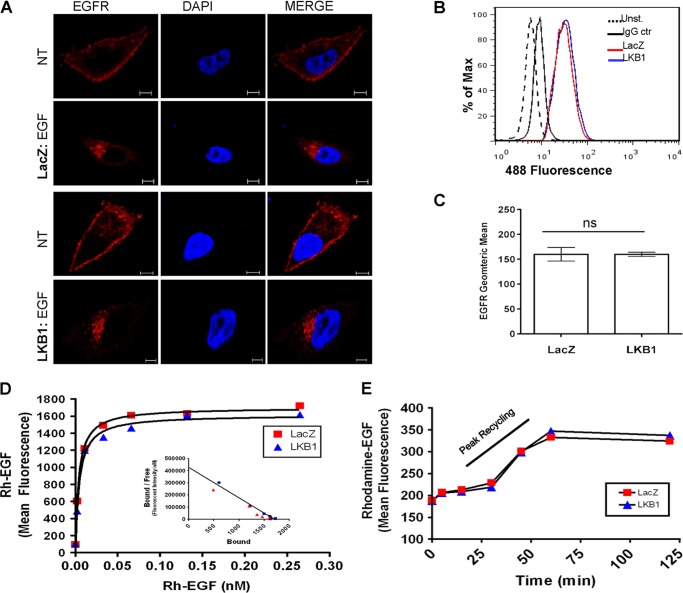
Despite changes in phosphorylation status, total receptor levels remained unaffected as demonstrated by Western blot data (Fig. 2). To further exclude differences in receptor availability, total EGFR expression and localization in the presence or absence of LKB1 was investigated. Confocal microscopy and flow cytometry data revealed comparable EGFR expression in LKB1-null or positive cells (Fig. 3, A–C). Under basal conditions, EGFR was predominately expressed at or near the cell surface in both LacZ and LKB1 transfected cells. However, upon EGF stimulation, the receptor was internalized to a similar degree across LKB1-expressing or null cells (Fig. 3A).
FIGURE 3.
Total EGFR level is not impacted by LKB1. A, representative images from confocal microscopy for total EGFR (red). The nucleus was stained with DAPI (blue). Both LKB1 and LacZ-transfected A549 cells displayed similar distribution of EGFR under non-stimulatory and EGF-stimulated conditions (scale bar, 5 μm). B and C, as measured by flow cytometry, under non-stimulatory conditions, total EGFR expression in LacZ and LKB1-treated cells remained the same (unpaired t test, p = 0.9912). D, Rh-EGF binding of cell surface EGF receptors is similar in LacZ and LKB1-treated cells. Binding curve and Scatchard plot (inset) represent mean fluorescent intensities. Both LacZ and LKB1-treated cells show similar binding curves as well as biochemical parameters (Bmax, 1700 and 1615; Kd, 0.0040 and 0.0045 nm for LacZ and LKB1, respectively). E, binding of Rh-EGF was assessed at various time points after stimulation with unlabeled EGF. LacZ and LKB1-treated cells showed similar recycling kinetics, peaking within 30 to 60 min. NT, no treatment; Unst., unstained; IgG ctr, IgG control.
EGFR Availability and Recycling Not Impacted by LKB1
We next assessed cell surface availability of the receptor under basal conditions via rhodamine-EGF (Rh-EGF) binding assay. Similar binding curves and biochemical parameters (Bmax, 1700 and 1615; Kd, 0.0040 and 0.0045 nm for LacZ and LKB1, respectively) were observed, indicating that LKB1 has minimal effects on EGF receptor availability (Fig. 3D). Next, we examined whether LKB1 altered the recycling of EGFR, thereby attenuating receptor availability at the cell surface. Non-labeled exogenous EGF was initially applied to achieve internalization of the receptor. Subsequent Rh-EGF binding at the cell surface of LKB1-null or positive cells resulted in similar mean fluorescent intensities for each time point, with peak recycling occurring within 30–60 min (Figs. 3E). This indicates that the rate of EGFR recycling is unaffected by LKB1. Together, these data demonstrate that total receptor levels and availability remain unaffected in the presence of LKB1.
