Background: Leucine deprivation decreases fat mass via enhancing energy expenditure; the involvement of leptin signaling is unknown.
Results: Leucine deprivation promoted leptin signaling, and the increased energy expenditure was blocked in leptin signaling-disrupted mice.
Conclusion: Leptin signaling is required for leucine deprivation-increased energy expenditure.
Significance: Our studies reveal a physiological mechanism linking leptin signaling with leucine deprivation-enhanced energy expenditure.
Keywords: Energy Metabolism, Hypothalamus, Leptin, STAT3, Uncoupling Proteins, Fat Loss, Leucine Deprivation
Abstract
Leptin signaling in the hypothalamus is crucial in energy homeostasis. We have previously shown that dietary deprivation of the essential amino acid leucine in mice stimulates fat loss by increasing energy expenditure. The involvement of leptin signaling in this regulation, however, has not been reported. Here, we show that leucine deprivation promotes leptin signaling in mice maintained on an otherwise normal diet and restores leptin responses in mice maintained on a high fat diet, a regimen known to induce leptin resistance. In addition, we found that leucine deprivation stimulated energy expenditure, and fat loss was largely blocked in db/db mice homozygous for a mutation in leptin receptor and a knock-in mouse line Y3F with abrogation of leptin receptor Tyr1138-mediated signal transducer and activator transcript 3 signaling. Overall, our studies describe a novel link between hypothalamic leptin signaling and stimulation of energy expenditure under leucine deprivation.
Introduction
Obesity is a complex chronic disease, and perturbed leptin signaling in the hypothalamus, the major area in the brain regulating energy homeostasis, is known to contribute to its development (1). Leptin, a peptide hormone secreted from white adipose tissue, works through the leptin receptor (Ob-R), a class 1 cytokine receptor (2). In mice, at least five forms of the receptor have been reported to be produced from the Ob-R gene, including four short isoforms (Ob-Ra, Ob-Rc, Ob-Rd, and Ob-Re) and a long isoform (Ob-Rb) (3). Although Ob-Rb is detectable in many tissues, it is only highly expressed in the hypothalamus (4), specifically in the arcuate nucleus of hypothalamus (ARC),3 ventromedial hypothalamus (VMH), and dorsomedial hypothalamus (DMH), with lower levels in the paraventricular nucleus of hypothalamus and the lateral hypothalamic area (4–6). Binding of leptin to Ob-Rb stimulates the phosphorylation of receptor Tyr1138, which activates the intracellular Janus kinase (JAK)2/signal transducer and activator of transcription (STAT) 3 pathway to reduce food intake and increase energy expenditure (7, 8). Leptin resistance, which blocks the anorexic and weight-reducing effects of leptin (9) and reduces phosphorylation of STAT3 in the ARC (10), has been observed in obese mice (11) and human (12) models.
Leptin signaling is transcriptionally regulated (13, 14) and significantly affected by nutritional status. Both high fat diets (HFD) and high fructose diets have been shown to cause leptin resistance (15–19). In addition, dietary protein content also influences leptin signaling (20, 21). Recent studies have demonstrated that increased serum levels of amino acids are also closely related to human obesity (22), which is usually associated with leptin resistance (23). These results indicate a possible role of essential amino acids in leptin signaling.
Our laboratory has shown previously that dietary deprivation of leucine stimulates fat loss largely via increasing energy expenditure (24). Given the importance of leptin signaling in the regulation of energy homeostasis, we speculated that hypothalamic leptin signaling is required for the stimulation of energy expenditure and fat loss during leucine deprivation. The aim of this study was to investigate this possible link. In this study, we show that leptin signaling is directly involved in the regulation of energy expenditure and fat loss during leucine deprivation, and the present evidence suggests that the signaling is mediated by the leptin receptor Tyr1138-mediated STAT3 pathway.
EXPERIMENTAL PROCEDURES
Animals and Diets
Male C57BL/6J mice and leptin receptor-deficient (db/db) mice were obtained from Shanghai Laboratory Animal Co., Ltd. (Shanghai, China). Y3F mice, with abrogated hypothalamic activation of STAT3 by leptin, were described previously (25). Both db/db and Y3F mice are in the C57BL/6J background. Mice were maintained on a 12-h light/dark cycle at 25 °C. Control (nutritionally complete amino acid), (−)leu (leucine-deficient), high fat diet (containing 60% of calories as fat), and high fat diet without leucine diets were obtained from Research Diets, Inc. (New Brunswick, NJ). Eight-to-10-week-old WT, db/db, and Y3F mice were randomly divided into control and (−)leu diet groups, with free access to diets for 7 days. Four-week-old mice received high fat diet for 16 weeks to generate the leptin-resistant mouse model (26). At the end of the experiments, animals were killed by CO2 inhalation. Tissues were isolated, snap-frozen, and stored at −80 °C for future analysis. These experiments were conducted in accordance to the guidelines of the Institutional Animal Care and Use Committee of the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, and University of Chinese Academy of Sciences.
Indirect Calorimetry
Mice were maintained in a comprehensive laboratory animal monitoring system (CLAMS, Columbus Instruments, Columbus, OH) for 20 h to allow them to adapt to this environment, and the volume of O2 consumption and CO2 production was continuously recorded during the next 24 h according to the manufacturer's instructions.
Rectal Temperature Measurement
Rectal temperatures of mice were measured at 3 p.m. (basal metabolic state) using a rectal probe attached to a digital thermometer (Physitemp Inc., Clifton, NJ).
Leptin Sensitivity Assay in Vivo
Leptin was administered after mice were maintained on a control or (−)leu diet for 7 days. For control and (−)leu-treated mice, mice were intraperitoneally (i.p.) injected with either PBS or leptin (2 or 3 mg/kg) (27, 28). For HFD-fed mice, mice were i.p. injected with either PBS or leptin (5 mg/kg) (29). Before each study day, mice were fasted for 24 h, and leptin was administered at 9:00 a.m. Food intake and body weight were measured at 1 and 4 h post-injection of leptin.
Real Time Quantitative Reverse Transcription-PCR (RT-PCR)
RT-PCR was performed using RNA isolated from the hypothalamus of mice after i.p. injected with 2 mg/kg leptin or PBS for 45 min, as described previously (30). The sequences of primers used in this study are available upon request.
