Background: HARE mediates systemic clearance of 14 ECM- or stress-derived ligands. Hyaluronan uptake activates NF-κB.
Results: Heparin, dermatan sulfate, and acetylated LDL also activated NF-κB; chondroitin sulfates types A, C, D, and E did not.
Conclusion: A subset of HARE ligands activates signaling and gene expression.
Significance: HARE may be a systemic tissue-stress sensor that responds to abnormal ECM turnover and damage.
Keywords: Cell Signaling, Extracellular Matrix, Ligand-binding Protein, NF-κB Transcription Factor, Scavenger Receptor, Luciferase, Stabilin-2
Abstract
The hyaluronan (HA) receptor for endocytosis (HARE; Stab2) clears 14 systemic ligands, including HA and heparin. Here, we used NF-κB promoter-driven luciferase reporter assays to test HARE-mediated intracellular signaling during the uptake of eight ligands, whose binding sites in the HARE ectodomain were mapped by competition studies (Harris, E. N., and Weigel, P. H. (2008) Glycobiology 18, 638–648). Unique intermediate size Select-HATM, heparin, dermatan sulfate, and acetylated LDL stimulated dose-dependent HARE-mediated NF-κB activation of luciferase expression, with half-maximal values of 10–25 nm. In contrast, chondroitin sulfate types A, C, D, and E did not stimulate NF-κB activation. Moreover, degradation of endogenous IkB-α (an NF-κB inhibitor) was stimulated only by the signaling ligands. The stimulatory activities of pairwise combinations of the four signaling ligands were additive. The four nonstimulatory chondroitin sulfate types, which compete for HA binding, also effectively blocked HA-stimulated signaling. Clathrin siRNA decreased clathrin expression by ∼50% and completely eliminated NF-κB-mediated signaling by all four ligands, indicating that activation of signaling complexes occurs after endocytosis. These results indicate that HARE not only binds and clears extracellular matrix degradation products (e.g. released normally or during infection, injury, tumorigenesis, or other stress situations) but that a subset of ligands also serves as signaling indicator ligands. HARE may be part of a systemic tissue-stress sensor feedback system that responds to abnormal tissue turnover or damage as a danger signal; the signaling indicator ligands would reflect the homeostatic status, whether normal or pathological, of tissue cells and biomatrix components.
Introduction
Glycosaminoglycans (GAGs)2 are long unbranched polysaccharides of repeating disaccharide units covalently attached to the core protein of a proteoglycan (except for HA). GAGs are a major component of the extracellular matrix (ECM) that can affect the activity and stability of proteins and signaling molecules within the matrix. Both soluble and insoluble GAGs interact with numerous proteins, including proteases, adhesion molecules, growth factors, and cytokines, and participate in the regulation of multiple cellular events, such as cell adhesion, proliferation, differentiation, and migration (1). GAGs can bind to growth factors and cell surface receptors, thereby affecting the cell function and morphology of cells (2, 3). ECM remodeling is important for several biological activities such as normal embryonic development, wound healing, and tumorigenesis. During tumor progression, soluble GAGs or fragments of GAG chains are released due to abnormal turnover of ECM, a process highly controlled by the coordinated actions of proteases and endoglycosidases (4). The altered ECM and released GAG chains cannot only contribute to angiogenesis and tumor invasion but also regulate the release of proangiogenic growth factors and cytokines sequestered by GAGs. The soluble GAGs and growth hormones bind to various cell surface receptors and initiate numerous downstream cell signaling pathways.
HARE/Stab2 is a single transmembrane protein preferentially and highly expressed in the sinusoidal endothelial cells (Secs) of liver and lymph node (5–8), the tissues responsible for systemic HA clearance. HARE/Stab2 is also highly expressed in Secs of spleen (5) and bone marrow (9), perhaps mediating local HA turnover, and is also found in macrophages (10), corneal and lens epithelium, mesenchymal heart valve cells, ependymal brain ventricle cells, prismatic epithelial cells covering renal papillae, and oviduct (11). HARE begins at Ser1135 and ends at the C-terminal Leu2551 of full-length Stab2 (5, 12), and this 190-kDa fully functional isoform is not a splice variant of Stab2; it is generated by proteolysis (13). The receptor was first characterized by Laurent and co-workers (14–17) as a systemic clearance receptor that removes HA and chondroitin sulfate (CS) from the vascular and lymphatic circulatory systems. We know now that HARE and Stab2 function as primary scavenger receptors for systemic clearance of 14 different ligands and also have other functions, including recently discovered cell signaling leading to downstream gene expression changes (10, 18, 19).
Although HARE is one of the scavenger receptors for systemic clearance of matrix components released during abnormal turnover or synthesis in many pathological situations, few studies have examined cell signaling activated by these matrix degradation products and their effects in cellular functions. Small-to-intermediate HA can induce cell signaling pathways and modulate biological responses such as angiogenesis, wound healing, and tumorigenesis (20–22). Park et al. (10) found that phagocytosis of apoptotic cells by activated macrophages is mediated by HARE/Stab2 binding to PS, and this interaction stimulates intracellular signaling leading to the synthesis and release of the anti-inflammatory cytokine TGF-β. We recently found that HARE binding to some, but not all, sizes of HA can stimulate cell signaling, leading to activation of the MAPK ERK1/2 and NF-κB-mediated gene expression; both responses occur in a dose- and time-dependent manner (18, 19). We characterized the HA sizes that can activate HARE-mediated cell signaling, and we found that only HARE uptake of small-intermediate size HA (40–400 kDa) activates downstream cell signaling pathways (18, 19). Although HARE binds to and clears 12 other ligands (other than HA and PS), including Hep, DS, and CS types and AcLDL (7, 12, 23, 24), no studies have been reported on HARE-mediated cell signaling in response to these ligands.
Here, we used a Dual-LuciferaseTM (LUC) reporter assay to test eight different HARE ligands (HA, Hep, DS, CS types A, C, D, and E, and AcLDL) for their ability to stimulate HARE-mediated NF-κB activation of gene expression. Four HARE ligands (HA, Hep, DS, and AcLDL) stimulated NF-κB activation of gene expression in a dose-dependent manner. These four ligands also stimulated endogenous HARE-mediated NF-κB activation as assessed by degradation of IκB-α. Mixtures of HA, Hep, DS, and AcLDL showed additive stimulatory effects. In contrast, the CS types A, C, D, and E did not stimulate HARE-mediated NF-κB activation. In fact, these nonsignaling CS types, which compete for HA binding to HARE, blocked HA-stimulated cell signaling. Clathrin siRNA treatment decreased clathrin expression by ∼50% and eliminated NF-κB-mediated signaling by the four ligands, indicating that endocytosis is required to activate HARE-ligand signaling complexes. These and previous findings on uptake (7, 12, 23, 24) and cell signaling (18) show that HARE not only binds and clears ECM degradation products but that HARE-mediated signaling and gene activation may be part of a feedback response system that senses abnormal biomatrix turnover as a danger signal. These findings support the possible role of HARE as a “tissue-stress sensor” during various physiological challenges (25).
EXPERIMENTAL PROCEDURES
Cells, Plasmids, and Reagents
HEK Flp-In 293 cells, Lipofectamine 2000, Lipofectamine LTX and PLUS reagents, glutamate, plasmid expression vectors, super-competent TOP10 Escherichia coli, Zeocin, hygromycin B, DMEM, and FBS were from Invitrogen. Stable Flp-In 293 (HEK) cell lines expressing 190-kDa human HARE or empty vector (EV) were generated as described previously (18, 23, 26). These cells are engineered to contain a selectable recombinase-mediated insertion site, and the cell lines used were verified to contain a single insertion at the correct site. Plasmid vector pGL4.32(luc2P/NF-κB-RE/Hygro), Dual-Luciferase reporter assay system (E1960), and Glomax 20/20 luminometer were from Promega (Madison, WI). Renilla LUC plasmid pRL-TK was kindly provided by Dr. K. Mark Coggeshall (Oklahoma Medical Research Foundation). Limulus amebocyte lysate reagent (Endosafe KTA0.03 endotoxin units/ml) was from Charles River (Charleston, SC). Monodisperse Select-HATM of different sizes was purchased from Hyalose (Oklahoma City, OK). Clinical grade hyaluronan (HA) made by streptococcal fermentation was from Genzyme (Cambridge, MA) or Lifecore (Chaska, MN), and various small, intermediate, or large HA preparations with narrow polydispersity were prepared by SEC fractionation and analyzed by SEC-MALLS (18, 23). Unfractionated Hep was from Celsus (Cincinnati, OH) or Sigma. CS-A, CS-C, CS-D, and CS-E were from Seikagaku (Japan); Streptomyces hyalurolyticus hyaluronidase (lyase), chloroquine diphosphate, and DS were from Sigma, and AcLDL was from Kalen Biomedicals (Montgomery, MD). Chemically sulfated bacterially derived CS-C (verified by fluorophore-assisted carbohydrate electrophoresis analysis) synthesized by Choncept (Oklahoma City, OK) was a gift from Dr. Paul L. DeAngelis. Antibodies to IκB-α (mouse monoclonal), α-actin (mouse monoclonal), and clathrin heavy chain (rabbit polyclonal antibody) were from Cell Signaling Technology (St. Louis, MO). Clathrin ON-TARGETplus SMART pool (L-004001-01) or siGENOME scramble-like nontargeting (D-001210-02) siRNAs were from Thermo Scientific (Waltham, MA). Nitrocellulose was from Millipore (Billerica, MA). Goat anti-rabbit IgG-HRP, donkey anti-goat IgG-HRP, and donkey anti-mouse IgG-HRP were from Santa Cruz Biotechnology (Santa Cruz, CA). Sodium 125I-iodide was from American Radiolabeled Chemicals (St. Louis, MO), and 125I-HA was prepared as described previously (27). Other materials, reagents, and kits were obtained as described recently (18). Unless specified, all other reagents were from Sigma.
Cell Culture Medium and Buffers
Compete Medium contained DMEM supplemented with 8% fetal bovine serum and 100 μg/ml hygromycin B. Transfection medium contained DMEM containing 8% fetal bovine serum without antibiotics. The compositions of PBS and other buffers were prepared as described previously (18).
Size-exclusion Chromatography, MALLS Analysis, and Endotoxin Detection
Various purified fractions of HA and samples of Hep, DS, and CS types were analyzed by SEC-MALLS to determine weight-average molar mass and size distribution (23, 28). The weight-average molar mass values (in parentheses) of the ligands used here were as follows: AcLDL (500 kDa); CS-A (14 kDa); commercial CS-C (41 kDa); synthetic CS-C (110 kDa); CS-D (36 kDa); CS-E (128 kDa); DS (17 kDa); HA (51, 107, and 133 kDa), and Hep (14 kDa). Endotoxin levels were determined using the Limulus amebocyte lysate assay. Endotoxin levels in all purified HA samples were <1 endotoxin unit/mg.