Full-length, Functional LKB1 Protein Is Critical to Phospho-RTK Attenuation
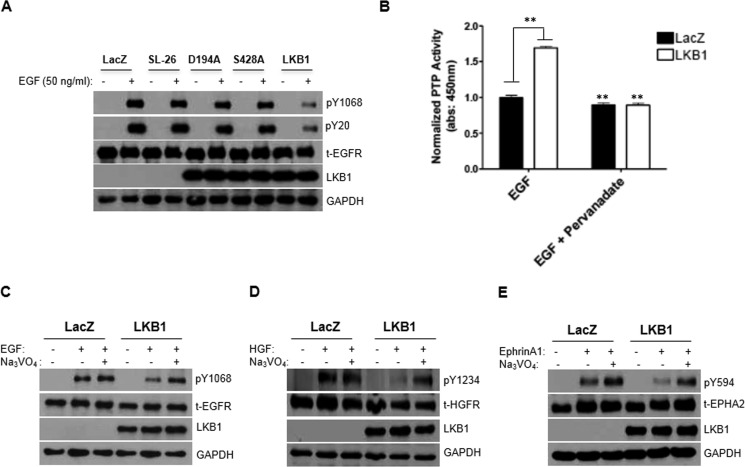
To define specific domain(s) within the LKB1 gene critical for receptor abrogation, we transfected A549 cells using wild-type LKB1 vector, in parallel with various LKB1 mutants. Our data suggest that a fully functional LKB1 protein is required for phospho-RTK abrogation, as LKB1 mutations that sequester LKB1 to the nucleus (SL-26) inactivate the kinase domain (D194A) or prevent serine 428 phosphorylation (S428A) resulted in the recovery of EGFR activation (Fig. 4A).
FIGURE 4.
Phosphatase activation contributes to rapid receptor attenuation instigated by LKB1. A, characterization of LKB1 domain critical to phospho-RTK abrogation suggests that full-length LKB1 is required. Unlike wild-type LKB1, the constitutive nuclear-localized (SL-26), kinase-dead (D194A), or serine 428 phosphorylation (S428A) mutants were unable to inhibit EGFR phosphorylation in A549 cells. B, increased global phosphatase activity was demonstrated by LKB1-expressing cells in comparison with LKB1-null cells or pervanadate treatment. **, p < 0.001. Data were normalized with non-treated control cells. 1 mm Sodium orthovanadate (Pervanadate), a phosphatase inhibitor, was used. C–E, LKB1-mediated abrogation of phospho-RTK (pY1068-EGFR, pY1234-HGFR, and pY594-EPHA2) was rescued upon pervanadate treatment.
Phosphatase Activity Is Involved with Rapid RTK Dephosphorylation Instigated by LKB1
Regulatory balance between kinase and phosphatase activity contributes to homeostasis of RTK activation (3, 4) and prompted investigation of phosphatase activity. Enhanced phosphatase activity was demonstrated by LKB1-expressing cells compared with null cells (Fig. 4B). As would be expected, treatment with pervanadate, a phosphatase inhibitor, resulted in the loss of phosphatase activity in the presence or absence of LKB1 (Fig. 4B). Biochemical analysis confirmed recovery of EGFR phosphorylation in LKB1-expressing cells following pervanadate treatment (Fig. 4C). Rescue of additional phospho-RTKs (HGFR and EphA2) upon pervanadate treatment was further demonstrated (Fig. 4, D and E). The data indicate that LKB1 expression confers significant enhancement of phosphatase activity, promoting rapid abrogation of RTK phosphorylation.