Western Blot Analysis
After fasting for 24 h, mice were subjected to i.p. injection with vehicle (PBS) or leptin (at 3 or 5 mg/kg) for 45 min before the hypothalami were removed for analysis. Western blot analysis was performed as described previously (24). Primary antibodies phospho-STAT3, anti-total-STAT3 (Cell Signaling Technology, Beverly, MA), and anti-actin antibody (Sigma) were incubated overnight at 4 °C, and specific proteins were visualized by ECL (Amersham Biosciences).
Immunohistochemistry Staining
Immunohistochemistry staining was performed as described previously (16). Briefly, brain coronal sections of 25 μm were cut using a frozen microtome (Leica Microsystems, Germany), incubated with primary antibody anti-phospho-STAT3 (Tyr(P)705) (Cell Signaling Technology, Beverly, MA), and pictures were taken by using an Olympus BX61 microscope (Olympus, Japan). Sections ranging from Bregma −1.34 to −2.06 mm, which contain the ARC, VMH, and DMH nuclei, were chosen for quantitative measurement of STAT3 phosphorylation levels, with the third ventricle used as the landmark. The intensity of pSTAT3-positive signals was evaluated for each nucleus using the Image-Pro Plus software program (Media Cybernetics, Inc.).
Statistical Analysis
All data are expressed as means ± S.E., with the numbers of mice included in each group in each experiment indicated. Significant differences were assessed by two-tailed Student t test or one-way ANOVA followed by the Student-Newman-Keuls (SNK) test. p < 0.05 was considered statistically significant.
RESULTS
Leucine Deprivation Increases Leptin Signaling in Mice
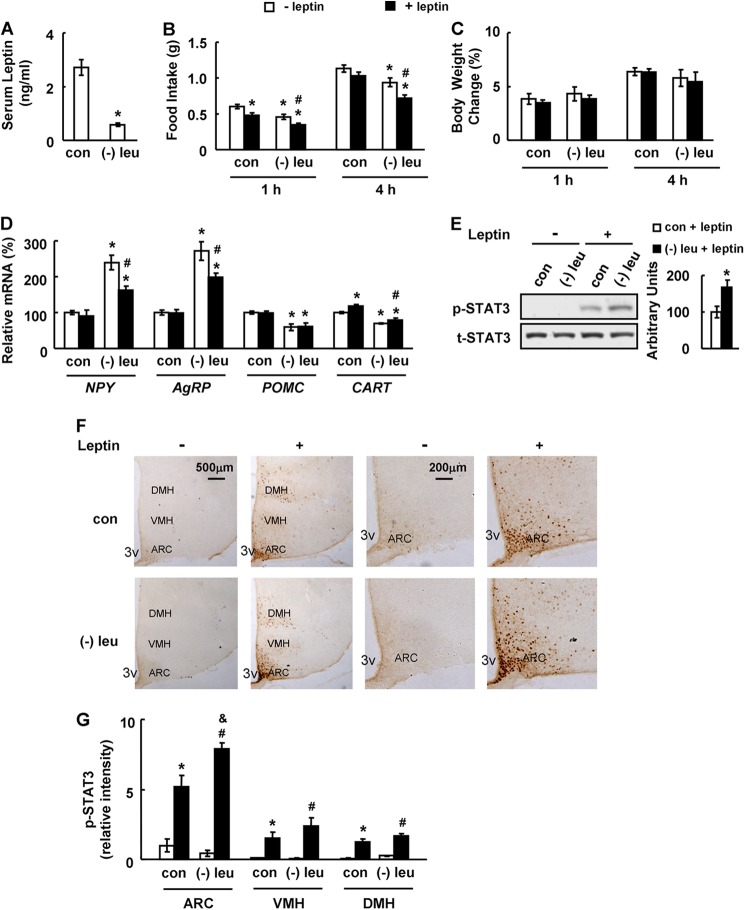
To investigate the effects of leucine deprivation on leptin signaling, C57BL/6J wild-type (WT) mice were maintained on a leucine-deficient diet or control diet for 7 days, as described in our previous studies (24, 30, 31). Leucine deprivation significantly decreased serum leptin levels compared with mice fed a control diet (Fig. 1A). The effect of leucine deprivation on leptin signaling was examined by measuring food intake and body weight following i.p. injecting leptin (3 mg/kg) (28). Although food intake was inhibited to a similar extent in both leucine-deprived and control mice 1 h after leptin injection, it was significantly decreased in leucine-deprived mice, but not in the control group, 4 h following leptin injection (Fig. 1B). In contrast, body weight was not significantly changed in either group following leptin injection (Fig. 1C), which is possibly caused by the continuous water drinking during the treatment.
FIGURE 1.
Leucine deprivation increases leptin signaling. Mice were fed a control (con) or leucine-deficient ((−)leu) diet for 7 days, followed by measuring serum leptin levels in A; mice were fasted for 24 h prior to intraperitoneally injecting PBS (− leptin) or 3 mg/kg leptin (+ leptin). This was followed by measuring food intake and body weight 1 and 4 h post-injection in B and C or analyzing STAT3 phosphorylation 45 min post-injection in E and F, or mice were fasted for 24 h prior to i.p. injecting PBS (− leptin) or 2 mg/kg leptin (+ leptin), followed by analyzing levels of mRNA 45 min post-injection in D. Data are mean ± S.E. (n = 5–6 for each group in A–E; n = 3 or 4 in F and G). Statistical significance was determined by Student's t test. *, p < 0.05 for the effect of (−)leu versus control diet for A and E, or by ANOVA followed by the SNK test. *, p < 0.05 for the effect of any group versus PBS treated-control mice. #, p < 0.05 for the effect of leptin-treated (−)leu group versus PBS-treated (−)leu group. &, p < 0.05 for the effect of leptin-treated (−)leu group versus leptin-treated control group for B–D and G. A, serum leptin levels; B, food intake; C, body weight change; D, hypothalamic neuropeptide changes; E, hypothalamic STAT3 proteins (upper panel, Western blot; lower panel, quantitative measurements of p-STAT3 protein relative to total STAT3); F, immunohistochemistry staining for p-STAT3 in hypothalamus. 3V, third ventricle. Images shown are representative of several animals for each group. Scale bar, 500 and 200 μm. G, quantitation of the intensities of positive p-STAT3 signals within the ARC, VMH, and DMH regions as marked in F. Relative signal intensities are quantified using Image-Pro Plus software from sections at Bregma −1.34 to −2.06 mm for each indicated treatment group.