Cell Culture and Transient Transfection with LUC Plasmids
HEK Flp-In 293 cells stably expressing HARE (WT) or EV were grown to confluence in Complete Medium, plated in 12-well tissue culture plates, and maintained in Complete Medium for at least 48 h prior to experiments. At 50–60% confluence, the medium was replaced with Transfection Medium, 10 min prior to transfection. Transfection complexes were generated in serum-free medium by mixing Lipofectamine LTX and PLUS reagents with 1 μg/ml firefly LUC vector pGL4.32 (luc2P/NF-κB-RE/Hygro) and 0.5 μg/ml Renilla LUC vector (pRL-TK). Transiently transfected cells were grown for 18 h before use.
Stimulation of Cell Lines with Ligands
Cells expressing HARE or EV were transiently transfected with firefly and Renilla LUC vectors as above, washed once each with sterile PBS and DMEM without serum, and then preincubated with fresh serum-free DMEM for 1 h at 37 °C. Medium was aspirated, and cells were incubated with appropriate ligand resuspended in serum-free DMEM as indicated. To determine the HA, Hep, CS types, or AcLDL concentration dependence for HARE- and NF-κB-mediated reporter gene expression, cells were incubated at 37 °C for 4 h with increasing concentrations of low endotoxin containing ligands in DMEM. To assess the effects of two signaling ligands, the cells were incubated for 4 h with 5 nm of HA, Hep, DS, or AcLDL alone or with one of the six possible pairwise combinations (each at 5 mm) of the four signaling ligands. After the indicated time, the medium was aspirated, and cells were processed (below) to determine HARE-mediated NF-κB-activated gene expression.
Dual-LuciferaseTM Reporter Assays
After incubation with one or more HARE ligands or TNF-α (as a positive control) for 4 h, the cells were washed with sterile PBS, scraped, and harvested in serum-free medium. Cells were centrifuged at 12,000 × g for 1 min, and the supernatant was aspirated. Cell pellets were resuspended in 150 μl of serum-free medium and assayed for LUC activity by using a Dual-Luciferase assay system following the manufacturer's protocol. Expression of the firefly enzyme gene is controlled by a highly regulated NF-κB promoter, whereas the Renilla enzyme gene is constitutively expressed. NF-κB is normally inhibited by binding to IκB, which results in a cytoplasmic localization. The activation of NF-κB (e.g. by cytokines such as TNF-α) is due to phosphorylation of IκB, which leads to degradation of the protein (29). The decreased level of IκB then leads to the dissociation and activation of NF-κB, which in turn stimulates expression of genes regulated by NF-κB promoters, including the firefly LUC recorder gene. The level of firefly LUC reflects inducible expression mediated by activation of NF-κB, and the level of Renilla LUC reflects basal constitutive expression. The amount of firefly and Renilla LUC activity in each sample was measured and recorded as relative light units (RLU) using a Glomax 20/20 luminometer (Promega). The ratio of firefly LUC to Renilla LUC activities in each lysate sample was calculated and normalized to the untreated cells as control (defined as 1.0). The results are expressed as the mean ± S.E. fold-change in firefly/Renilla LUC activity, and the concentration dependence for HARE- and NF-κB-mediated reporter gene expression was plotted as a curve generated by nonlinear regression analysis using SigmaPlot version 10 (Systat Software, Inc., Point Richmond, CA).
Endocytosis of 125I-HA and Hyperosmolar or Chloroquine Treatment
HARE or EV cells were grown to confluence and processed for 125I-HA endocytosis as described previously (26, 30). Cells were preincubated with 0.45 m sucrose (hyperosmolar conditions) or 100 μm chloroquine in DMEM for 30 min at 37 °C. The cells were then incubated with inhibitors and 1 μg/ml 125I-HA for 3 h at 37 °C in the presence or absence of a 100-fold excess of unlabeled HA; specific HA uptake was calculated as fmol/106 cells (total uptake minus nonspecific uptake in the presence of excess HA).
IκB-α Degradation Assay
Cells expressing HARE were grown to confluence in 6-well plates, washed with sterile PBS, and incubated with serum-free DMEM for 1 h. Cells were then incubated with 1 ng/ml TNF-α, 100 nm HA, Hep, DS, or AcLDL or 250 nm CS-A, CS-C, CS-D, or CS-E for 45 min and processed as described previously (18). Equal amounts of cell lysate protein were resolved on 10% SDS-PAGE, transferred to nitrocellulose, and probed with anti-IκB-α antibody (IgG). The same membrane was reprobed for actin, and the ratios of IκB-α/actin in each sample were determined from the digital images.
Clathrin Knockdown with siRNA
HARE Flp-In 293 cells were transfected with 100 nm scramble-like control or human clathrin heavy chain siRNA in 1 ml of serum-free DMEM using Lipofectamine2000, according to the manufacturer's instructions. After 5 h at 37 °C, 5% CO2, medium was replaced with DMEM containing 8% FBS, and the cells were incubated for 2 days. Cells were harvested and analyzed for relative clathrin levels by resolving equal amounts of protein in SDS-PAGE followed by Western blotting using antibodies to actin and clathrin. For LUC reporter gene assays, the NF-κB and Renilla plasmids were transfected 2 days after siRNA treatment. LUC activity assays were performed 3 days after siRNA transfection.
Statistical Analysis
Data are presented as the mean ± S.E. based on three independent experiments, each performed in triplicate (n = 9), unless noted otherwise. For statistical comparison, data were first analyzed by a one-way analysis of variance, and any significant difference in the group was then assessed by individual pairwise post hoc Tukey's HSD tests using GraphPad Prism version 5 statistical software (GraphPad Software, Inc., San Diego). Pairwise comparisons were made between EV and HARE cells treated with the same ligand concentration and then with HARE cells plus ligand versus EV cells without ligand. Only those sample sets considered statistically significant in both cases are indicated with a symbol (*, p < 0.05; **, p < 0.005; ***, p < 0.001).
RESULTS
HA Endocytosis by HARE Mediates NF-κB-activated Gene Expression
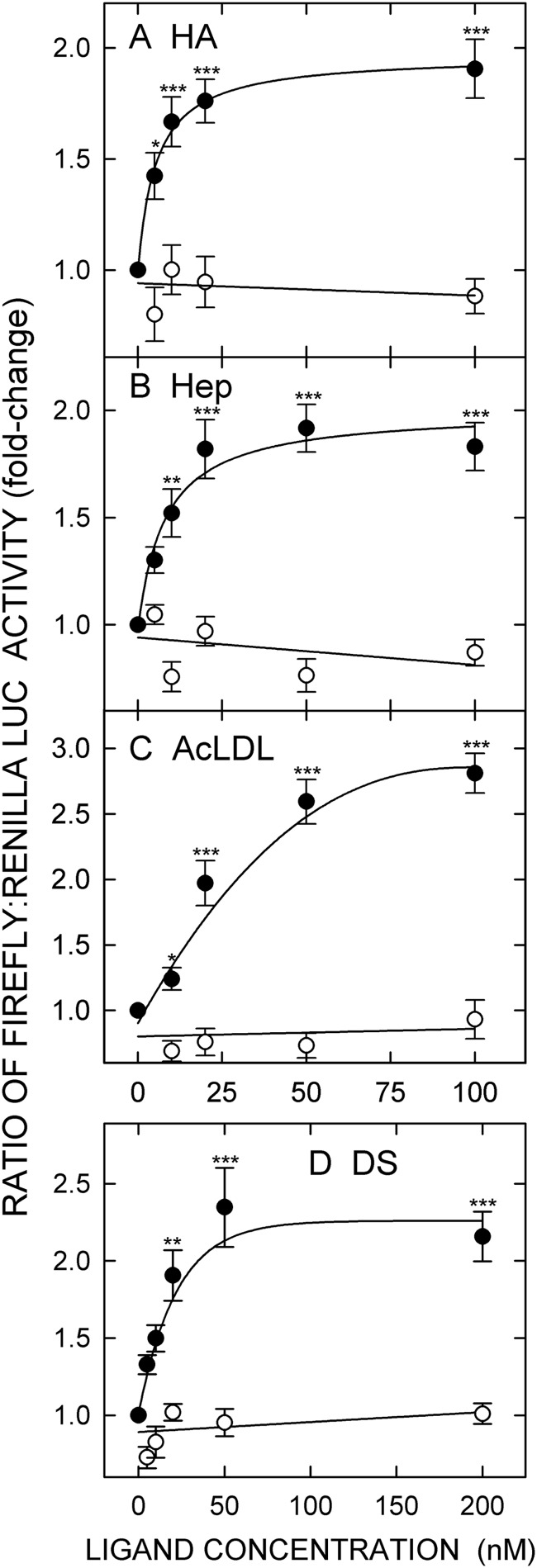
We determined for the first time the HA size dependence for HARE-mediated cell signaling using narrow dispersity (polydispersity, 1.05–1.15) HA samples purified by SEC fractionation and characterized by SEC-MALLS for their weight-average molar mass, size distribution, and concentration (18). We found that sHA-iHA of 40–400-kDa HA stimulates HARE-mediated ERK1/2 activation and NF-κB-activated gene expression. Here, we assessed the ability of a nearly monodisperse (PD = 1.01) 133-kDa Select-HATM to stimulate HARE-mediated NF-κB activation. NF-κB activation of recorder gene expression was stimulated in a dose-dependent manner in cells expressing HARE but not EV (Fig. 1A). The dose-response curve was hyperbolic and leveled off at an ∼2-fold stimulation at ≥20 nm (p < 0.001) with an apparent Km ∼10 nm, which is nearly identical to the dissociation constant for HA-HARE complexes determined in cells or with purified protein (13, 31). The results are consistent with the previous NF-κB activation observed using a narrow dispersity 107-kDa iHA preparation (18).
FIGURE 1.
HARE-mediated NF-κB-activated gene expression is stimulated in a dose-dependent manner by Select-HATM, heparin, acetylated LDL, and dermatan sulfate. Cells expressing HARE (●) or EV (○) were grown and transiently transfected with plasmids encoding firefly and Renilla LUC for 18 h in Transfection Medium. Cells were washed, incubated in serum-free medium for 1 h, washed again, and incubated for 4 h with the indicated concentration of: A, 133-kDa iHA (Select-HATM); B, unfractionated Hep; C, AcLDL; D, DS. Cells were then processed and analyzed for their relative ratios of the two LUC activities as described under “Experimental Procedures.” Results are normalized to the untreated control and expressed as a fold-change in the ratio of firefly-to-Renilla LUC activity. Values are means ± S.E. (n = 9) from three independent experiments. Values for p compare HARE and EV cells at each ligand concentration and HARE cells plus ligand versus EV cells without ligand. Only sample sets with significant differences in both cases are marked: *, p < 0.05; **, p < 0.005; ***, p < 0.001.