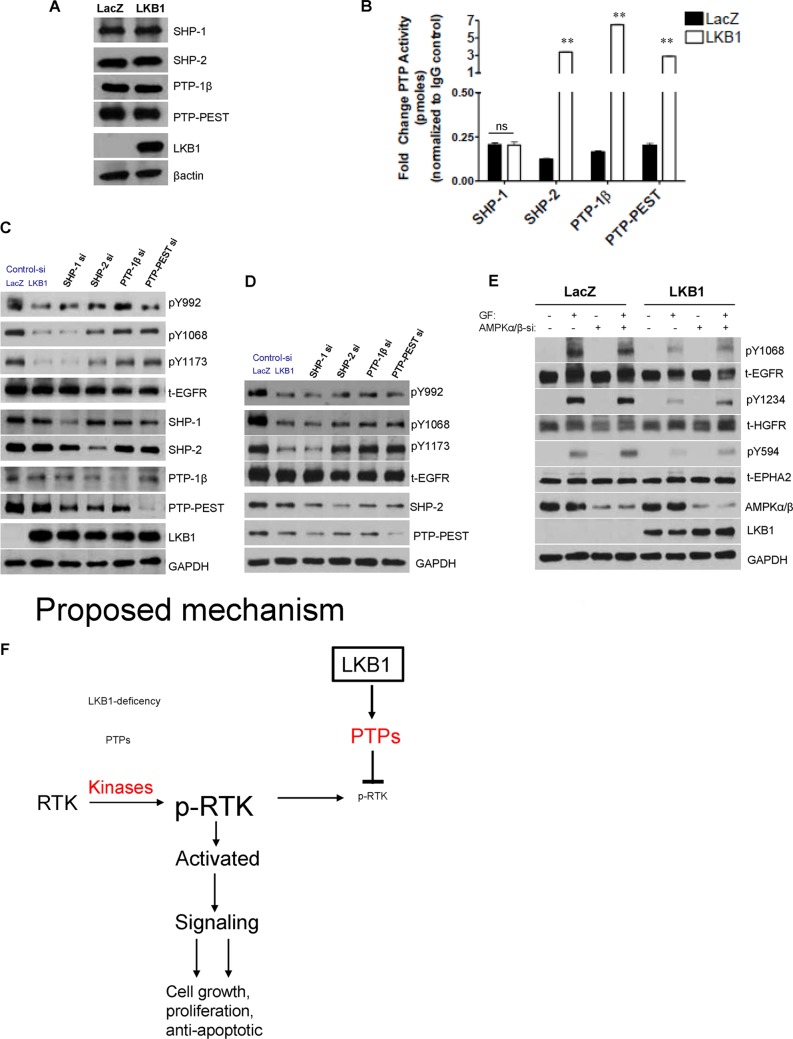
LKB1 Promotes SHP-2, PTP-1β, and PTP-PEST activity
To identify specific PTPs modulated by LKB1, we tested expression of cytosolic phosphotyrosine PTPs, namely SHP-1, SHP-2, PTP-1β, and PTP-PEST (3, 4). Expression of the selected PTPs was not affected by LKB1 (Fig. 5A). We next pulled down and measured activity of respective PTPs from LKB1-null or positive cells. Specific up-regulation of SHP-2, PTP-1β, and PTP-PEST activity (but not SHP-1) was evident in LKB1-cells (Fig. 5B). Finally, confirmation of PTP-mediated receptor attenuation was confirmed following depletion (siRNA) of specific PTPs in the presence of LKB1. In both A549 and HeLaS3 cell lines, variable recovery of phosphotyrosine residues was evident upon knockdown of specific PTPs (Fig. 5, C and D), and in agreement with distinct or combinatorial receptor modulation by PTPs (3, 4). Subsequent investigations evaluated whether the major LKB1 substrate, AMPK, played a role in phospho-RTK attenuation (22, 26). We silenced predominant α/β AMPK subunits in LKB1-null or positive cells, and in the LKB1-deficient cells, AMPK expression failed to abrogate receptor tyrosine activation (Fig. 5E). Importantly, phospho-RTK inhibition mediated by LKB1 was enhanced in the presence of AMPK (Fig. 5E).
FIGURE 5.
LKB1 promotes activity of specific PTPs. A, protein expression of intracellular PTPs involved in regulation of tyrosine phosphorylation was not affected by LKB1 in A549 cells. B, the activities of SHP-2, PTP-1β, and PTP-PEST were strongly up-regulated by LKB1 but not SHP-1. With the exception of SHP-1 activity, PTPs activity in LKB1 cells was significantly enhanced compared with LacZ cells; **, p < 0.001. C and D, knockdown of respective PTPs in LKB1-transfected A549 or HeLaS3 cells confirm differential recovery of phosphotyrosine residues. E, in A549 cells, LKB1-mediated phospho-RTK attenuation is further enhanced by AMPK. F, proposed mechanism of rapid phospho-RTK abrogation instigated by LKB1 via enhanced PTP activity. GF, growth factor.