We next examined hypothalamic expression of several key neuropeptides that regulate energy balance (27). These included orexigenic neuropeptide Y, agouti-related peptide, anorexigenic pro-opiomelanocortin, and cocaine and amphetamine-regulated transcript, which have previously been shown to be inhibited or stimulated, following i.p. administration of leptin (2 mg/kg) for 45 min (27). Leptin significantly inhibited hypothalamic expression of Npy and Agrp in leucine-deprived mice but not in control mice (Fig. 1D). We did not see the reported changes in neuropeptide expression following leptin injection in control mice; however, possibly due to the lower dose of leptin used in our study, which varies among published experiments (27, 28). In addition, leptin increased Cart expression, but it had no effect on Pomc expression in the hypothalamus of mice maintained on either control or leucine-deficient diets (Fig. 1D). Consistent with a much stronger effect of leptin in leucine-deprived mice, leptin produced a much more pronounced phosphorylation of STAT3 (the “gold standard” marker of cellular leptin action (10)) in the hypothalamus of these mice, especially in the ARC area, compared with control mice as shown by immunohistochemistry staining (Fig. 1, E–G).
Leucine Deprivation Restores Leptin Signaling in Mice under Conditions of Leptin Resistance Induced by Maintenance on HFD
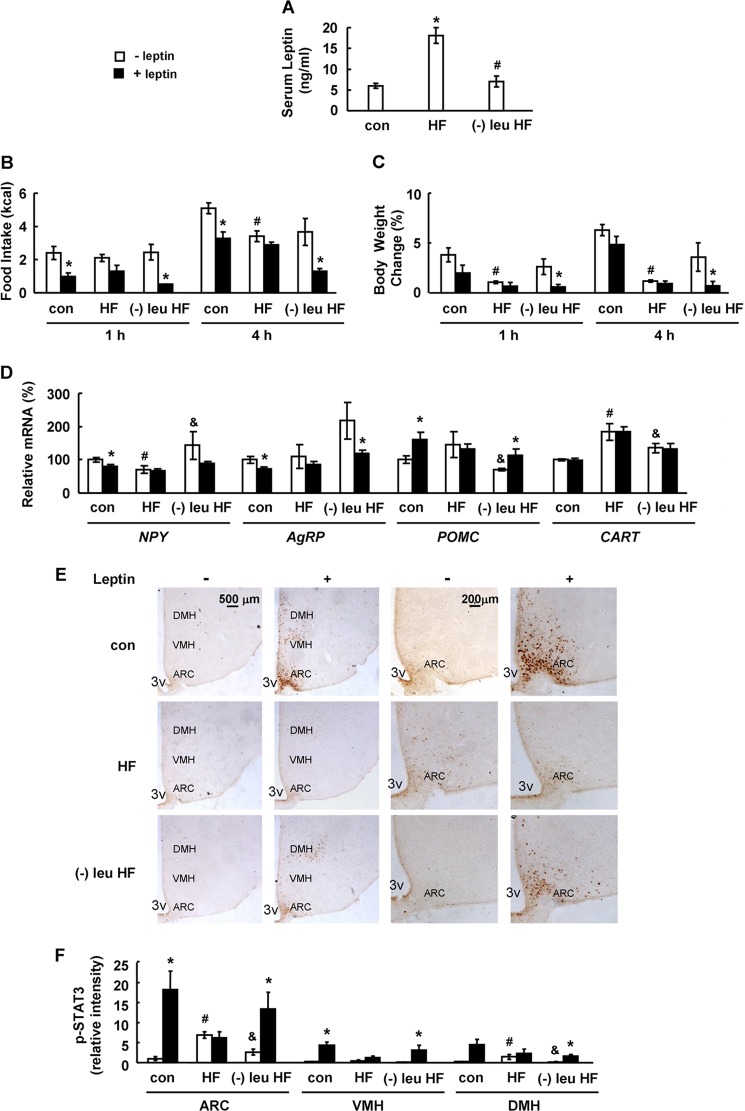
To test if leucine deprivation also stimulates leptin signaling under conditions of leptin resistance, we examined its effect in mice maintained on a HFD for 16 weeks, a regimen known to induce leptin resistance (26). These mice were subsequently maintained on either a HFD or a leucine-deficient HFD for 7 days before PBS or leptin injection. HFD increased serum leptin levels compared with the control group, and this increase was significantly reversed by leucine-deficient HFD (Fig. 2A). Resistance to leptin in HFD-fed mice was demonstrated by the loss of leptin-induced anorexia, corresponding to neuropeptide changes in the hypothalamus and STAT3 phosphorylation in the ARC compared with mice maintained on a control diet (Fig. 2, B and D–F). In contrast, leucine-deficient HFD largely restored leptin's ability to reverse the above markers of leptin resistance (Fig. 2, B and D–F). Body weight was not obviously decreased in control or HFD-fed mice, but it was significantly reduced in mice maintained on a leucine-deficient HFD, following leptin injection for 1 and 4 h (Fig. 2C).
FIGURE 2.
Leucine deprivation restores leptin signaling in mice under conditions of leptin resistance induced by HFD. Mice were fed a control or HFD for 16 weeks, followed by feeding control diet (con), HFD (HF), or a HFD without leucine ((−)leu HF) for 7 days. These mice were measured by serum leptin levels (A) or were fasted for 24 h prior to intraperitoneally injecting PBS (− leptin) or 5 mg/kg leptin (+ leptin), followed by measuring food intake and body weight 1 and 4 h post-injection (B and C), or analyzing levels of mRNA and STAT3 phosphorylation for 45 min post-injection in D and E. Data are mean ± S.E. (n = 5–7 for each group in A–D; n = 3 or 4 in E and F). Statistical significance was determined by ANOVA followed by the SNK test. *, p < 0.05 for the effect of HFD diet versus control diet. #, p < 0.05 for the effect of leucine-deprived HFD versus HFD for A. *, p < 0.05 for the effect of leptin-treated group versus PBS-treated group under the same diet. #, p < 0.05 for the effect of PBS-treated HFD versus PBS-treated control diet. &, p < 0.05 for the effect of PBS-treated (−)leu HFD versus PBS-treated HFD for B–F. A, serum leptin levels; B, food intake; C, body weight change; D, hypothalamic neuropeptide changes; E, immunohistochemistry staining for p-STAT3 in hypothalamus. 3V, third ventricle. Images shown are representative of several animals for each group. Scale bar, 500 and 200 μm. F, quantitation of the intensities of positive p-STAT3 signals within the ARC, VMH, and DMH regions as marked in E. Relative signal intensities are quantified using Image-Pro Plus software from sections at Bregma −1.34 to −2.06 mm for each indicated treatment group.