Endocytosis of Hep Stimulates HARE-mediated NF-κB-activated Gene Expression
To determine whether Hep can stimulate HARE-mediated NF-κB activated gene expression, we incubated HARE or EV cells with increasing concentrations of unfractionated Hep. HARE cells, but not EV cells, treated with Hep showed a dose-dependent activation of NF-κB-mediated gene expression (Fig. 1B). The response curve increased to ∼2-fold and leveled off at 50 nm (p < 0.001) with an apparent Km value of ∼20 nm. The response results are consistent with the binding affinity of unfractionated Hep for HARE (Kd ∼20–60 nm) as assessed using stable cell lines or purified receptor ectodomain (32). These and other ongoing findings3 indicate that Hep binding to, and uptake by, HARE stimulates both ERK1/2 phosphorylation and the NF-κB-mediated cell signaling pathway leading to altered gene expression.
AcLDL Endocytosis by HARE Stimulates NF-κB-activated Gene Expression
Multiple known and unknown scavenger receptors are involved in ∼95% of the uptake of modified LDL by macrophages via clathrin-mediated endocytosis (33). Binding of oxidized LDL or AcLDL to scavenger receptors such as SR-A, LOX-1, and MACRO induce cell signaling events (e.g. involving PI3K, MAPK, and NF-κB) and modulate various biological responses (34–36). HARE is the macrophage apoptotic cell receptor (10, 37) and also binds and internalizes modified LDLs via clathrin-mediated endocytosis (12, 24, 38), but its role in cell signaling has not been determined yet. To test this, we assessed the ability of AcLDL to stimulate HARE-mediated NF-κB-activated reporter gene expression (Fig. 1C). Interestingly, as for iHA and Hep, AcLDL also stimulated gene expression in a dose-dependent manner. The response curve showed a robust ∼3-fold increase in HARE-mediated reporter gene expression at ≥50 nm (p < 0.001) with half-maximal stimulation at ∼25 nm, compared with untreated HARE cells. In contrast, no signaling activity was detected in EV cells.
DS Endocytosis by HARE Stimulates NF-κB-activated Gene Expression
To explore further the HARE ligands that might stimulate HARE-mediated NF-κB activation, we treated HARE or EV cells with increasing concentrations of DS. HARE-mediated uptake of DS activated NF-κB and reporter gene expression in a dose-dependent manner, whereas no response occurred with EV cells (Fig. 1D). The dose response was also hyperbolic with a maximum stimulation of 2–2.5-fold at 50 nm (p < 0.001) and an apparent Km ∼20 nm.
The TNF-α responsiveness of the EV and HARE cell lines used here are identical, indicating that the NF-κB response is not affected by HARE expression (18). Recorder LUC gene expression levels stimulated by TNF-α are 20-fold at 0.5–1 ng/ml, compared with 2–3-fold stimulation mediated by HARE signaling in response to the four signaling ligands.
HARE Does Not Stimulate NF-κB Activation in Response to Endocytosis of CS-A, CS-C, CS-D, or CS-E
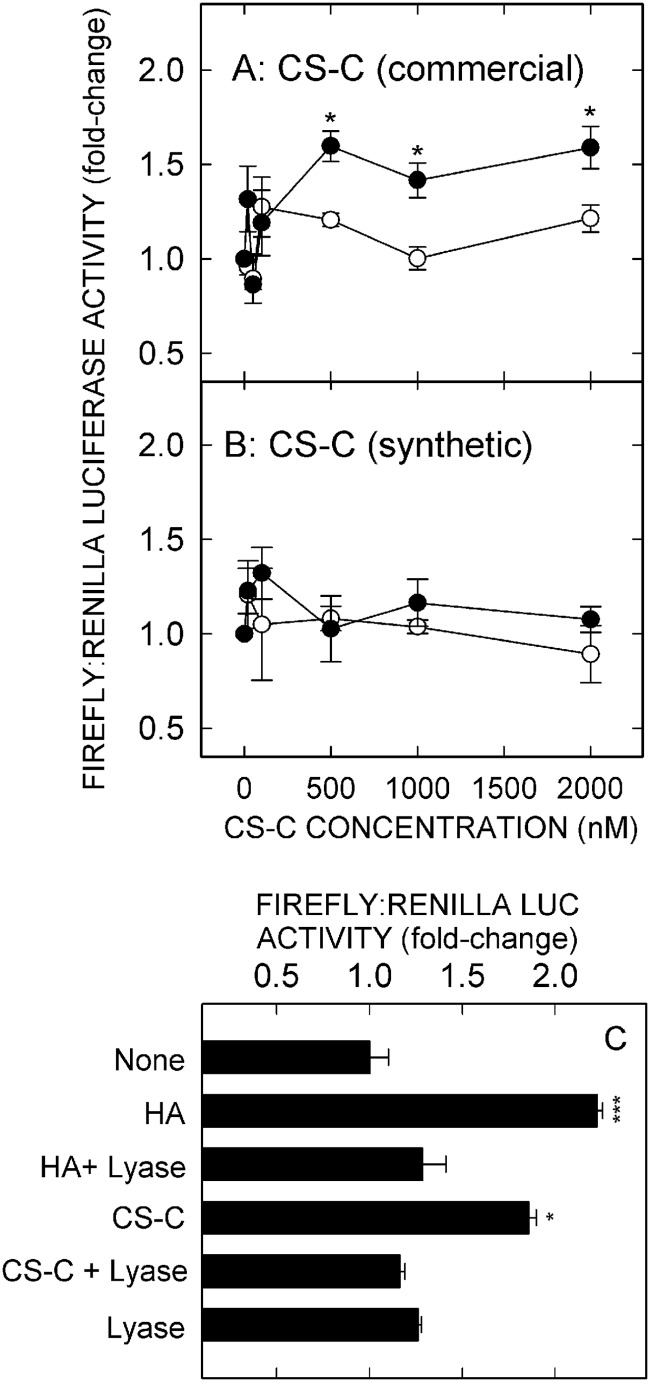
To characterize further the HARE ligands that stimulate NF-κB activation, we incubated HARE or EV cells with increasing concentrations of CS-A, CS-C, CS-D, or CS-E. Surprisingly, none of the CS types stimulated NF-κB-activated gene expression in either HARE or EV cells up to 100 nm, well above the saturating concentrations for the above four signaling ligands. We then explored higher concentrations of CS types A, D, and E (up to 2 μm), but no significant signaling was stimulated by these three CS types (Fig. 2, A–C). However, CS-C showed modest but significant (p < 0.05) HARE-mediated activation of NF-κB at 1–2 μm (Fig. 3A).
FIGURE 2.
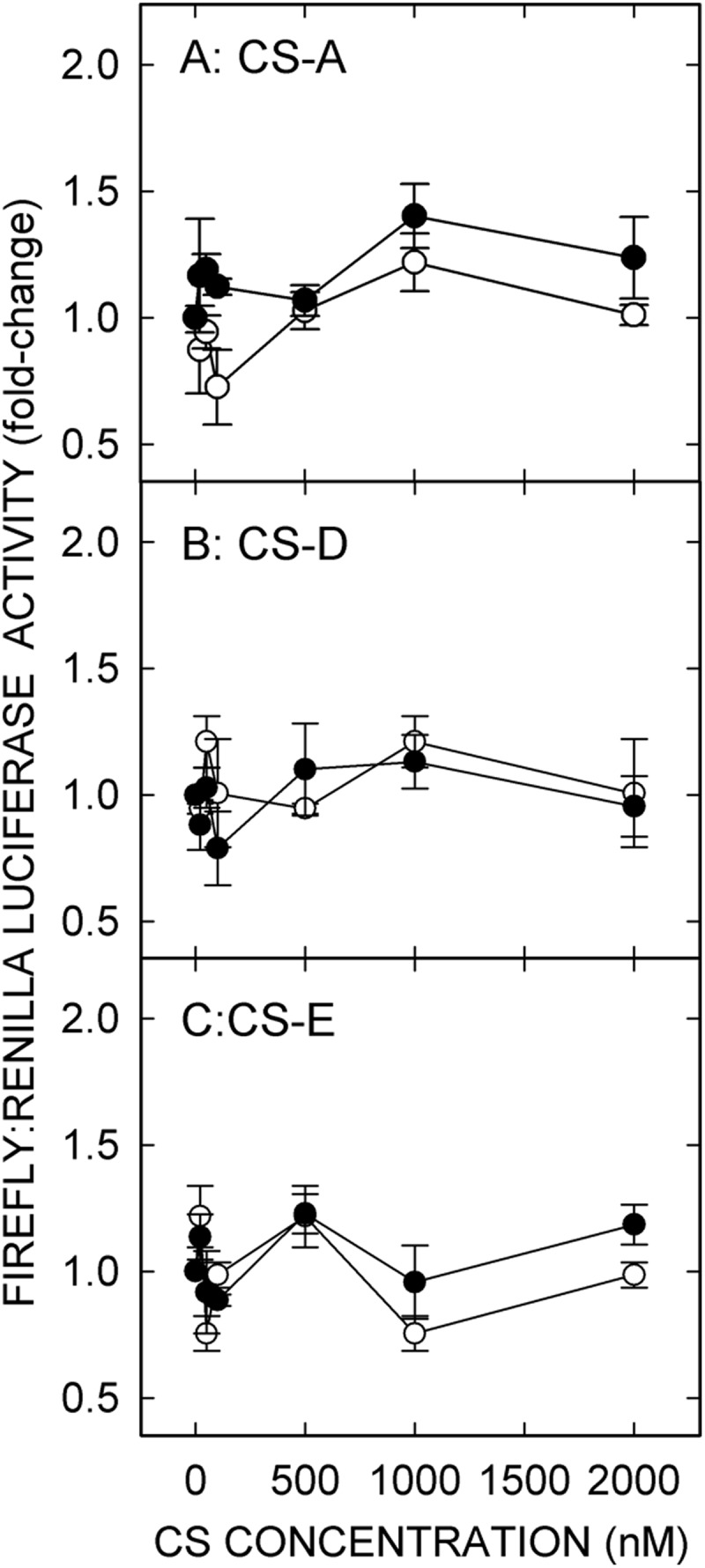
Chondroitin sulfate types A, D, and E do not stimulate NF-κB-mediated gene expression in HARE-expressing cells. HARE (●) or EV (○) cells were incubated with increasing concentrations of CS-A (A), CS-D (B), or CS-E (C) and processed for relative luciferase activities as in Fig. 1 (n = 9).
