Abstract
Background
Even though highly effective drugs are available in South Africa, multidrug resistant tuberculosis (MDR-TB) patients with HIV infection have higher mortality compared to HIV-uninfected MDR-TB patients. This trend has been observed in similar countries with high HIV prevalence. This study sought to determine excess mortality attributable to HIV among MDR-TB patients in South Africa using relative survival methods.
Methods
Data available were from a cohort of 2079 MDR-TB patients enrolled in a Standardized Programmatic Management of MDR-TB from 2000 to 2004 in South Africa. A Poisson-based model adjusted for age, gender, year of diagnosis, TB history, and resistance to ethambutol, anti-TB injectable drugs and fluoroquinolones antibiotics was constructed to assess the excess mortality among HIV co-infected MDR-TB patients. Excess hazard ratios (EHRs) were used to describe the effect of the predictors on net mortality, controlling for the general mortality in the South African population.
Results
Death was recorded on 1619 patients, of whom 367 (22.7%) had died within 2 years. Out of the 1413 patients that tested for HIV infection, 554 (39.2%) tested positive. Excess mortality was higher in HIV infected, compared to HIV uninfected, MDR-TB patients (adjusted excess hazard ratio, 5.6 [95% CI, 3.2–9.7]); in patients whose TB isolates’ resistance to ethambutol and kanamycin was unknown (3.7 [2.1–6.2] and 4.87 [1.9–13.3], respectively) vs. known. There were no differences in excess mortality between age and gender of the patient, year of diagnosis and TB history.
Conclusion
Adjusting for some important predictors, MDR-TB patients with HIV infection experienced higher excess mortality compared to HIV-uninfected MDR-TB patients, after accounting for the general mortality in South Africa. An appropriate, though complex method has produced predictor effect estimates similar to those obtained from classical methods. Thus, the use of relative survival methods should be encouraged in the analysis of causespecific mortality, when ascertainment of cause of death is inaccurate or unknown.
Keywords: HIV, Tuberculosis, Relative mortality, South Africa
Introduction
Drug resistant tuberculosis (TB) has become a major public health problem in many high TB burden countries such as South Africa. Thirty-six percent of incidence TB cases are estimated to have multidrug resistant TB (MDR-TB), which is caused by Mycobacterium tuberculosis strains resistant to at least isoniazid (INH) and rifampin (RIF), and can result from primary infection or develop during treatment against TB [1]. MDR-TB global burden was estimated at 310,000 incidence cases in 2011, with India, China and the Russian Federation accounting for nearly 60% of the cases [2]. It is estimated that a third of MDR-TB patients died from the disease in 2008. South Africa, with an estimated 13,000 incidence MDR-TB cases, is among the few sub-Saharan African countries in the top 27 high MDR-TB burden states that accounts for 85% of the global MDR-TB burden [2].
The high rates of MDR-TB among high burden TB countries are hampering the progress regarding TB care and control (WHO [1]). This has been fueled by an increase in the TB incidence, which has been associated with the emergence of human immunodeficiency virus (HIV) [3,4]. Individuals with latent TB are more likely to reactivate and experience rapidly progressive TB and drug resistance when co-infected with HIV. HIV-infected patients are more susceptible to TB infection and mortality due to their compromised immune systems. The diagnosis of MDR-TB among HIV patients is also compromised as they cannot cough out sputum, which is a gold standard for testing TB bacterium.
There have been several studies examining risk factors for mortality in MDR-TB in South Africa [5–7]. Furthermore, large meta-analyses of MDR-TB outcomes have also been undertaken [8,9]. Thus, risk factors for MDR-TB outcomes are well-known and documented in the literature. Most of these previous studies assessed MDR-TB related mortality using verbal autopsies and hospital records. However, the accuracy of verbal autopsies in identifying probable causes of deaths has been the subject of much debate [10]. Even medical assessment of death, though accurate and objective, can still contain incorrect data in settings where resources are limited and scarce [11]. In such settings, reliance on verbal autopsies and medical records to ascertain death can lead to over- or under- estimation of incidence of deaths that are attributable to MDR-TB.
Most of these studies analyzed MDR-TB death using standard statistical methods such as logistic and proportional hazard regressions [6,7,12]. These are usually based on overall mortality where the cause of death is irrelevant. If used for cause-specific survival analysis, data pertaining to other causes are not used in the estimation of survival fractions. Furthermore, they largely rely on accurate assessment of cause of death. Thus, even application of standard models may give biased estimates of MDR-TB specific death rates. This paper provides an alternative statistical model using relative survival, which do not necessarily depend on accurate cause of death identification. We carry out an investigation of risk factors for MDR-TB mortality using the alternative method and compare the results to those obtained from the classical methods.