Leptin Signaling Is Required for the Enhancement of Energy Expenditure under Leucine Deprivation
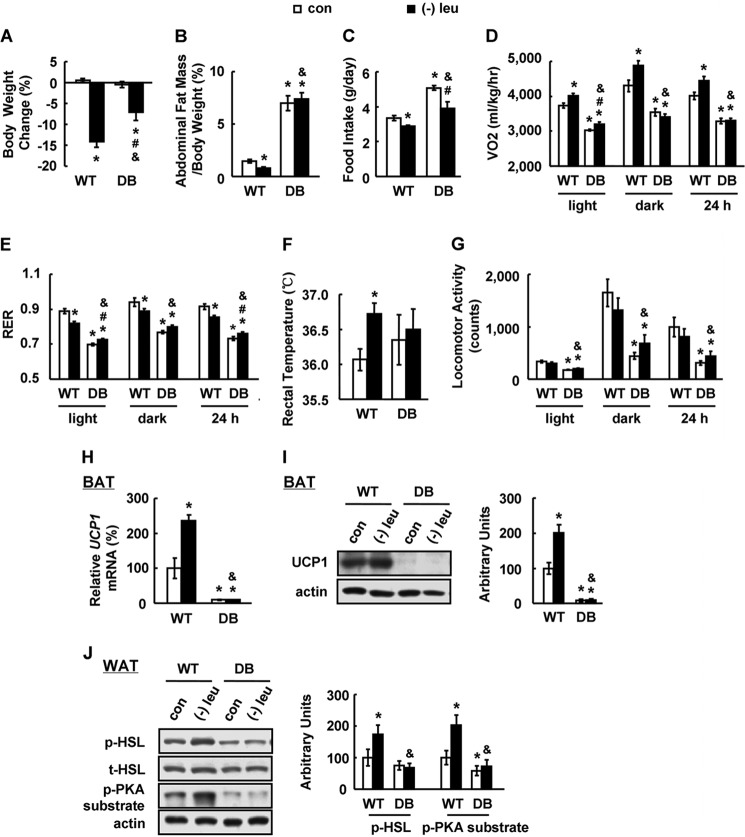
We have previously shown that leucine deprivation stimulates fat loss largely by increasing energy expenditure (24). To investigate a possible role of leptin signaling in the stimulation of energy expenditure and fat loss during leucine deprivation, we fed WT mice and mice homozygous for a mutated leptin receptor (db/db) a control or leucine-deficient diet for 7 days.
Consistent with our previous results (24), leucine deprivation significantly decreased body weight and fat mass in WT mice, but both of these effects were largely blocked in db/db mice (Fig. 3, A and B). The absence of leucine deprivation-induced fat loss in db/db mice could be the result of an increase in food intake, a decrease in energy expenditure, or both. A similar reduction in food intake, however, was observed in both WT and db/db mice maintained on a leucine-deficient diet (Fig. 3C). We therefore measured energy expenditure by indirect calorimetry, rectal temperature, and physical activity. The total energy expenditure (24 h O2 consumption, normalized to lean body mass) was markedly increased, and the respiratory exchange ratio (RER, VCO2/VO2) was low during both the dark and light phases in WT mice maintained on a leucine-deficient diet compared with WT mice maintained on a control diet (Fig. 3, D and E). By contrast, the effects of leucine deprivation on energy expenditure and RER were absent in db/db mice (Fig. 3, D and E). Consistent with changes in energy expenditure, the increase in body temperature observed in WT mice following leucine deprivation was significantly blocked in db/db mice (Fig. 3F). We did not see significant differences in physical activity between WT and db/db mice following leucine deprivation; however, basal activity in db/db mice was decreased compared with WT mice (Fig. 3G).
FIGURE 3.
Leptin signaling is required for leucine deprivation-increased energy expenditure. Wild-type (WT) and db/db mice with leptin receptor mutation (DB) were fed a control (con) or leucine-deficient ((−)leu) diet for 7 days. Energy expenditure was measured by indirect calorimetry. Data are mean ± S.E. (n = 5–8 for each group). Statistical significance was determined by ANOVA followed by the SNK test. *, p < 0.05 for the effect of any group versus WT mice with control diet. #, p < 0.05 for the effect of DB mice with (−)leu diet versus DB mice with control diet. &, p < 0.05 for the effect of DB mice with (−)leu diet versus WT mice with (−)leu diet. A, body weight change; B, adipose tissue mass in proportion to body weight; C, daily food intake; D, 24 h oxygen consumption (VO2); E, respiratory exchange ratio (RER); F, rectal temperature; G, physical activity; H, Ucp1 mRNA expression in BAT; I, UCP1 protein in BAT (upper panel, Western blot; lower panel, quantitative measurements of UCP1 protein relative to actin); J, p-HSL and p-PKA substrate protein in white adipose tissue (upper panel, Western blot; lower panel, quantitative measurements of p-HSL and p-PKA substrate protein relative to total HSL and actin, respectively).
Increased energy expenditure in leucine-deprived mice has previously been shown to correlate with an increase in uncoupling protein (UCP)1 expression in brown adipose tissue (BAT) and phosphorylation of protein kinase A (PKA) and the rate-limiting lipase hormone-sensitive lipase (HSL) in white adipose tissue (24). Here, we found that increases in UCP1 expression in BAT, as well as phosphorylation of HSL and PKA substrate, were blocked in db/db mice following leucine deprivation and that basal levels of these mRNAs and proteins were lower in db/db mice (Fig. 3, H–J).