FIGURE 3.
HARE-mediated NF-κB activation by commercial chondroitin sulfate type C is due to contamination by signaling HA. HARE (●) or EV (○) cells were incubated with increasing concentrations of high purity synthetic CS-C (n = 6) (B) or commercial CS-C (A), either untreated or treated with S. hyalurolyticus hyaluronidase (C) and processed as in Fig. 1. Values are means ± S.E. (n = 6–9) from 2 to 3 independent experiments. Values for p compare WT with EV at each CS-C concentration shown: *, p < 0.05; ***, p < 0.0001.
Because such high concentrations of CS-C were needed to observe this modest increase in gene expression, we considered that the product, which was derived from shark cartilage and ∼90% pure, could be contaminated with a low level of signaling sHA-iHA (e.g. 1% contamination would be ∼10 nm HA at 1 μm CS-C). Two strategies were used to test the possibility that HA contamination in the CS-C was responsible for the signaling response. First, we found that high purity chemically synthesized CS-C did not activate NF-κB in HARE or EV cells, even at very high doses (Fig. 3B). Second, treatment of the CS-C preparation with the specific HA lyase from S. hyalurolyticus eliminated the stimulatory activity (Fig. 3C). These latter two results demonstrate that the modest HARE-mediated activation of NF-κB at high concentrations of CS-C derived from shark cartilage was due to HA contamination. Thus, all the above results demonstrate that unlike HA, Hep, DS, and AcLDL, none of the CS types tested can stimulate HARE-mediated NF-κB activation.
Stimulation of HARE-mediated NF-κB Activation by Hep, DS, and AcLDL Is Not Due to HA Contamination
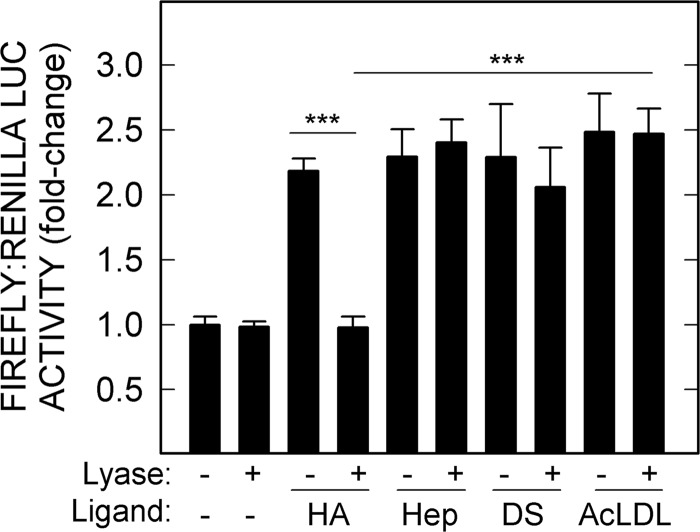
The possibility exists that Hep, DS, and AcLDL used in this study might have trace amounts of signaling HA as contamination, as noticed in commercial CS-C (Fig. 3). To test this, we treated HA, Hep, DS, and AcLDL preparations with the specific HA lyase to eliminate potential HA-dependent activity. As expected, we observed no decrease in stimulatory activity of Hep, DS, or AcLDL (Fig. 4). In contrast, the signaling activity of control HA was eliminated by the lyase (p < 0.001).
FIGURE 4.
HARE-mediated NF-κB activation by Hep, DS, or AcLDL is not due to HA contamination. HARE cells were incubated for 4 h with samples (20 nm) of 51-kDa HA, Hep, DS, or AcLDL, which had been treated with or without S. hyalurolyticus hyaluronidase, and processed for relative luciferase activities as described under “Experimental Procedures” and Fig. 1 (n = 9): ***, p < 0.001.
HA and Hep Stimulate HARE-mediated NF-κB Activation in an Additive Manner at Nonsaturating Concentrations
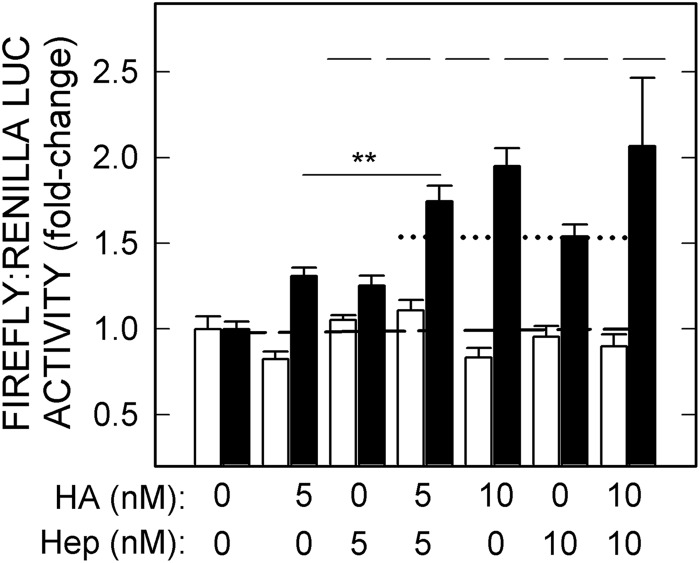
To investigate the additive or synergistic effects of the four ligands capable of stimulating HARE-mediated NF-κB-activated gene expression, we first tested ligand mixtures at concentrations giving maximal individual responses, as in Fig. 1. There were no additive responses under these saturating conditions, as seen in the example for HA and Hep at 10 nm (Fig. 5, top dashed line), indicating that at least in this model cell system the HARE-mediated signaling cascade leading to NF-κB-activated gene expression cannot respond to a second ligand if the first is already saturating the response. We then treated HARE or EV cells with 5 nm of either HA or Hep alone or combined (Fig. 5). The lower ligand concentrations, which individually gave significant but nonsaturating stimulation of HARE-mediated NF-κB activation in HARE but not EV cells, gave additive activation responses (Fig. 5, lower dotted line; p < 0.005) when combined, compared with the same concentrations alone.
FIGURE 5.
Effects of HA and Hep on HARE-mediated NF-κB activation are additive. EV (white bars) or HARE (black bars) cells were incubated with 5 or 10 nm 107-kDa HA (18) or Hep alone or in pairwise combinations and processed as in Fig. 1. Values are means ± S.E. (n = 9). The baseline for activation (1.0-fold) is indicated by a short dashed line; the calculated additive response at 5 nm is indicated by the dotted line, and the calculated additive response at 10 nm is the long dashed line (top). Values for p compare paired combinations at 5 nm with either HA or Hep alone for EV and WT: **, p < 0.005.
All Pairwise Combinations of HA, Hep, DS, and AcLDL Show Additive Effects for HARE-mediated NF-κB Activation
To investigate if other combinations of the four signaling ligands show similar additive or synergistic effects to the above HA-Hep result, HARE-mediated NF-κB-activated gene expression was determined with the remaining five different pairwise combinations of HA, Hep, DS, and AcLDL (Fig. 6, A–E). All pairwise combinations of the four signaling ligands at nonsaturating concentrations showed a similar additive activation as observed for HA and Hep. When two ligands were mixed, compared with either ligand alone, HARE-mediated NF-κB-activated gene expression was significantly increased to the level or above the level predicted to be an additive response (Fig. 6, A–E, dotted lines). Additive effects were seen for HA-Hep (p < 0.005, Fig. 5), HA-DS (p < 0.05, Fig. 6A), and HA-AcLDL (p < 0.005, Fig. 6B). The combinations of Hep-DS (p < 0.005, Fig. 6C), Hep-AcLDL (p < 0.005, Fig. 6D), and DS-AcLDL (p < 0.005, Fig. 6E) showed greater NF-κB-activated gene expression responses than the calculated additive response, indicating that these ligand combinations might be synergistic. The overall results demonstrate that HARE-mediated binding and internalization of combinations of HA, Hep, DS, and AcLDL can result in additive or synergistic NF-κB-activated gene expression.
FIGURE 6.
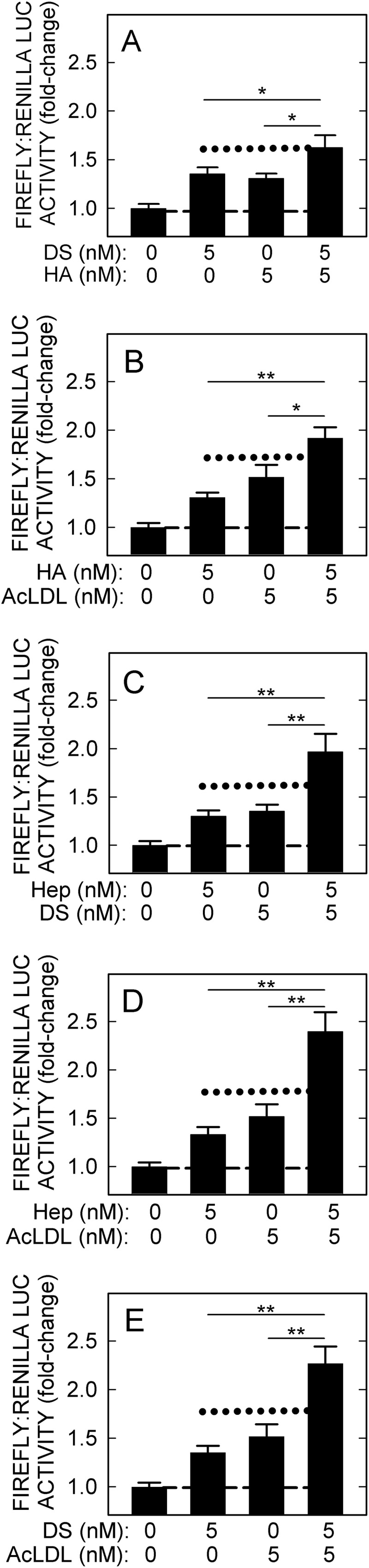
Pairwise combinations of the four signaling ligands are additive for HARE-mediated NF-κB activation. Cells expressing HARE were incubated with 5 nm of each signaling ligand alone or in pairwise combinations and processed as in Fig. 1. The pairwise combinations are as follows: A, HA and DS; B, HA and AcLDL; C, Hep and DS; D, Hep and AcLDL; and E, DS and AcLDL. Values are means ± S.E. (n = 9) from three independent experiments. The short dashed lines indicate the baseline (1.0-fold), and dotted lines represent the calculated additive responses. Values for p compare HARE cells treated with two ligands to cells treated with each signaling ligand alone: *, p < 0.05; **, p < 0.005.