DISCUSSION
A plethora of cellular processes and downstream signal transduction pathways are modulated by RTKs. However, dysregulated RTK activation has been implicated in several pathological conditions, including human neoplasms. The regulatory mechanisms governing RTK activation are complex and include autocrine, paracrine, or juxtacrine events, ligand specificity, as well as duration or amplitude of stimulation (1, 27). In addition, signaling outcomes are variable and cell-type/context-dependent (1, 27). Here, we identify novel regulation of a repertoire of phospho-RTKs by the LKB1 tumor suppressor in lung and cervical cancer cell lines. The consequences of frequent LKB1 mutations and loss-of-function in human tumors have been widely described (5–9, 28). We report that the loss of LKB1 expression in cancer cells contributes to aberrant receptor activation, RTK-activated pathways and tumor growth (Fig. 5F). Proliferation profile of LKB1-null or positive cells in the presence or absence of growth factor stimulation was measured in real time using electrode-coated plates and was consistent with growth inhibition by LKB1 under all conditions (data not shown).
As readout for receptor activation, we assessed phosphorylation profiles of RTKs in the presence or absence of LKB1. Re-expression of LKB1 into the LKB1-deficient A549 cells demonstrated dramatic inhibition against several tumor-enhancing RTKs, including EGFR, ErbB2, HGFR, and EphA2. The activation of additional RTKs such as, Axl, RET, insulin-like growth factor I receptor, ROR, and MuSK was also blocked. Attenuation of selected RTKs was subsequently validated in A549, H1792, H1975 (lung adenocarcinoma), and HeLaS3 (cervical) cancer cell lines. Activating EGFR mutations significantly contribute to aberrant signaling by the receptor. However, enhanced LKB1 expression in a H1975 cell line that possesses a primary EGFR mutation (exon 21) was sufficient to block its activation. Importantly, phospho-RTK attenuation was further enhanced by AMPK, suggesting that AMPK may play a role in receptor modulation.
Mechanistic insight of RTK attenuation revealed that LKB1 expression correlated with increased PTP activity. The modulation of PTP is a dynamic and reversible process and subject to regulatory phosphorylation, which can result in the activation or inhibition of PTPs (3, 4, 29). It can also be regulated by reversible oxidation of cysteine in the active site of PTPs (30). Cellular oxidation states impacted by reactive oxygen or nitrogen species production result in the modulation of PTPs. Reactive oxygen or nitrogen species production can be influenced by several factors, including natural redox states or growth factor stimulation (29, 30). Interestingly, LKB1 has been described as a master kinase associated with the activation of 13 kinases of the AMPK subfamily (21). AMPK is at the cross-roads of two important processes, namely metabolic (bioenergetics) and redox regulation (31). Similar to the activation of LKB1 enzyme, AMPK responds to stress conditions such as serum deprivation or hypoxia (1, 26). AMPK-based redox signaling has been linked with redox-related processes, including autophagy and apoptosis (31). It is therefore conceivable that redox or metabolic alterations that frequently occur in cancer cells has impact upon reactive oxygen species or reactive nitrogen species production as well as AMPK activity (31–36). In turn, reactive oxygen or nitrogen species production play critical roles in PTP regulation, providing a link for phospho-RTK modulation by LKB1. The regulatory mechanisms of PTPs via AMPK and/or other kinases of the AMPK subfamily are a current area of research interests.
Aberrant expression of several RTKs such as EGFR, ErbB2, HGFR, EphA2, and RET have been associated with tumor development, progression, or advanced disease, and often correlate with adverse prognosis (37–41). Persistent problems with drug resistance against tyrosine kinase inhibitors in addition to cross-activation of multiple RTKs by different growth factors represent a major challenge for effective cancer treatment. Therefore, inhibition of a repertoire of RTKs by a single gene, LKB1, may provide an attractive option for effective targeting of aberrant RTK activity and improve drug sensitivity in a subset of RTK-driven tumors. Our preliminary data has demonstrated modest sensitivity at low gefitinib concentrations (0.02, 0.05, and 0.1 μm) in the presence of LKB1 but requires further characterization (data not shown). Beyond cancer, these findings merit further investigation in other RTK-driven pathological conditions, especially the receptors identified in this study. For instance, muscle-specific tyrosine kinase has been implicated in neuromuscular junction defects through reciprocal tyrosine phosphorylation with Abl (42), whereas insulin-like growth factor I receptors and insulin receptor are intimately linked with diabetes (43–45). Finally, PTPs such as PTP-1β and SHP-2 have been associated with cancer, diabetes, restenosis, and cardiac hypertrophy (29, 30), providing potential therapeutic interventions by LKB1.