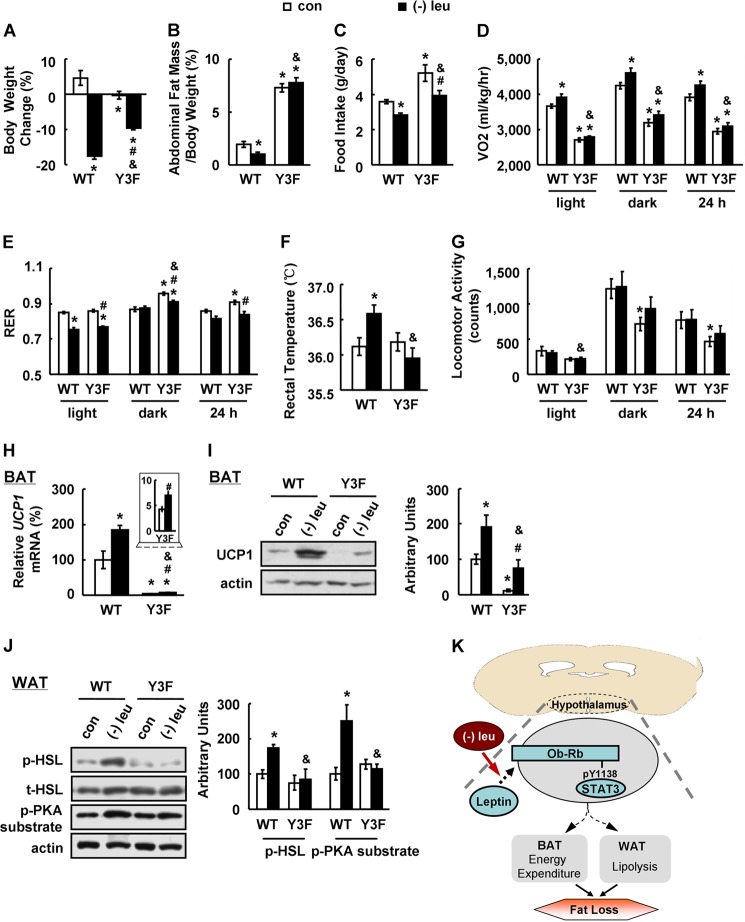
Ob-Rb Tyr1138-mediated STAT3 Signaling Is Required for Increased Energy Expenditure during Leucine Deprivation
One of the major pathways mediating the effects of leptin depends on STAT3, as evidenced by the observation that neuron-specific deletion of Stat3 recapitulates the obese phenotype of db/db mice (32). To explore the possible role of hypothalamic STAT3 signaling during leucine deprivation, we used knock-in Y3F mice, in which hypothalamic activation of STAT3 by leptin is prevented by a tyrosine-to-phenylalanine substitution at Tyr1138 of Ob-Rb (25). Y3F and WT control mice were maintained on a leucine-deficient or control diet for 7 days prior to examination of related metabolic parameters. Phenotypically, the response to leucine deficiency in Y3F mice closely matched that of db/db mice (Fig. 4, A–J). Although UCP1 expression in BAT was slightly induced in Y3F mice following leucine deprivation, UCP1 levels were much lower compared with those in leucine-deprived WT mice (Fig. 4, H and I).
FIGURE 4.
Hypothalamic Ob-Rb Tyr1138-mediated STAT3 is required for leucine deprivation-increased energy expenditure. Wild-type (WT) and knock-in mouse line with abrogated leptin receptor Tyr1138-mediated STAT3 signaling (Y3F) were fed a control (con) or leucine-deficient ((−)leu) diet for 7 days. Energy expenditure was measured by indirect calorimetry. Data are mean ± S.E. (n = 5–6 for each group). Statistical significance was determined by ANOVA followed by the SNK test. *, p < 0.05 for the effect of any group versus WT mice with control diet. #, p < 0.05 for the effect of Y3F mice with (−)leu diet versus Y3F mice with control diet. &, p < 0.05 for the effect of Y3F mice with (−)leu diet versus WT mice with (−)leu diet. A, body weight change; B, adipose tissue mass in proportion to body weight; C, daily food intake; D, 24 h oxygen consumption (VO2); E, respiratory exchange ratio (RER); F, rectal temperature; G, physical activity; H, Ucp1 mRNA expression in BAT; I, UCP1 protein in BAT (upper panel, Western blot; lower panel, quantitative measurements of UCP1 protein relative to actin); J, p-HSL and p-PKA substrate protein in white adipose tissue (upper panel, Western blot; lower panel, quantitative measurements of p-HSL and p-PKA substrate protein relative to total HSL and actin, respectively); K, working model.
DISCUSSION
In addition to transcriptional and/or translational regulation of genes related to leptin signaling (13, 14), nutritional status may also influence leptin signaling. It is well known that HFD causes leptin resistance (15–17). Chronic fructose diets have also been shown to be associated with increased plasma leptin levels and to induce leptin resistance prior to the onset of obesity (17–19). Moreover, fructose-free but high fat diets can reverse high fructose/high fat diet-induced leptin resistance in rats, suggesting that fructose in the diet is the bioactive ingredient that causes leptin resistance (33).
Several lines of evidence suggest a relationship between essential amino acids and leptin secretion and signaling. For example, leucine activates leptin expression in rat adipose cells (34) and increases satiety by stimulating leptin secretion in rats (35). It has also been reported that leucine promotes leptin receptor expression in mouse C2C12 myotubes (36). In addition, dietary supplementation of arginine or histidine has been reported to suppress serum leptin levels (37, 38). A direct effect of essential amino acids on leptin signaling, however, has not previously been reported.
In this study, we show for the first time that dietary deficiency of leucine has a significant effect on leptin signaling, as demonstrated by decreased food intake, body weight, adiposity, change of neuropeptide, and phosphorylation of STAT3 in ARC area following leptin stimulation in mice maintained on a leucine-deficient diet. In contrast to our observation that leucine deprivation improves leptin signaling in mice, it has been reported that a high protein diet decreases caloric intake in humans, possibly mediated via increased leptin sensitivity (20), and a low protein diet increases food intake and serum leptin levels in rats, possibly reflecting a state of leptin resistance (21). These results provide important information for understanding the nutritional regulation of leptin signaling. However, the effects of individual amino acids should not be equated with high or low levels of proteins and deserve independent investigation.