HA-HARE Stimulation of NF-κB-mediated Gene Expression is Blocked by CS-A, CS-C, CS-D, or CS-E
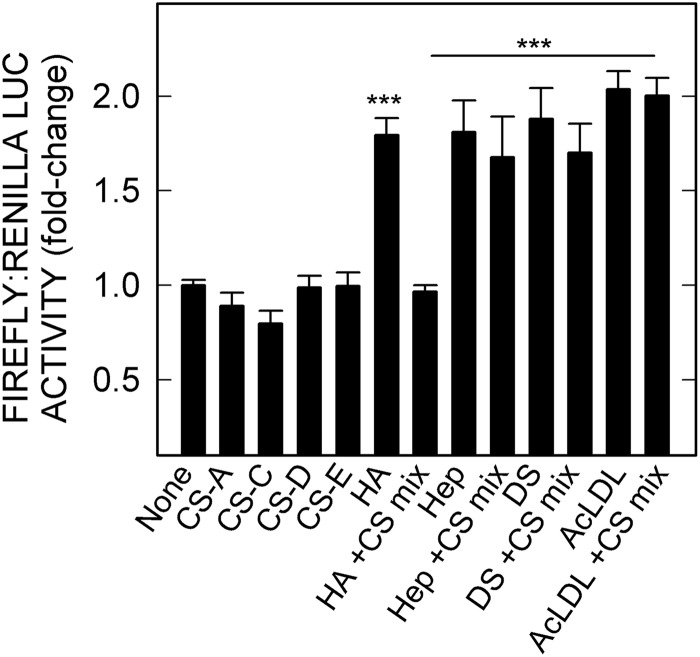
We previously reported that HARE binding to HA and various CS types requires the ∼93-amino acid Link domain and that HA can block CS binding or vice versa (24). Thus, the inability of the four CS types to stimulate NF-κB-activated gene expression led to a prediction that these GAGs would block HA-mediated activation of NF-κB, by inhibiting HA binding to HARE. This was first tested by adding increasing amounts of various CS types to cells expressing HARE in the presence of 10 nm HA (data not shown). The ∼1.8-fold NF-κB activation response stimulated by HA alone was decreased by either CS-A, CS-C, CS-D. or CS-E. In each case, the effective CS concentration needed to block HA-HARE-mediated signaling was relatively high (>500 nm), which is consistent with a lower affinity binding of HARE to the smaller CS types, compared with HA (31). Similarly, a mixture of the four nonsignaling CS types (250 nm each) significantly blocked HA-HARE-mediated cell signaling leading to NF-κB activation (p < 0.001, Fig. 7). In contrast, these four CS types are noncompeting for the other ligands and did not block HARE-mediated cell signaling and NF-κB activation stimulated by Hep, DS, or AcLDL.
FIGURE 7.
CS types A, C, D, and E block HA-stimulated HARE-mediated NF-κB activation of gene expression but not activation by Hep, DS, or AcLDL. HARE cells were incubated with 10 nm 51-kDa HA, Hep, DS, or AcLDL alone or in a mixture of the four CS types (250 nm each) and processed as in Fig. 1. Values for p compare 10 nm HA, Hep, DS, or AcLDL samples without versus with the CS mixture (***, p < 0.001; n = 9).
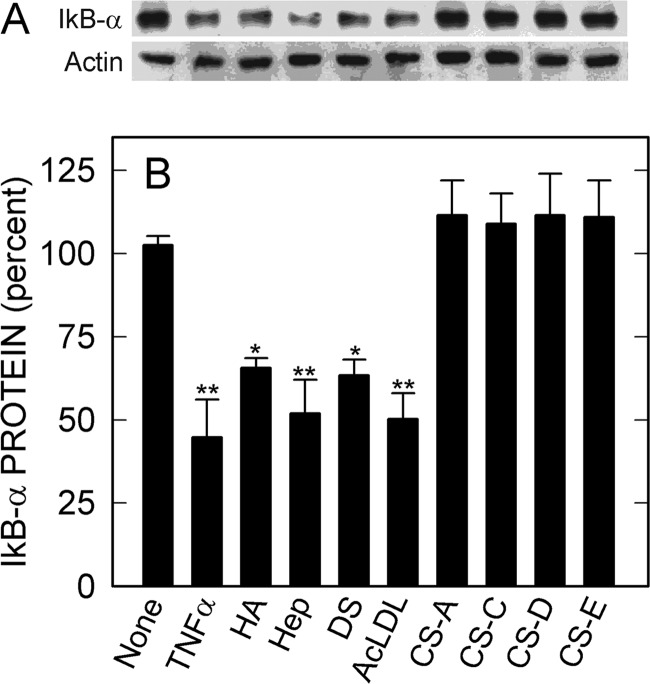
Signaling, but Not Nonsignaling, Ligands Stimulate Degradation of the Endogenous NF-κB Inhibitor IκB-α
In the absence of recorder gene plasmids, HA-HARE-mediated signaling leads to degradation of ∼50% of endogenous cellular IκB-α protein within 1 h (18). This result confirms that the relatively low level of signaling observed with the LUC recorder gene assay is robust enough to remove half of the NF-κB inhibitory protein even in the absence of the LUC plasmids. We then assessed the effects of the other signaling and nonsignaling ligands on degradation of endogenous IκB-α protein, with no LUC plasmids present (Fig. 8). The results confirm that all four signaling ligands stimulate HARE-mediated signaling by activating endogenous NF-κB pathways and thus validate the use of NF-κB promoter-based LUC gene expression assays to monitor signaling responses. Nonsignaling ligands were inactive.
FIGURE 8.
HARE-activating ligands stimulate degradation of endogenous IκB-α. HARE cells were grown, pretreated, and then incubated with 1 ng/ml TNFα, 100 nm signaling ligands (HA, Hep, DS, or AcLDL), or 250 nm nonsignaling ligands (CS-A, CS-C, CS-D, or CS-E) as described under “Experimental Procedures.” A, Western blot analyses were performed with an antibody (IgG) against IκB-α (upper panel) and, after stripping the membrane, with anti-actin antibody (IgG) (lower panel). B, blots from 3 to 4 independent experiments were digitized by scanning, and densitometric analysis was performed to determine the IκB-α/actin ratios for each sample. Normalized data for the IκB-α/actin ratios are presented as mean ± S.E. (n = 3–4) percent, relative to the no treatment value as 100%: *, p < 0.05; **, p < 0.005.
Signaling-competent HARE-Ligand Complexes May Be Intracellular
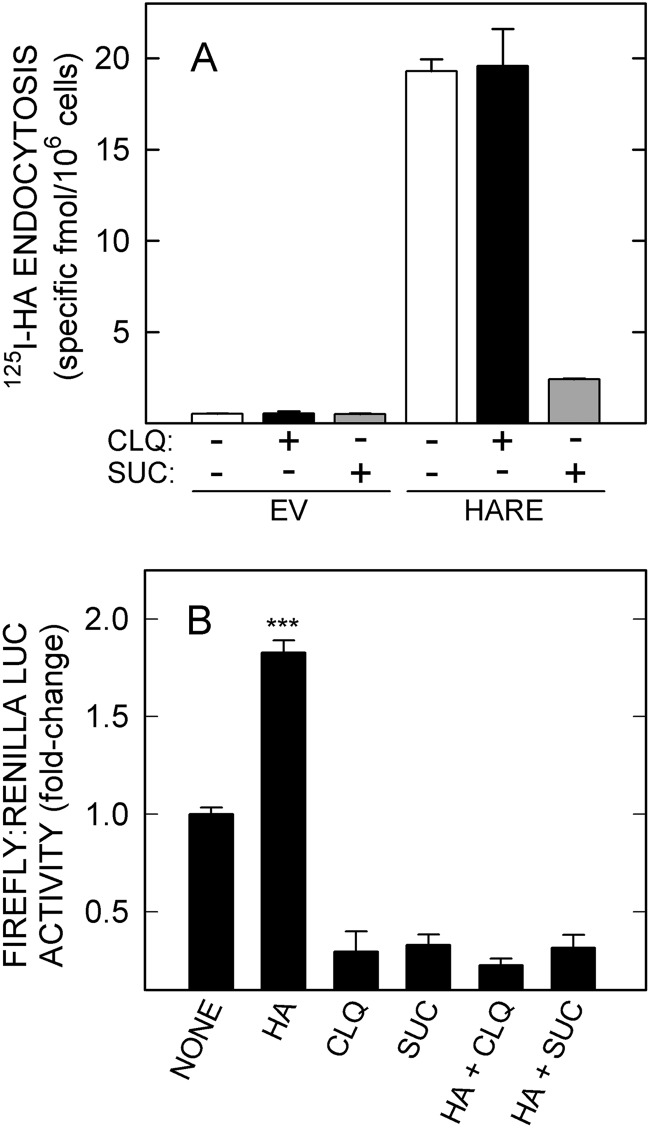
We tried multiple approaches to answer the basic question of whether active signaling complexes are at the cell surface, in endosomes, or both, including inhibition of signaling or coated pit dynamics using MEK inhibitors, dynasore, ammonium chloride, and dominant negative dynamin. In each case, confounding factors precluded obtaining meaningful results. As reported for HA-HARE interactions (18), for example, NF-κB signaling is altered even in EV control cells by solvents alone, DMSO or ethanol. A similar effect occurred using hyperosmolar sucrose, which disrupts clathrin assembly (39), or chloroquine, which disrupts intracellular pH gradients and vesicle trafficking (Fig. 9).
FIGURE 9.
Chloroquine or hyperosmolarity impairs basal NF-κB activation in the absence of ligand. A, EV or HARE cells were treated with medium only (white bars) or with medium plus 100 μm chloroquine (black bars) or 0.45 m sucrose (gray bars) for 30 min at 37 °C and then incubated with 1 μg/ml 125I-HA in the presence or absence of 100-fold excess unlabeled HA for 3 h at 37 °C before measuring specific 125I-HA uptake as described under “Experimental Procedures.” Values are means ± S.E. (n = 6) from two independent experiments. B, HARE cells were washed, incubated in serum-free medium for 1 h at 37 °C, washed again, and preincubated with 100 μm chloroquine (CLQ) or 0.45 m sucrose (SUC) for 30 min. The medium was aspirated, and cells were incubated for an additional 4 h with 20 nm HA (51 kDa), with or without the inhibitors as indicated and processed as in Fig. 1 for LUC expression. Values are means ± S.E. (n = 9) from three independent experiments; ***, p < 0.001.