Acknowledgments
We thank the Oklahoma Medical Research Foundation Imaging Facility for assistance with confocal microscopy. We also thank the University of Oklahoma Health Sciences Center flow cytometry laboratory for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants HL079584, HL080499, HL074399, HL089920, HL096032, HL105157, and HL110488 (to M.-H. Z.).
- RTK
- receptor tyrosine kinase
- LKB1
- liver kinase B1
- AMPK
- AMP-activated protein kinase
- EGFR
- epidermal growth factor receptor
- HGFR
- hepatocyte growth factor receptor
- RET
- rearranged during transfection
- PTP
- protein tyrosine phosphatase
- pRTK
- receptor tyrosine kinase phosphorylation
- RPI-1
- 1,3-dihydro-5,6-dimethoxy-3-[(4-hydroxyphenyl)methylene]-H-indol-2-one)
- Rh-EGF
- rhodamine-labeled EGF.
REFERENCES
- 1. Roskoski R. (2004) The ErbB/HER receptor protein-tyrosine kinases and cancer. BBRC 319, 1–11 [DOI] [PubMed] [Google Scholar]
- 2. Gullick W. J. (1991) Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br Med. Bull. 47, 87–98 [DOI] [PubMed] [Google Scholar]
- 3. Sorenson C. M., Sheibani N. (2002) Altered regulation of SHP-2 and PTP 1B tyrosine phosphatases in cystic kidneys from bcl-2 -/- mice. Am. J. Physiol. Renal. Physiol. 282, 442–450 [DOI] [PubMed] [Google Scholar]
- 4. Tiganis T. (2002) Protein Tyrosine Phosphatases: Dephosphorylating the Epidermal Growth Factor Receptor. IUBMB Life 53, 3–14 [DOI] [PubMed] [Google Scholar]
- 5. Shah U., Sharpless N. E., Hayes D. N. (2008) LKB1 and lung cancer: more than the usual suspects. Cancer Res. 68, 3562–3565 [DOI] [PubMed] [Google Scholar]
- 6. Zhang S., Schafer-Hales K., Khuri F. R., Zhou W., Vertino P. M., Marcus A. I. (2008) The Tumor Suppressor LKB1 Regulates Lung Cancer Cell Polarity by Mediating cdc42 Recruitment and Activity. Cancer Res. 68, 740–748 [DOI] [PubMed] [Google Scholar]
- 7. Zhuang Z. G., Di G. H., Shen Z. Z., Ding J., Shao Z. M. (2006) Enhanced expression of LKB1 in breast cancer cells attenuates angiogenesis, invasion, and metastatic potential. Mol. Cancer Res. 4, 843–849 [DOI] [PubMed] [Google Scholar]
- 8. Contreras C. M., Gurumurthy S., Haynie J. M., Shirley L. J., Akbay E. A., Wingo S. N., Schorge J. O., Broaddus R. R., Wong K. K., Bardeesy N., Castrillon D. H. (2008) Loss of Lkb1 provokes highly invasive endometrial adenocarcinomas. Cancer Res. 68, 759–766 [DOI] [PubMed] [Google Scholar]
- 9. Wingo S. N., Gallardo T. D., Akbay E. A., Liang M. C., Contreras C. M., Boren T., Shimamura T., Miller D. S., Sharpless N. E., Bardeesy N., Kwiatkowski D. J., Schorge J. O., Wong K. K., Castrillon D. H. (2009) Somatic LKB1 mutations promote cervical cancer progression. PLoS One 4, e5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahoney C. L., Choudhury B., Davies H., Edkins S., Greenman C., Haaften G. v., Mironenko T., Santarius T., Stevens C., Stratton M. R., Futreal P. A. (2009) LKB1/KRAS mutant lung cancers constitute a genetic subset of NSCLC with increased sensitivity to MAPK and mTOR signalling inhibition. Br. J. Cancer 100, 370–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ding L., Getz G., Wheeler D. A., Mardis E. R., McLellan M. D., Cibulskis K., Sougnez C., Greulich H., Muzny D. M., Morgan M. B., Fulton L., Fulton R. S., Zhang Q., Wendl M. C., Lawrence M. S., Larson D. E., Chen K., Dooling D. J., Sabo A., Hawes A. C., Shen H., Jhangiani S. N., Lewis L. R., Hall O., Zhu Y., Mathew T., Ren Y., Yao J., Scherer S. E., Clerc K., Metcalf G. A., Ng B., Milosavljevic A., Gonzalez-Garay M. L., Osborne J. R., Meyer R., Shi X., Tang Y., Koboldt D. C., Lin L., Abbott R., Miner T. L., Pohl C., Fewell G., Haipek C., Schmidt H., Dunford-Shore B. H., Kraja A., Crosby S. D., Sawyer C. S., Vickery T. (2008) Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsumoto S., Iwakawa R., Takahashi K., Kohno T., Nakanishi Y., Matsuno Y., Suzuki K., Nakamoto M., Shimizu E., Minna J. D., Yokota J. (2007) Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene 26, 5911–5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nony P., Gaude H., Rossel M., Fournier L., Rouault J. P., Billaud M. (2003) Stability of the Peutz-Jeghers syndrome kinase LKB1 requires its binding to the molecular charperones Hsp90/Cdc37. Oncogene 22, 9165–9175 [DOI] [PubMed] [Google Scholar]