Leptin resistance has been identified as one of the major contributing factors in obesity, based on observations in db/db mice, HFD-fed mic, and fructose-fed mice (15–17, 19, 39). Various strategies have been proposed to promote leptin signaling, including overexpression of Src homology-2B (13), inhibition of protein-tyrosine phosphatase 1B expression (14), treatment with drugs such as metformin (40), or feeding a fish-rich diet (41). Here, we showed that dietary leucine deprivation can also efficiently reverse the decreased leptin signaling in a leptin-resistant mouse model. These results further indicate a role for dietary amino acid content in leptin sensitivity and suggest that manipulation of dietary amino acids may be an effective way to improve leptin signaling and thereby decrease body weight. Furthermore, because of the importance of amino acids in leptin signaling, we speculate that the attenuated leptin signaling in obese human patients might be caused by the increased serum levels of amino acids such as leucine.
Our previous work has shown that leucine deprivation stimulates fat loss largely via increasing energy expenditure (24). It is known that leptin increases energy expenditure and promotes fat metabolism in ob/ob mice, which are deficient in leptin secretion (42). Furthermore, it has been shown that central injection of leptin efficiently augments BAT UCP1 and prevents weight gain in HFD-fed rats (43). In our study, a role for leptin signaling in the regulation of energy expenditure during leucine deprivation was confirmed by the observation of leucine deprivation-mediated decreases in fat mass, and increases in energy expenditure were blocked in db/db mice. Our observation that leptin levels are decreased in leucine-deprived mice, compared with a control group, confirm that enhanced leptin signaling is responsible for the stimulation of energy expenditure during leucine deprivation. Lower leptin levels in leucine-deprived mice could result from decreased fat mass or from a direct effect of leucine deprivation on leptin secretion. Consistent with the latter possibility, it has been reported that leucine stimulates leptin secretion (34, 35).
Leptin functions by activating STAT3-dependent and -independent intracellular signaling pathways (16, 25). Binding of leptin to its membrane receptor Ob-Rb stimulates receptor Tyr1138, Tyr985, and Tyr1077 phosphorylation (44). Phosphorylated Tyr1138 recruits STAT3 and activates the JAK2/STAT3 pathway, which modulates energy homeostasis (7, 8, 45). By using Y3F mice with abrogated Tyr1138-mediated STAT3 signaling (25), we provide evidence that hypothalamic STAT3 contributes to leptin-dependent regulation of energy expenditure during leucine deprivation. Consistent with our results, STAT3 signaling has been shown to be critical for leptin regulation of UCP1 expression in BAT (8).
One of the downstream targets in mediating the effects of hypothalamic STAT3 may be ribosomal protein S6 kinase 1 (S6K1), the downstream target of the mammalian target of rapamycin kinase (46). Our previous study has shown that S6K1 activity is decreased in the hypothalamus and acts as a major regulator of increased thermogenesis and fat loss during leucine deprivation (31). By contrast, S6K1 activity is not decreased in the hypothalamus of leucine-deprived db/db and Y3F mice compared with control groups.4 In addition to the Tyr1138-mediated STAT3-dependent pathway, intracellular signaling pathways, including ERK, SOCS3, and STAT5, which are regulated by phosphorylation of Ob-Rb at other tyrosine sites, have been identified as important regulators of energy homeostasis (47–49). The possible role of these effectors in the leucine and leptin-mediated regulation of energy homeostasis need to be investigated in the future.
The mechanism by which leucine deprivation regulates leptin signaling, however, is not yet understood. Previous studies have indicated SH2B1, SOCS3, and protein-tyrosine phosphatase 1B as important regulators (13, 14, 50) for leptin signaling. We did not, however, observe any changes in the hypothalamic expression of Sh2b, Socs3, and Ptp1b by leucine deprivation (data not shown). Recent studies have demonstrated that Rho kinase (ROCK)1, in which the activity is influenced by nutritional status (51, 52), increases phosphorylation of JAK2 and downstream activation of STAT3 to regulate leptin action (45). A possible role for ROCK1 in connecting leucine deprivation with the leptin pathway will be studied in the future.
Taken together, our results show that leucine deprivation promotes leptin signaling in mice maintained on an otherwise normal diet and restores the responses to leptin under leptin-resistant conditions in HFD-fed mice. In addition, we show that leptin signaling is directly involved in the stimulation of energy expenditure and fat loss under leucine deprivation and that this effect is likely to be mediated by the STAT3-dependent pathway (Fig. 4K). These results describe a novel link between hypothalamic leptin/STAT3 signaling and stimulation of energy expenditure under leucine deprivation, and they also provide a new perspective for understanding the nutritional control of leptin signaling and the role of leptin signaling in energy homeostasis under deprivation of an essential amino acid. Future studies, however, will be required to elucidate mechanisms underlying leucine deprivation control of improved leptin signaling in the hypothalamus and to identify specific STAT3-expressing neuron responses for leucine deprivation-increased energy expenditure.
This work was supported in part by the Ministry of Science and Technology of China 973 Program 2010CB912502 and 2009CB919001, National Natural Science Foundation Grants 81130076, 31271269, and 81100615, Key Program of Shanghai Scientific and Technological Innovation Action Plan Grant 13JC1409000, Knowledge Innovation Program of Chinese Academy of Sciences Grant KSCX2-EW-R-09, Chinese Academy of Sciences Project 2011KIP307.
Q. Zhang, B. Liu, Y. Cheng, Q. Meng, T. Xia, L. Jiang, S. Chen, Y. Liu, and F. Guo, unpublished observations.
- ARC
- arcuate nucleus
- HFD
- high fat diet
- VMH
- ventromedial hypothalamus
- DMH
- dorsomedial hypothalamus
- ANOVA
- analysis of variance
- SNK
- Student-Newman-Keuls
- HSL
- hormone-sensitive lipase
- BAT
- brown adipose tissue.