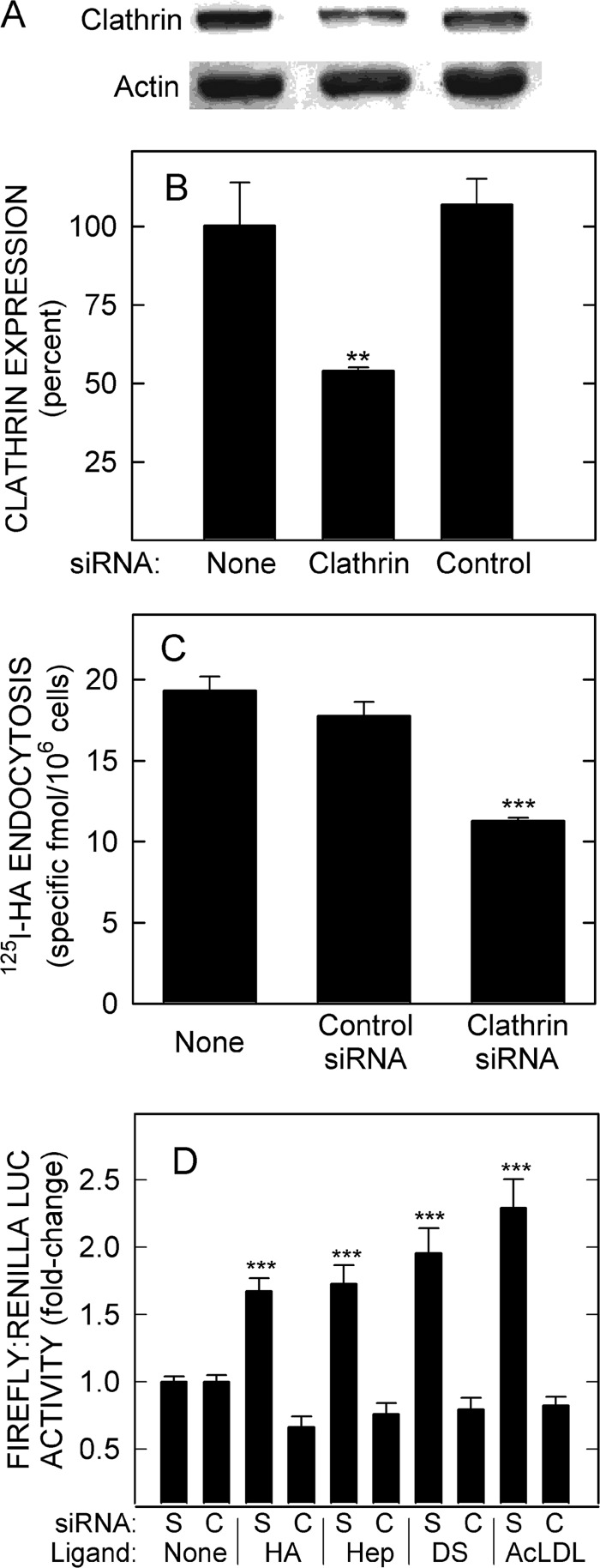
We then used siRNA to knock down clathrin heavy chain expression (Fig. 10A). Protein levels were decreased ∼50% by clathrin siRNA treatment (Fig. 10B; p < 0.005), with no effect by control siRNA. Cells treated with clathrin siRNA also showed a significant decrease (∼40%; p < 0.001) in the ability to endocytose 125I-HA (Fig. 10C). Clathrin knockdown also eliminated HARE-mediated NF-κB activation by HA as well as activation by Hep, DS, and AcLDL (Fig. 10D). The results indicate that the formation of signal-competent HARE-ligand complexes is dependent on coated pit-mediated endocytosis and thus occurs intracellularly, presumably in endosomes.
FIGURE 10.
Depletion of endogenous clathrin expression disrupts HARE-mediated endocytosis and NF-κB-activated gene expression. A, cells expressing HARE were transfected with siRNA targeting clathrin heavy chain or scramble-like control siRNA, cultured for 2 days, lysed, and processed for Western blotting of clathrin (upper panel) or actin (lower panel) as described under “Experimental Procedures.” B, blots from three independent experiments were digitized and quantified, and clathrin levels were expressed as the mean ± S.E. (n = 3) percent relative to untreated cells (as 100%). C, cells transfected with clathrin or control siRNA were washed, incubated in serum-free medium for 1 h, washed again, and incubated with 1 μg/ml 125I-HA in the presence or absence of a 100-fold excess of unlabeled HA for 3 h at 37 °C before measuring cell-associated 125I-HA. Values are the mean ± S.E. (n = 6) specific 125I-HA uptake. D, cells were transfected with scramble-like control (S) or clathrin (C) siRNA as above and then transfected on day 2 with firefly and Renilla LUC plasmids and cultured for 18 h. The cells were washed, pretreated, incubated with 20 nm HA, Hep, DS, or AcLDL for 4 h at 37 °C and then processed as in Fig. 1. Values are mean ± S.E. (n = 9) from three independent experiments. Statistical significance for B–D is as follows: **, p < 0.005; ***, p < 0.001.
DISCUSSION
Full-length Stab2 and 190-kDa HARE, generated by proteolysis, are the main scavenger receptors for the systemic clearance of at least 14 structurally different ligands as follows: HA, Hep, chondroitin, CS types (A, C, D, and E), DS, advanced glycation end products, AcLDL (and oxidized LDL), collagen N-terminal pro-peptides, αMβ2 integrin (23, 31, 38, 40–44), apoptotic cells (and debris) displaying clustered PS (10), and bacteria (42) via an unidentified surface ligand. Both receptor isoforms are strategically expressed on the two main cell types mediating macromolecular or particle turnover, mobile macrophages and fixed Secs. HARE/Stab2 are expressed at very high levels in fenestrated Secs of lymph nodes and liver; functional HARE copy number in primary rat liver Secs is 2–5 × 105 (45). Receptor-ligand complexes are rapidly endocytosed from blood and lymph by the coated pit mediated pathway, with receptor recycling times of <10 min; so this very high throughput receptor system is designed for rapid, large scale clearance. In addition, HARE is able to bind and internalize several different ligands simultaneously (e.g. HA and Hep), because there are multiple independent binding sites within the large ectodomain (24). Piggyback effects also contribute to the simultaneous uptake of ligands (e.g. hyaladherins bound to HA or growth factors bound to CS).
All the above ligands reflect normal processes (e.g. collagen biosynthesis-assembly, normal GAG turnover, and cell apoptosis) or abnormal processes (e.g. infection, oxidative damage resulting in modified LDL, and high circulating glucose resulting in glycated ligands) that might occur in one or more tissues at any time. The only ligand that may not be readily apparent as an indicator of tissue function or integrity is αMβ2 integrin, which is the major leukocyte integrin expressed on neutrophils. Although the physiological significance is not yet clear, αMβ2-HARE interactions mediate leukocyte adhesion to hepatic Secs and clearance of aged erythrocytes by hepatic Secs (41, 46, 47).
Tissue biomatrices contain dozens, perhaps hundreds, of different proteins (including splice variants) and a greater number of discrete proteoglycans (because different GAG chain length, sulfation, and attachment patterns on each core protein create many variants). Although PubMed lists almost 3900 articles (as of October 2013) under the search term “matrix homeostasis,” little is known about the normal synthesis and degradation (i.e. homeostasis) of any biomatrix. What we know in most cases is the turnover characteristics of an individual matrix component, either from a particular tissue or for the whole body. As with normal cells, each matrix component in a healthy tissue is characterized by specific ongoing and equal rates of both degradation and replacement by biosynthesis, so that the net amount of the component remains the same over relatively long times. Normal aging processes may slowly alter this balance, so that the amount of some matrix components increases and others decrease. However, an imminent threat to survival of an affected tissue(s) or the organism itself arises from processes that can rapidly alter this balance (e.g. over hours to months), such as wounding, infection, oxidative damage, extensive glycation, rapidly growing tumors, and metastasis. In the extreme case of tissue necrosis, all the cell and matrix components are being degraded, and the levels of the resulting degradation product “ligands” in the lymphatic and vascular circulations are much higher than normal.
Recycling clearance receptors (e.g. HARE, LDL, mannose, and asialoglycoprotein receptors) are generally viewed as performing relatively uninteresting, although important, housekeeping functions by removing bio-trash molecules from the circulation and salvaging their building block monomers by lysosomal degradation (e.g. sugars, fatty acids, and amino acids). This latter ligand-recycling (monomer salvage) function is important because it allows mammals to survive brief periods of starvation, greatly reduces our daily calorie intake need, and avoids usually fatal storage diseases. In addition to these obvious functions, however, a system could have also developed for some clearance receptors to provide feedback about their ligand burden via ligand-dependent intracellular signals. For example, the macrophage scavenger receptor (34) and LDL receptor-related proteins, but not the LDL receptor, generate intracellular signals in response to ligand binding (48). Although the mannose and asialoglycoprotein recycling receptors do not signal in response to ligand binding and internalization, HARE clearly does.
As the list of biomatrix and tissue-stress ligands recognized by HARE/Stab2 grew, we recognized that these receptors might be uniquely able to monitor the levels of sets of ligands (i.e. pattern profiles) that could reflect normal or pathological processes in distant tissues. Testing this idea in vitro proved difficult, because freshly isolated rat liver Secs lose HARE mRNA and protein with a half-life of 1 day (45); we also found no established cell lines that express HARE, including those derived privately or commercially from rat or human liver Secs. Other cell lines derived from tissues known to express the protein in vivo (e.g. lens epithelial cells) also lose expression when established in culture. Even among the 21 of 63 NCI cell lines derived from tumors with high in vivo Stab2 levels, we found very low message and no detectable protein (e.g. in DU145, HCEC, K562, SKOV3, and U251 cells). Thus, we established Flp-In 293 cell lines expressing various HARE isoforms and variants (13, 18, 19, 26, 31) to study ligand binding, cellular processing, and possible signal transduction. We found that, in the absence of ligand, HARE is associated with the kinases ERK1/2, JNK, and p38 and that binding and uptake of HA, the first reported ligand (17, 49), stimulates HARE-mediated ERK1/2 activation (19). We then determined that gene expression changes could be stimulated by HARE uptake of HA, mediated by activation of NF-κB (18), and that this response was very dependent on the HA size. Strikingly, only sHA-iHA of 40–400 kDa stimulates cell signaling leading to both ERK1/2 activation and NF-κB-activated reporter gene expression. We do not know if these two signaling pathways are dependent, related, or separate parallel transduction processes with different cellular outcomes.