- 14. Sanchez-Cespedes M. (2007) A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene 26, 7825–7832 [DOI] [PubMed] [Google Scholar]
- 15. Shaw R. J., Bardeesy N., Manning B. D., Lopez L., Kosmatka M., DePinho R. A., Cantley L. C. (2004) The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6, 91–99 [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez-Nieto S., Sanchez-Cespedes M. (2009) BRG1 and LKB1: tales of two tumor suppressor genes on chromosome 19p and lung cancer. Carcinogenesis 30, 547–554 [DOI] [PubMed] [Google Scholar]
- 17. Ylikorkala A., Rossi D. J., Korsisaari N., Luukko K., Alitalo K., Henkemeyer M., Mäkelä T. P. (2001) Vascular Abnormalities and deregulation of VEGF in Lkb1-Deficient Mice. Science 293, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 18. Fan D., Ma C., Zhang H. (2009) The molecular mechanisms that underlie the tumor suppressor function of LKB1. Acta Biochim. Biophys. Sin. 41, 97–107 [DOI] [PubMed] [Google Scholar]
- 19. Boudeau J., Baas A. F., Deak M., Morrice N. A., Kieloch A., Schutkowski M., Prescott A. R., Clevers H. C., Alessi D. R. (2003) MO25α/β interact with STRADα/β enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 22, 5102–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boudeau J., Scott J. W., Resta N., Deak M., Kieloch A., Komander D., Hardie D. G., Prescott A. R., van Aalten D. M., Alessi D. R. (2004) Analysis of the LKB1-STRAD-MO25 complex. J. Cell Sci. 117, 6365–6375 [DOI] [PubMed] [Google Scholar]
- 21. Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. (2004) LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hardie D. G., Carling D., Gamblin S. J. (2011) AMP-activated protein kinase: also regulated by ADP? Trends Biochem. Sci. 36, 470–477 [DOI] [PubMed] [Google Scholar]
- 23. Karuman P., Gozani O., Odze R. D., Zhou X. C., Zhu H., Shaw R., Brien T. P., Bozzuto C. D., Ooi D., Cantley L. C., Yuan J. (2001) The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol. Cell 7, 1307–1319 [DOI] [PubMed] [Google Scholar]
- 24. Xie Z., Dong Y., Zhang J., Scholz R., Neumann D., Zou M. (2009) Identification of the Serine 307 of LKB1 as a Novel Phosphorylation Site Essential for Its Nucleocytoplasmic Transport and Endothelial Cell Angiogenesis. Mol. Cell Biol. 29, 3582–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sordella R., Bell D. W., Haber D. A., Settleman J. (2004) Gefitinib-Sensitizing EGFR Mutations in Lung Cancer Activate Anti-Apoptotic Pathways. Science 305, 1163–1167 [DOI] [PubMed] [Google Scholar]
- 26. Hardie D. G. (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 744–785 [DOI] [PubMed] [Google Scholar]
- 27. Amit I., Citri A., Shay T., Lu Y., Katz M., Zhang F., Tarcic G., Siwak D., Lahad J., Jacob-Hirsch J., Amariglio N., Vaisman N., Segal E., Rechavi G., Alon U., Mills G. B., Domany E., Yarden Y. (2007) A module of negative feedback regulators defines growth factor signaling. Nat. Genet. 39, 503–512 [DOI] [PubMed] [Google Scholar]
- 28. Ji H., Ramsey M. R., Hayes D. N., Fan C., McNamara K., Kozlowski P., Torrice C., Wu M. C., Shimamura T., Perera S. A., Liang M. C., Cai D., Naumov G. N., Bao L., Contreras C. M., Li D., Chen L., Krishnamurthy J., Koivunen J., Chirieac L. R., Padera R. F., Bronson R. T., Lindeman N. I., Christiani D. C., Lin X., Shapiro G. I., Jänne P. A., Johnson B. E., Meyerson M., Kwiatkowski D. J., Castrillon D. H., Bardeesy N., Sharpless N. E., Wong K. K. (2007) LKB1 modulates lung cancer differentiation and metastasis. Nature 448, 807–811 [DOI] [PubMed] [Google Scholar]