For analyses of time-to-event data, relative survival methods [13–17], have widely been used in cancer studies [18–19], and recently, in HIV/AIDS studies [20,21]. Even though these methods are available in standard statistical packages such as STATA and SAS, their uptake in the general public health domain has been very low. We used relative survival method to estimate excess mortality in a cohort of MDR-TB patients in South Africa. The method offers an alternative approach to gain insight in the estimation of mortality attributable to various factors in the cohort.
Materials and Methods
Data
Data were obtained from a cohort of MDR-TB patients (n = 2079) enrolled in a study of programmatic management of MDR-TB in South Africa from 2000 to 2004. All patients who were diagnosed with a first MDR-TB episode and admitted to one of the MDR-TB treatment centers were enrolled into the study. These included patients who had either converted from TB to MDR-TB or were infected with MDR-TB without having had TB before. Case finding, through culture and drug susceptibility testing, and treatment routines are described in Farley et al. [22] and Van der Walt et al. [23]. In particular, patients received a standardized regimen which included testing for ethambutol drug resistance to determine whether it should form part of the second line drug regimen.
The data collected included patients’ baseline demographic, treatment and clinical variables (for example; gender, age at diagnosis, HIV status, baseline and follow-up CD4 counts, height, weight) as well as monthly treatment and clinical variables. Adverse drug reactions data for most commonly identified drug reactions related to second line drugs treatment were also collected and are analyzed descriptively in Van der Walt et al. [23]. Other treatment outcome variables collected included completion or cure, failure, default and death, which are analysed using competing risks model in Farley et al. [22].
Ethics statement
The ethics approval for the original study was obtained from the South African Medical Research Council’s Ethics Board and the South African Provincial Departments of Health in all of the nine provinces of South Africa (Western Cape, Northern Cape, Eastern Cape, Gauteng, Mpumalanga, Kwa-Zulu Natal, Limpopo, North West, and Free State), and the Institutional Review Board of The Johns Hopkins University Bloomberg School of Public. Written informed consent was obtained from each study participant before enrolment and the informed consent process was approved by the respective ethical or review boards [22,23].
Relative survival
In traditional survival analyses such as when using the Cox survival model, the cause of death is irrelevant to the analysis; thus overall survival is used to estimate the proportion of subjects still alive at a certain point in time. For a cohort of patients suffering from a particular disease, the interest lies in the deaths due to the disease rather than all the causes. Cause-specific survival analysis is thus employed to measure disease-specific survival, which is often done by performing a competing risks survival analysis.
However, a slightly more complex form for performing a causespecific survival analysis is based on relative survival analysis. This method is based on using two mortality rates: the overall mortality rate observed in a cohort of patients and the expected mortality rate in the general population. The assumption is that the number of deaths due to the disease of interest equals the total number of deaths less the expected number of deaths in the general population [14–17]. Thus, relative survival estimates the net survival when analyzing disease-specific time-to-death when the cause of death is not readily available or not accurately known.
In our settings, it is difficult to classify each patient’s death into either ‘entirely due to MDR-TB’ or ‘entirely unrelated to MDR-TB’. Thus, within these contexts, the use of relative survival methods provides us an opportunity to reliably compare the observed proportions surviving in this group of MDR-TB patients to the expected proportion that would have survived in a group of people with the same age, sex and the calendar period from the general population of South Africa. The net survival rates are now assumed to be MDR-TB specific. We want to investigate the differences in the net survival between HIV-infected and non-HIV MDR-TB infected patients after controlling for the effects of relevant explanatory variables. We do not need information on cause of death as is the case in the other cause-specific survival models.
Modelling relative survival
When modelling relative survival, the hazard function at time t since diagnosis for persons diagnosed with MDR-TB is modelled as the sum of the expected hazard λ☆(t) and the excess hazard v(t) due to a diagnosis of MDR-TB.
where the expected hazard is estimated from the general population mortality rates.
The model is known as an additive hazards model or relative survival model, since it can be written as
Where S(t), S☆(t) and r(t) represent cumulative observed, expected and relative survival, respectively. Such models are generally biologically more plausible and provide a better fit to the data than multiplicative models. The hazards are assumed to be constant within pre-specified sub intervals of follow-up time (i.e. piecewise constant hazards). These intervals are typically of length 1 year, although it is common to use shorter intervals early in the follow up (e.g. during the first year) and longer intervals later in the follow up (e.g. after 10 years). A set of indicator variables is constructed (one indicator variable for each interval excluding the reference interval) and incorporated into the covariate vector.