Here, we used a LUC reporter gene assay to determine whether any of the other GAG ligands or AcLDL can activate HARE-mediated gene expression. Hep, DS, and AcLDL stimulated NF-κB activated gene expression as effectively as, or to a greater extent than, HA. These four ligands also stimulated endogenous HARE-mediated NF-κB activation, as assessed by degradation of IκB-α. Multiple signaling ligands in combination were additive for HARE-mediated NF-κB activation of gene expression. In contrast, CS-A, CS-C, CS-D, and CS-E did not activate NF-κB or stimulate degradation of IκB-α and they actually blocked HA-mediated signaling. A previous study to map the binding sites of these eight ligands showed there are two discrete sites (24). HA, CS-A, CS-C, and CS-D compete for binding to the ∼93-amino acid Link domain region, and their binding is eliminated if the Link domain is deleted. In contrast, Hep, CS-E, DS, and AcLDL do not affect the binding of HA or the four nonsignaling CS ligands and only partially compete with one another to varying extents for binding to HARE. The ability of HARE to recognize some CS types but not others indicates a high degree of selectivity in the binding domains and is consistent with earlier findings (13, 24, 31) that not all GAGs are ligands for this receptor (e.g. keratan sulfate and heparan sulfate). HARE is able to differentiate among the sulfation patterns presented by various GAGs to enable selective binding and signaling responses.
The model to explain the HA size dependence for signaling (18) is that only an optimum HA length can cross-link two or more HARE proteins and stabilize interactions between their cytoplasmic domains. If HARE monomers (and thus their cytoplasmic domains) normally interact only weakly, then a bound HA of appropriate size could bring HARE monomers together to form a new stabilized cytoplasmic domain oligomer complex. This new protein complex can then be bound by signaling proteins. Unlike the findings for HA, however, there is no size dependence for signaling by Hep, DS, or AcLDL, although the latter is large enough to be multivalent and possibly engage multiple HARE proteins. The Hep used here is 14 kDa and the DS is 17 kDa; yet both small polymers signal as well as, or better than, 133-kDa HA. The inability of the CS types to signal is also not because they are too small, because CS-E is 128 kDa and synthetic CS-C is 110 kDa; both are appropriate sizes for active HA signaling. Thus, the HA size dependence for signaling appears to be a novel feature of HARE and other HA receptors.
Many signaling receptor-ligand complexes become activated only after coated pit-mediated endocytosis and endosome formation (50–52). HARE also appears to be similarly activated to signal competence only after internalization, based on the loss of signaling capability by clathrin siRNA knockdown (Fig. 10). Many alternative approaches to disrupt endocytosis and obtain supporting evidence for this conclusion were inconclusive, because they also altered basal NF-κB signaling, even in EV control cells.
The results regarding HA-mediated signaling and the nonsignaling CS types may indicate that HARE is designed to sense the ratio of HA and these four CS types, which would be most prevalent in CS proteoglycans. HA signaling may be designed to occur only when there is more HA turnover and degradation (resulting in smaller products) relative to CS. This may make physiological sense because the normal continuous and ongoing release of ≥1 MDa of HA from tissue matrices includes the release of bound proteoglycans (53); HA complexes with bound CS proteoglycans could present two ligands to HARE in lymph nodes, neither of which would be signaling (large HA and most CS types). Because the normal HA size in plasma is ∼100 kDa (i.e. signaling HA), a decrease in the normal ratio of signaling HA/inactive CS types could decrease signaling, whereas an increased ratio could increase signaling.
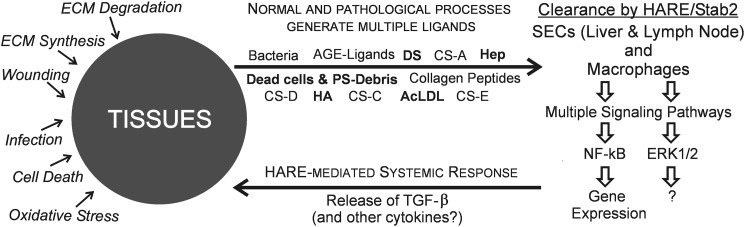
A recently proposed tissue-stress sensor hypothesis for HARE function (25) is well supported by the above results showing that HARE senses and responds (by intracellular signaling mechanisms) to at least five different ECM or stress indicator ligands during their clearance as follows: HA, Hep, DS, AcLDL, and apoptotic cells (10). Other HARE ligands, as yet untested, might also serve as indicator ligands in this model (Fig. 11), in which HARE continually senses and responds to the physiological status of cells and biomatrices in the body's tissues, as reflected in the pattern and amounts of ECM- and stress-related ligands in the lymphatic and vascular circulations. In addition to its well known uptake-clearance function, HARE can also capture information that reflects the steady-state levels of the key indicator ligands, their stability in tissues and their normal ongoing turnover (i.e. normal homeostasis). This whole body monitoring system could also detect elevated levels of these indicator ligands (reflecting abnormal ECM turnover and cell death) to recognize and respond to the causes of homeostasis disruption that alter tissue biomatrix composition or turnover or tissue cell viability (e.g. infection, wounds, tumorigenesis, and autoimmune or other diseases).
FIGURE 11.
Tissue-stress sensor model. The scheme depicts a feedback response system in which HARE senses abnormal biomatrix turnover to help tissues respond to various insults. The left and upper portions of the scheme illustrate the normal processes (e.g. homeostatic ECM synthesis and degradation) and abnormal or pathological processes (e.g. wounding, infection, or oxidative stress) that result in the release of ECM and cellular degradation products (e.g. HA, Hep, and dead cells or debris containing PS) into the lymphatic and vascular circulatory systems from tissues throughout the body. These different ligands are endocytosed and cleared by HARE expressed in migratory macrophages and stationary Secs of lymph node and liver. A subset of cleared ligands (i.e. HA, Hep, DS, AcLDL, and PS; in boldface), which may serve as indicator ligands, selectively initiate HARE responses that stimulate two cell signaling pathways as follows: activation of ERK1/2 and activation of NF-κβ-mediated gene expression (right portion of scheme). The relationship between these two signaling pathways is not known, but the outcome of HARE/Stab2 activation of NF-κB-mediated gene expression in macrophages is the synthesis and secretion of the anti-inflammatory cytokine TGF-β (10) that can then influence processes in distant tissues (lower portion of scheme). A prediction of this model is that additional cytokines and other factors will be identified as part of the HARE-mediated signaling and gene activation response by the two surveillance cell types.
A HARE/Stab2-mediated sensing system would use both a migratory surveillance component, macrophages, and fixed surveillance components, Secs, in liver and lymph nodes. Both cell surveillance components would coordinate and link multiple housekeeping (clearance) functions with intracellular responses through signal transduction and gene activation pathways. HARE/Stab2 is the macrophage apoptotic cell receptor, which binds PS patches on dead cells and debris; macrophages stimulate intracellular signaling and secretion of TGF-β in response to this interaction. Although we are unable to test HARE signaling in Secs, the activation of gene expression responses in cell lines expressing HARE indicates that the basic cellular machinery for signaling may be common among different cell types if the receptor is present. Further studies are needed to test the validity of the tissue-stress sensor model and to identify the cytokines and other proteins secreted in response to signaling during clearance of the HARE indicator ligands.
Acknowledgments
We thank Bruce Baggenstoss and Jennifer Washburn for HA reagents and technical support for experiments.
This work was supported, in whole or in part, by National Institutes of Health Grant GM69961 from NIGMS. This work was also supported by Oklahoma Center for the Advancement of Science and Technology Grant HR10-074 from the State of Oklahoma.
M. S. Pandey and P. H. Weigel, unpublished results.
- GAG
- glycosaminoglycan
- AcLDL
- acetylated low density lipoprotein
- CS
- chondroitin sulfate
- DS
- dermatan sulfate
- EV
- empty vector
- ECM
- extracellular matrix
- HA
- hyaluronic acid, hyaluronate, hyaluronan
- HARE
- hyaluronic acid receptor for endocytosis
- iHA
- intermediate HA (>100 to 1,000 kDa)
- Hep
- heparin
- LUC
- luciferase
- MALLS
- multiangle laser light scattering
- PS
- phosphatidylserine
- sHA
- small HA (>10 to 100 kDa)
- SEC
- size-exclusion chromatography
- Secs
- sinusoidal endothelial cells
- Stab2
- Stabilin-2.