REFERENCES
- 1. Sahu A. (2004) Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology 145, 2613–2620 [DOI] [PubMed] [Google Scholar]
- 2. Tartaglia L. A. (1997) The leptin receptor. J. Biol. Chem. 272, 6093–6096 [DOI] [PubMed] [Google Scholar]
- 3. Lee G. H., Proenca R., Montez J. M., Carroll K. M., Darvishzadeh J. G., Lee J. I., Friedman J. M. (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635 [DOI] [PubMed] [Google Scholar]
- 4. Fei H., Okano H. J., Li C., Lee G. H., Zhao C., Darnell R., Friedman J. M. (1997) Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl. Acad. Sci. U.S.A. 94, 7001–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mercer J. G., Hoggard N., Williams L. M., Lawrence C. B., Hannah L. T., Trayhurn P. (1996) Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 387, 113–116 [DOI] [PubMed] [Google Scholar]
- 6. Elmquist J. K., Bjørbaek C., Ahima R. S., Flier J. S., Saper C. B. (1998) Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 395, 535–547 [PubMed] [Google Scholar]
- 7. Bates S. H., Stearns W. H., Dundon T. A., Schubert M., Tso A. W., Wang Y., Banks A. S., Lavery H. J., Haq A. K., Maratos-Flier E., Neel B. G., Schwartz M. W., Myers M. G., Jr. (2003) STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 [DOI] [PubMed] [Google Scholar]
- 8. Bates S. H., Dundon T. A., Seifert M., Carlson M., Maratos-Flier E., Myers M. G., Jr. (2004) LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53, 3067–3073 [DOI] [PubMed] [Google Scholar]
- 9. Dardeno T. A., Chou S. H., Moon H. S., Chamberland J. P., Fiorenza C. G., Mantzoros C. S. (2010) Leptin in human physiology and therapeutics. Front. Neuroendocrinol. 31, 377–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Myers M. G., Jr., Leibel R. L., Seeley R. J., Schwartz M. W. (2010) Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol. Metab. 21, 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tam J., Cinar R., Liu J., Godlewski G., Wesley D., Jourdan T., Szanda G., Mukhopadhyay B., Chedester L., Liow J. S., Innis R. B., Cheng K., Rice K. C., Deschamps J. R., Chorvat R. J., McElroy J. F., Kunos G. (2012) Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 16, 167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelesidis T., Kelesidis I., Chou S., Mantzoros C. S. (2010) Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann. Intern. Med. 152, 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Z., Zhou Y., Carter-Su C., Myers M. G., Jr., Rui L. (2007) SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr(813) phosphorylation-dependent and -independent mechanisms. Mol. Endocrinol. 21, 2270–2281 [DOI] [PubMed] [Google Scholar]
- 14. Zabolotny J. M., Bence-Hanulec K. K., Stricker-Krongrad A., Haj F., Wang Y., Minokoshi Y., Kim Y. B., Elmquist J. K., Tartaglia L. A., Kahn B. B., Neel B. G. (2002) PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2, 489–495 [DOI] [PubMed] [Google Scholar]
- 15. Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. (2008) Hypothalamic IKKβ/NF-κaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. You J., Yu Y., Jiang L., Li W., Yu X., Gonzalez L., Yang G., Ke Z., Li W., Li C., Liu Y. (2010) Signaling through Tyr(985) of leptin receptor as an age/diet-dependent switch in the regulation of energy balance. Mol. Cell. Biol. 30, 1650–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang B. W., Chiang M. T., Yao H. T., Chiang W. (2004) The effect of high fat and high fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes. Metab. 6, 120–126 [DOI] [PubMed] [Google Scholar]
- 18. Lee Y. C., Ko Y. H., Hsu Y. P., Ho L. T. (2006) Plasma leptin response to oral glucose tolerance and fasting/re-feeding tests in rats with fructose-induced metabolic derangements. Life Sci. 78, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 19. Shapiro A., Mu W., Roncal C., Cheng K. Y., Johnson R. J., Scarpace P. J. (2008) Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high fat feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1370–R1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weigle D. S., Breen P. A., Matthys C. C., Callahan H. S., Meeuws K. E., Burden V. R., Purnell J. Q. (2005) A high protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 82, 41–48 [DOI] [PubMed] [Google Scholar]
- 21. Du F., Higginbotham D. A., White B. D. (2000) Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J. Nutr. 130, 514–521 [DOI] [PubMed] [Google Scholar]
- 22. Newgard C. B., An J., Bain J. R., Muehlbauer M. J., Stevens R. D., Lien L. F., Haqq A. M., Shah S. H., Arlotto M., Slentz C. A., Rochon J., Gallup D., Ilkayeva O., Wenner B. R., Yancy W. S., Jr., Eisenson H., Musante G., Surwit R. S., Millington D. S., Butler M. D., Svetkey L. P. (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukuda M., Williams K. W., Gautron L., Elmquist J. K. (2011) Induction of leptin resistance by activation of cAMP-Epac signaling. Cell Metab. 13, 331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng Y., Meng Q., Wang C., Li H., Huang Z., Chen S., Xiao F., Guo F. (2010) Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 59, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang L., You J., Yu X., Gonzalez L., Yu Y., Wang Q., Yang G., Li W., Li C., Liu Y. (2008) Tyrosine-dependent and -independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proc. Natl. Acad. Sci. U.S.A. 105, 18619–18624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Münzberg H., Flier J. S., Bjørbaek C. (2004) Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145, 4880–4889 [DOI] [PubMed] [Google Scholar]
- 27. Enriori P. J., Evans A. E., Sinnayah P., Jobst E. E., Tonelli-Lemos L., Billes S. K., Glavas M. M., Grayson B. E., Perello M., Nillni E. A., Grove K. L., Cowley M. A. (2007) Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 5, 181–194 [DOI] [PubMed] [Google Scholar]