Our primary interest is in the excess hazard component v(t), which is assumed to be an exponential function exp(zβ), where z is a vector of the predictor variables with the corresponding vector of coefficients β. The basic relative survival model therefore can be written as
In this way, the excess hazard component is modelled as proportional hazards model. The estimates are excess hazard ratios (EHRs), the interpretation of which are similar to that of the familiar Cox hazard ratios (HRs), only that an EHR is obtained after accounting for expected background mortality.
A Poisson model [14–15,17], which uses the above additive hazard model for estimating the parameters was implemented. Follow up intervals of 1 year, and up to a maximum of two 1-year intervals from 0, were used. A set of indicator variables were constructed, one indicator variable for each interval, and incorporated into the covariate vector.
We assume that patients are grouped into k strata based on a combination of the relevant predictors. Furthermore assume that there are i intervals of follow up. Following Dickman et al. [18], let the number of deaths, dki, be distributed as a Poisson distribution, dki ~ Poisson (μki) where μki = λki yki and yki is person time at risk for the observations in stratum k in the interval i. If we denote d☆ki as the expected number of deaths (due to causes other than MDR-TB and estimated from the general population mortality rates) in stratum k in interval i then
| (1) |
This is a Generalised Linear Model (GLM) with outcome dki distributed as a Poisson random variable using a link function ln(μki−d☆ki) with offset ln(yki). This is not a standard link function so fitting the model requires software which allows user defined link functions.
The STATA macro strs [available from http://www.pauldickman.com] was used to calculate expected survival (hence d☆ki) based on the Ederer II method [17], using life tables for South Africa [24–25]. The probabilities were obtained from the tables derived by the World Health Organization and the Statistics South Africa and were not given for every calendar year. As a result we imputed all the years that we did not have the probabilities. For each individual, censoring was at 2 years; and we estimated expected survival rate in each of the two follow up 1-year intervals. In addition, we calculated the exposure time in each interval and whether or not the subject died in the interval. We then aggregated these values for each combination of covariates in each follow up period (2 gender groups × 4 age groups × 5 periods of diagnosis × 3 HIV status groups = 120 combinations of covariate groups (2 follow up periods)).
Results
The definitions and distributions of the explanatory variables used are shown in Table 1. Log-rank test results for the equality of the survival rates between categories of the respective explanatory variables are also shown. A majority of the patients were males (62.1%), diagnosed between 2002 and 2003 (53.1%) and had prior TB disease (92.9%). Of the 1413 that were tested for HIV, 554 (39.2%) were HIV positive. Majority of the patients were within the age groups 25–34 and 35–44, accounting for about 63.0% of the patients. Data on ethambutol-resistant TB bacteria was known for 55.0% of the patients, and only for 21.0% and 15.7% on kanamycin and oxfloxacin or ciprofloxacin, respectively. In order to use the resistance data in the models, resistance patterns of TB isolates were categorized to either known or unknown. This might be an unreliable categorization, but in absence of reliable data, knowledge regarding the resistance behavior of TB isolates might influence treatment management and care.
Table 1.
Baseline characteristics and 2-year survival probabilities with 95% confidence intervals.