REFERENCES
- 1. Handel T. M., Johnson Z., Crown S. E., Lau E. K., Proudfoot A. E. (2005) Regulation of protein function by glycosaminoglycans–as exemplified by chemokines. Annu. Rev. Biochem. 74, 385–410 [DOI] [PubMed] [Google Scholar]
- 2. Adams J. C., Watt F. M. (1993) Regulation of development and differentiation by the extracellular matrix. Development 117, 1183–1198 [DOI] [PubMed] [Google Scholar]
- 3. Vlodavsky I., Korner G., Ishai-Michaeli R., Bashkin P., Bar-Shavit R., Fuks Z. (1990) Extracellular matrix-resident growth factors and enzymes: possible involvement in tumor metastasis and angiogenesis. Cancer Metastasis Rev. 9, 203–226 [DOI] [PubMed] [Google Scholar]
- 4. Sanderson R. D., Yang Y., Kelly T., MacLeod V., Dai Y., Theus A. (2005) Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J. Cell. Biochem. 96, 897–905 [DOI] [PubMed] [Google Scholar]
- 5. Zhou B., Weigel J. A., Fauss L., Weigel P. H. (2000) Identification of the hyaluronan receptor for endocytosis (HARE) J. Biol. Chem. 275, 37733–37741 [DOI] [PubMed] [Google Scholar]
- 6. Zhou B., Oka J. A., Singh A., Weigel P. H. (1999) Purification and subunit characterization of the rat liver endocytic hyaluronan receptor. J. Biol. Chem. 274, 33831–33834 [DOI] [PubMed] [Google Scholar]
- 7. Zhou B., Weigel J. A., Saxena A., Weigel P. H. (2002) Molecular cloning and functional expression of the rat 175-kDa hyaluronan receptor for endocytosis. Mol. Biol. Cell 13, 2853–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou B., McGary C. T., Weigel J. A., Saxena A., Weigel P. H. (2003) Purification and molecular identification of the human hyaluronan receptor for endocytosis. Glycobiology 13, 339–349 [DOI] [PubMed] [Google Scholar]
- 9. Qian H., Johansson S., McCourt P., Smedsrød B., Ekblom M., Johansson S. (2009) Stabilins are expressed in bone marrow sinusoidal endothelial cells and mediate scavenging and cell adhesive functions. Biochem. Biophys. Res. Commun. 390, 883–886 [DOI] [PubMed] [Google Scholar]
- 10. Park S. Y., Jung M. Y., Kim H. J., Lee S. J., Kim S. Y., Lee B. H., Kwon T. H., Park R. W., Kim I. S. (2008) Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 15, 192–201 [DOI] [PubMed] [Google Scholar]
- 11. Falkowski M., Schledzewski K., Hansen B., Goerdt S. (2003) Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular, tissues, and at solid/liquid interfaces. Histochem. Cell Biol. 120, 361–369 [DOI] [PubMed] [Google Scholar]
- 12. Harris E. N., Pandey M. S., Weigel P. H. (2009) in Hepatic Endocytosis (Berg T., Sporstol M., Mousavi S. A., eds) pp. 210–238, Transworld Research Network, Kerala, India [Google Scholar]
- 13. Harris E. N., Kyosseva S. V., Weigel J. A., Weigel P. H. (2007) Expression, processing, and glycosaminoglycan binding activity of the recombinant human 315-kDa HA receptor for endocytosis (HARE). J. Biol. Chem. 282, 2785–2797 [DOI] [PubMed] [Google Scholar]
- 14. Laurent T. C., Fraser J. R. (1992) Hyaluronan. FASEB J. 6, 2397–2404 [PubMed] [Google Scholar]
- 15. Laurent T. C., Fraser J. R. (1991) in Degradation of Bioactive Substances: Physiology and Pathophysiology (Henriksen J. H., ed) pp. 249–264, CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 16. Lebel L., Gabrielsson J., Laurent T. C., Gerdin B. (1994) Kinetics of circulating hyaluronan in humans. Eur. J. Clin. Invest. 24, 621–626 [DOI] [PubMed] [Google Scholar]
- 17. Fraser J. R., Laurent T. C., Pertoft H., Baxter E. (1981) Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem. J. 200, 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pandey M. S., Baggenstoss B. A., Washburn J., Harris E. N., Weigel P. H. (2013) The hyaluronan receptor for endocytosis (HARE) activates NF-κB-mediated gene expression in response to 40–400-kDa, but not smaller or larger, hyaluronans. J. Biol. Chem. 288, 14068–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kyosseva S. V., Harris E. N., Weigel P. H. (2008) The hyaluronan receptor for endocytosis (HARE) mediates hyaluronan-dependent signal transduction via extracellular signal-regulated kinases (ERK) J. Biol. Chem. 283, 15047–15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lokeshwar V. B., Selzer M. G. (2000) Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel, and vein-derived human endothelial cells. J. Biol. Chem. 275, 27641–27649 [DOI] [PubMed] [Google Scholar]
- 21. West D. C., Hampson I. N., Arnold F., Kumar S. (1985) Angiogenesis induced by degradation products of hyaluronic acid. Science 228, 1324–1326 [DOI] [PubMed] [Google Scholar]
- 22. Toole B. P. (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer 4, 528–539 [DOI] [PubMed] [Google Scholar]
- 23. Harris E. N., Weigel J. A., Weigel P. H. (2008) The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin. J. Biol. Chem. 283, 17341–17350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris E. N., Weigel P. H. (2008) The ligand-binding profile of HARE/Stabilin-2: hyaluronan and chondroitin sulfates A, C, and D bind to overlapping sites distinct from the sites for heparin, acetylated low-density lipoprotein and dermatan sulfate. Glycobiology 18, 638–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weigel P. H., Pandey M. S., Harris E. N. (2012) in Structure and Function of Biomatrix: Control of Cell Function and Gene Expression (Balazs E. A., ed) pp. 293–314, Matrix Biology Institute, Edgewater, NJ [Google Scholar]
- 26. Pandey M. S., Harris E. N., Weigel J. A., Weigel P. H. (2008) The cytoplasmic domain of the hyaluronan receptor for endocytosis (HARE) contains multiple endocytic motifs targeting coated pit-mediated internalization. J. Biol. Chem. 283, 21453–21461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raja R. H., LeBoeuf R. D., Stone G. W., Weigel P. H. (1984) Preparation of alkylamine and 125I-radiolabeled derivates of hyaluronic acid uniquely modified at the reducing end. Anal. Biochem. 139, 168–177 [DOI] [PubMed] [Google Scholar]
- 28. Baggenstoss B. A., Weigel P. H. (2006) Size exclusion chromatography-multiangle laser light scattering analysis of hyaluronan size distributions made by membrane-bound hyaluronan synthase. Anal. Biochem. 352, 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Senftleben U., Karin M. (2002) The IKK/NF-κB pathway. Crit. Care Med. 30, S18–S26 [PubMed] [Google Scholar]
- 30. McGary C. T., Raja R. H., Weigel P. H. (1989) Endocytosis of hyaluronic acid by rat liver endothelial cells: Evidence for receptor recycling. Biochem. J. 257, 875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris E. N., Weigel J. A., Weigel P. H. (2004) Endocytic function, glycosaminoglycan specificity, and antibody sensitivity of the recombinant human 190-kDa HA receptor for endocytosis (HARE) J. Biol. Chem. 279, 36201–36209 [DOI] [PubMed] [Google Scholar]
- 32. Harris E. N., Baggenstoss B. A., Weigel P. H. (2009) Rat and human HARE/Stabilin-2 are clearance receptors for high and low molecular weight heparin. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1191–G1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukuda S., Horiuchi S., Tomita K., Murakami M., Morino Y., Takahashi K. (1986) Acetylated low-density lipoprotein is endocytosed through coated pits by rat peritoneal macrophages. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 52, 1–13 [DOI] [PubMed] [Google Scholar]
- 34. Hsu H. Y., Chiu S. L., Wen M. H., Chen K. Y., Hua K. F. (2001) Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathways. J. Biol. Chem. 276, 28719–28730 [DOI] [PubMed] [Google Scholar]
- 35. Coller S. P., Paulnock D. M. (2001) Signaling pathways initiated in macrophages after engagement of type A scavenger receptors. J. Leukocyte Biol. 70, 142–148 [PubMed] [Google Scholar]
- 36. Matsunaga T., Hokari S., Koyama I., Harada T., Komoda T. (2003) NF-κB activation in endothelial cells treated with oxidized high density lipoprotein. Biochem. Biophys. Res. Commun. 303, 313–319 [DOI] [PubMed] [Google Scholar]
- 37. Park S. Y., Kang K. B., Thapa N., Kim S. Y., Lee S. J., Kim I. S. (2008) Requirement of adaptor protein GULP during stabilin-2-mediated cell corpse engulfment. J. Biol. Chem. 283, 10593–10600 [DOI] [PubMed] [Google Scholar]
- 38. Hansen B., Longati P., Elvevold K., Nedredal G.-I., Schledzewski K., Olsen R., Falkowski M., Kzhyshkowska J., Carlsson F., Johansson S., Smedsrød B., Goerdt S., Johansson S., McCourt P. (2005) Stabilin-1 and stabilin-2 are both directed into the early endocytic pathway in hepatic sinusoidal endothelium via interactions with clathrin/AP-2, independent of ligand binding. Exp. Cell Res. 303, 160–173 [DOI] [PubMed] [Google Scholar]
- 39. Oka J. A., Weigel P. H. (1988) Effects of hyperosmolarity on ligand processing and receptor recycling in the hepatic galactosyl receptor system. J. Cell. Biochem. 36, 169–183 [DOI] [PubMed] [Google Scholar]
- 40. Tamura Y., Adachi H., Osuga J., Ohashi K., Yahagi N., Sekiya M., Okazaki H., Tomita S., Iizuka Y., Shimano H., Nagai R., Kimura S., Tsujimoto M., Ishibashi S. (2003) FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J. Biol. Chem. 278, 12613–12617 [DOI] [PubMed] [Google Scholar]
- 41. Jung M. Y., Park S. Y., Kim I. S. (2007) Stabilin-2 is involved in lymphocyte adhesion to the hepatic sinusoidal endothelium via the interaction with αMβ2 integrin. J. Leukocyte Biol. 82, 1156–1165 [DOI] [PubMed] [Google Scholar]
- 42. Adachi H., Tsujimoto M. (2002) FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. J. Biol. Chem. 277, 34264–34270 [DOI] [PubMed] [Google Scholar]
- 43. Yannariello-Brown J., Zhou B., Weigel P. H. (1997) Identification of a 175-kDa protein as the ligand-binding subunit of the rat liver sinusoidal endothelial cell hyaluronan receptor. Glycobiology 7, 15–21 [DOI] [PubMed] [Google Scholar]
- 44. Smedsrød B., Malmgren M., Ericsson J., Laurent T. C. (1988) Morphological studies on endocytosis of chondroitin sulphate proteoglycan by rat liver endothelial cells. Cell Tissue Res. 253, 39–45 [DOI] [PubMed] [Google Scholar]
- 45. Weigel P. H., Yik J. H. (2002) Glycans as endocytosis signals: the cases of the asialoglycoprotein and hyaluronan/chondroitin sulfate receptors. Biochim. Biophys. Acta 1572, 341–363 [DOI] [PubMed] [Google Scholar]
- 46. Lee S. J., Park S. Y., Jung M. Y., Bae S. M., Kim I. S. (2011) Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood 117, 5215–5223 [DOI] [PubMed] [Google Scholar]
- 47. Kim S., Park S. Y., Kim S. Y., Bae D. J., Pyo J. H., Hong M., Kim I. S. (2012) Cross-talk between engulfment receptors stabilin-2 and integrin αvβ5 orchestrates engulfment of phosphatidylserine-exposed erythrocytes. Mol. Cell. Biol. 32, 2698–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. May P., Herz J., Bock H. H. (2005) Molecular mechanisms of lipoprotein receptor signalling. Cell. Mol. Life Sci. 62, 2325–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eriksson S., Fraser J. R., Laurent T. C., Pertoft H., Smedsrød B. (1983) Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp. Cell Res. 144, 223–228 [DOI] [PubMed] [Google Scholar]
- 50. Hupalowska A., Miaczynska M. (2012) The new faces of endocytosis in signaling. Traffic 13, 9–18 [DOI] [PubMed] [Google Scholar]
- 51. Platta H. W., Stenmark H. (2011) Endocytosis and signaling. Curr. Opin. Cell Biol. 23, 393–403 [DOI] [PubMed] [Google Scholar]
- 52. Sadowski L., Pilecka I., Miaczynska M. (2009) Signaling from endosomes: location makes a difference. Exp. Cell Res. 315, 1601–1609 [DOI] [PubMed] [Google Scholar]
- 53. Fraser J. R., Laurent T. C., Laurent U. B. (1997) Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 242, 27–33 [DOI] [PubMed] [Google Scholar]