- 28. Bewick G. A., Kent A., Campbell D., Patterson M., Ghatei M. A., Bloom S. R., Gardiner J. V. (2009) Mice with hyperghrelinemia are hyperphagic and glucose intolerant and have reduced leptin sensitivity. Diabetes 58, 840–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cristino L., Busetto G., Imperatore R., Ferrandino I., Palomba L., Silvestri C., Petrosino S., Orlando P., Bentivoglio M., Mackie K., Di Marzo V. (2013) Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc. Natl. Acad. Sci. U.S.A. 110, E2229–E2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng Y., Zhang Q., Meng Q., Xia T., Huang Z., Wang C., Liu B., Chen S., Xiao F., Du Y., Guo F. (2011) Leucine deprivation stimulates fat loss via increasing CRH expression in the hypothalamus and activating the sympathetic nervous system. Mol. Endocrinol. 25, 1624–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xia T., Cheng Y., Zhang Q., Xiao F., Liu B., Chen S., Guo F. (2012) S6K1 in the central nervous system regulates energy expenditure via MC4R/CRH pathways in response to deprivation of an essential amino acid. Diabetes 61, 2461–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao Q., Wolfgang M. J., Neschen S., Morino K., Horvath T. L., Shulman G. I., Fu X. Y. (2004) Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl. Acad. Sci. U.S.A. 101, 4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shapiro A., Tümer N., Gao Y., Cheng K. Y., Scarpace P. J. (2011) Prevention and reversal of diet-induced leptin resistance with a sugar-free diet despite high fat content. Br. J. Nutr. 106, 390–397 [DOI] [PubMed] [Google Scholar]
- 34. Roh C., Han J., Tzatsos A., Kandror K. V. (2003) Nutrient-sensing mTOR-mediated pathway regulates leptin production in isolated rat adipocytes. Am. J. Physiol. Endocrinol. Metab. 284, E322–E330 [DOI] [PubMed] [Google Scholar]
- 35. Lynch C. J., Gern B., Lloyd C., Hutson S. M., Eicher R., Vary T. C. (2006) Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am. J. Physiol. Endocrinol. Metab. 291, E621–E630 [DOI] [PubMed] [Google Scholar]
- 36. Mao X., Zeng X., Wang J., Qiao S. (2011) Leucine promotes leptin receptor expression in mouse C2C12 myotubes through the mTOR pathway. Mol. Biol. Rep. 38, 3201–3206 [DOI] [PubMed] [Google Scholar]
- 37. Fu W. J., Haynes T. E., Kohli R., Hu J., Shi W., Spencer T. E., Carroll R. J., Meininger C. J., Wu G. (2005) Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J. Nutr. 135, 714–721 [DOI] [PubMed] [Google Scholar]
- 38. Kasaoka S., Tsuboyama-Kasaoka N., Kawahara Y., Inoue S., Tsuji M., Ezaki O., Kato H., Tsuchiya T., Okuda H., Nakajima S. (2004) Histidine supplementation suppresses food intake and fat accumulation in rats. Nutrition 20, 991–996 [DOI] [PubMed] [Google Scholar]
- 39. Lin H. Y., Xu Q., Yeh S., Wang R. S., Sparks J. D., Chang C. (2005) Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes 54, 1717–1725 [DOI] [PubMed] [Google Scholar]
- 40. Kim Y. W., Kim J. Y., Park Y. H., Park S. Y., Won K. C., Choi K. H., Huh J. Y., Moon K. H. (2006) Metaformin restores leptin sensitivity in high fat-fed obese rats with leptin resistance. Diabetes 55, 716–724 [DOI] [PubMed] [Google Scholar]
- 41. Winnicki M., Somers V. K., Accurso V., Phillips B. G., Puato M., Palatini P., Pauletto P. (2002) Fish-rich diet, leptin, and body mass. Circulation 106, 289–291 [DOI] [PubMed] [Google Scholar]
- 42. Hwa J. J., Fawzi A. B., Graziano M. P., Ghibaudi L., Williams P., Van Heek M., Davis H., Rudinski M., Sybertz E., Strader C. D. (1997) Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am. J. Physiol. 272, R1204–R1209 [DOI] [PubMed] [Google Scholar]
- 43. Dube M. G., Beretta E., Dhillon H., Ueno N., Kalra P. S., Kalra S. P. (2002) Central leptin gene therapy blocks high fat diet-induced weight gain, hyperleptinemia, and hyperinsulinemia–Increase in serum ghrelin levels. Diabetes 51, 1729–1736 [DOI] [PubMed] [Google Scholar]
- 44. Kloek C., Haq A. K., Dunn S. L., Lavery H. J., Banks A. S., Myers M. G. (2002) Regulation of Jak kinases by intracellular leptin receptor sequences. J. Biol. Chem. 277, 41547–41555 [DOI] [PubMed] [Google Scholar]
- 45. Huang H., Kong D., Byun K. H., Ye C., Koda S., Lee D. H., Oh B. C., Lee S. W., Lee B., Zabolotny J. M., Kim M. S., Bjørbæk C., Lowell B. B., Kim Y. B. (2012) Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat. Neurosci. 15, 1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cota D., Proulx K., Smith K. A., Kozma S. C., Thomas G., Woods S. C., Seeley R. J. (2006) Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930 [DOI] [PubMed] [Google Scholar]
- 47. Banks A. S., Davis S. M., Bates S. H., Myers M. G. (2000) Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275, 14563–14572 [DOI] [PubMed] [Google Scholar]
- 48. Bjorbak C., Lavery H. J., Bates S. H., Olson R. K., Davis S. M., Flier J. S., Myers M. G., Jr. (2000) SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 275, 40649–40657 [DOI] [PubMed] [Google Scholar]
- 49. Gong Y., Ishida-Takahashi R., Villanueva E. C., Fingar D. C., Münzberg H., Myers M. G., Jr. (2007) The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J. Biol. Chem. 282, 31019–31027 [DOI] [PubMed] [Google Scholar]
- 50. Mori H., Hanada R., Hanada T., Aki D., Mashima R., Nishinakamura H., Torisu T., Chien K. R., Yasukawa H., Yoshimura A. (2004) Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 10, 739–743 [DOI] [PubMed] [Google Scholar]
- 51. Huang H., Lee S. H., Ye C., Lima I. S., Oh B. C., Lowell B. B., Zabolotny J. M., Kim Y. B. (2013) ROCK1 in AgRP neurons regulates energy expenditure and locomotor activity in male mice. Endocrinology 154, 3660–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chuang S. M., Juan Y. S., Long C. Y., Huang C. H., Levin R. M., Liu K. M. (2011) The effect of l-arginine on bladder dysfunction following ovariectomy in a rabbit model. Int. Urogynecol. J. 22, 1381–1388 [DOI] [PubMed] [Google Scholar]