- 29. Ostman A., Frijhoff J., Sandin A., Böhmer F. D. (2011) Regulation of protein tyrosine phosphatases by reversible oxidation. J. Biochem. 150, 345–356 [DOI] [PubMed] [Google Scholar]
- 30. Meng T. C., Lou Y. W., Chen Y. Y., Hsu S. F., Huang Y. F. (2006) Cys-oxidation of protein tyrosine phosphatases: its role in regulation of signal transduction and its involvement in human cancers. J. Cancer Mol. 2, 9–16 [Google Scholar]
- 31. Cardaci S., Filomeni G., Ciriolo M. R. (2012) Redox implications of AMPK-mediated signal transduction beyond energetic clues. J. Cell Sci. 125, 2115–2125 [DOI] [PubMed] [Google Scholar]
- 32. Emerling B. M., Weinberg F., Snyder C., Burgess Z., Mutlu G. M., Viollet B., Budinger G. R., Chandel N. S. (2009) Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic. Biol. Med. 46, 1386–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poels J., Spasić M. R., Callaerts P., Norga K. K. (2009) Expanding roles for AMP-activated protein kinase in neuronal survival and autophagy. Bioessays 31, 944–952 [DOI] [PubMed] [Google Scholar]
- 34. Piantadosi C. A. (2012) Regulation of mitochondrial process by protein S-nitrosylation. Biochim. Biophys. Acta 1820, 712–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tennant D. A., Durán R. V., Gottlieb E. (2010) Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer 10, 267–277 [DOI] [PubMed] [Google Scholar]
- 36. Trachootham D., Alexandre J., Huang P. (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 [DOI] [PubMed] [Google Scholar]
- 37. Waterman H., Yarden Y. (2001) Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 490, 142–152 [DOI] [PubMed] [Google Scholar]
- 38. Puri N., Salgia R. (2008) Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J. Carcinog. 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kinch M. S., Moore M. B., Harpole D. H., Jr. (2003) Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin. Cancer Res. 9, 613–618 [PubMed] [Google Scholar]
- 40. Faoro L., Singleton P. A., Cervantes G. M., Lennon F. E., Choong N. W., Kanteti R., Ferguson B. D., Husain A. N., Tretiakova M. S., Ramnath N., Vokes E. E., Salgia R. (2010) EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival, cell invasion, focal adhesions, and mammalian target of rapamycin activation. J. Biol. Chem. 285, 18575–18585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ju Y. S., Lee W. C., Shin J. Y., Lee S., Bleazard T., Won J. K., Kim Y. T., Kim J. I., Kang J. H., Seo J. S. (2012) A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 22, 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Finn A. J., Feng G., Pendergast A. M. (2003) Postsynaptic requirement for Abl kinases in assembly of the neuromuscular junction. Nat. Neurosci. 6, 717–723 [DOI] [PubMed] [Google Scholar]
- 43. Fernández A. M., Kim J. K., Yakar S., Dupont J., Hernandez-Sanchez C., Castle A. L., Filmore J., Shulman G. I., Le Roith D. (2001) Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 15, 1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nitert M. D., Chisalita S. I., Olsson K., Bornfeldt K. E., Arnqvist H. J. (2005) IGF-I/insulin hybrid receptors in human endothelial cells. Mol. Cell. Endocrinol. 229, 31–37 [DOI] [PubMed] [Google Scholar]
- 45. Varewijck A. J., Yki-Järvinen H., Schmidt R., Tennagels N., Janssen J. A. (2013) Concentrations of insulin glargine and its metabolites during long-term insulin therapy in type 2 diabetic patients and comparison of effects of insulin glargine, its metabolites, IGF-I, and human insulin on insulin and igf-I receptor signaling. Diabetes 62, 2539–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]