| Covariate | Category | Frequency n (%) | Deaths n (%)a | 2-Year Survival (95 CI%) | Log-rank test (p-value) |
|---|---|---|---|---|---|
| Overall | 2079 (100) | 367(22.67)/1619 (17.65) | 71 (66, 74) | ||
| Sex | Male | 1290 (62.14) | 215/998 (21.54) | 69 (63, 75) | 0.19 |
| Female | 786 (37.86) | 151/619 (24.39) | 72 (67, 77) | ||
| Age | 18–24 | 286 (13.89) | 45/232 (19.40) | 71 (56, 82) | <0.05 |
| 25–34 | 655 (31.81) | 120/495 (24.24) | 68 (60, 74) | ||
| 35–44 | 645 (31.33) | 133/492 (27.03) | 66 (58, 72) | ||
| > 45 | 473 (22.97) | 67/384 (17.45) | 79 (71, 86) | ||
| Year of diagnosis | 1996 – 2000 | 139 (6.69) | 20/104 (19.23) | 77 (61, 87) | 0.04 |
| 2001 | 359 (17.27) | 47/267 (17.60) | 78 (69, 84) | ||
| 2002 | 557 (26.79) | 102/453 (22.52) | 67 (56, 75) | ||
| 2003 | 546 (26.26) | 127/440 (28.86) | 68 (62, 73) | ||
| 2004 | 478 (22.99) | 71/355 (20.00) | 72 (60, 81) | ||
| TB/MDR-TB history | No | 145 (7.15) | 16/105 (15.24) | 85 (76, 91) | 0.12 |
| Yes | 1,8882 (92.85) | 348/1,479 (23.53) | 70 (65, 74) | ||
| HIV status | Negative | 859 (41.32) | 101/656 (15.40) | 76 (60, 82) | <0.05 |
| Positive | 554 (26.65) | 169/445 (37.98) | 54 (47, 61) | ||
| Status unknown | 666 (32.03) | 97/518 (26) | 79 (74, 83) | ||
| Ethambutol Resistance | Known | 1,444 (55.03) | 146/911 (16.03) | 78 (72, 82) | <0.05 |
| Unknown | 935 (44.97) | 221/708 (31.21) | 62 (56, 68) | ||
| Kanamycin Resistance | Known | 437 (20.97) | 62/382 (16.23) | 76 (68, 82) | <0.05 |
| Unknown | 1,643 (79.03) | 305/1,237 (24.66) | 69 (64, 73) | ||
| Ofloxacin/Ciprofloxacin Resistance | Known | 326 (15.68) | 57/286 (19.93) | 71 (62, 79) | 0.19 |
| Unknown | 1,753 (84.32) | 310/1,333 (23.26) | 71 (66, 75) |
Frequencies and percentages based on complete observed data, so the totals do not necessary equal those in the third column, which a gives a count of the subjects enrolled.
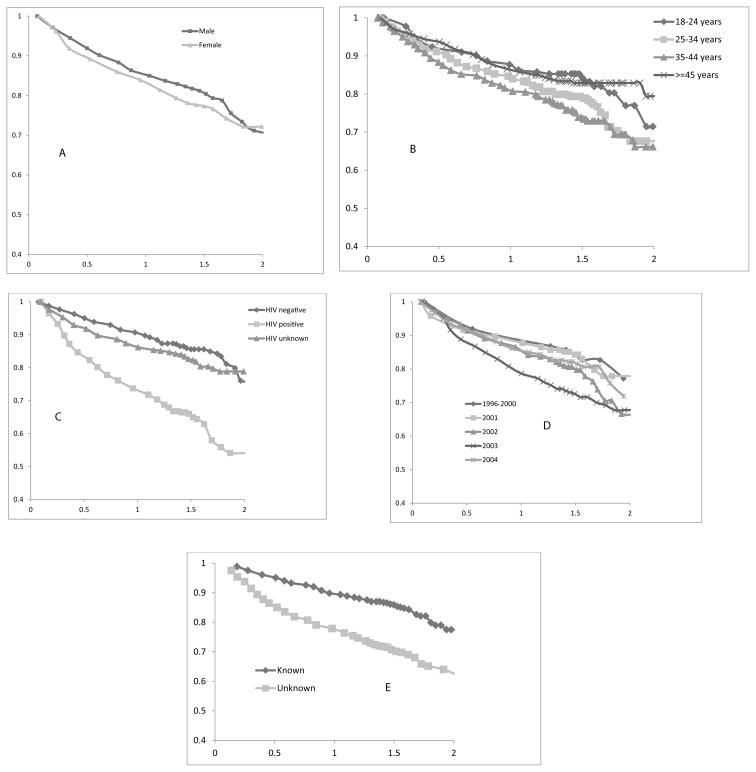
A total of 1619 patients had the data on death status within two years known, and of which 367 (22.7%) had died during the course of the treatment. The overall two-year survival rate was 70% ([95% CI: 60–80]) and was associated with age, year of diagnosis, HIV status, resistance knowledge to ethambutol and kanamycin (p-value < 0.05). However, it was not associated with gender, TB history, and resistance knowledge to ofloxacin or ciprofloxacin (p-value >0.05). The period of diagnosis had a significant effect on the survival chances of the patients, with those diagnosed in 2003 having the highest survival rates (p-value < 0.05). There was a significant difference in the survival of the patients in terms of their HIV status (p-value < 0.01). The Kaplan Meier survival curves for the MDR-TB patients classified by some of the analyzed predictor variables are shown in Figure 1.
Figure 1.
Survival fractions classified by (A) gender, (B) diagnosis age, (C) HIV status of the patient; (D) year of diagnosis and (E) resistance to ethambutol.
Other equally important predictors were not considered because of low numbers of complete data. For instance, baseline CD4 count and height were only available for 75 and 479 patients, respectively. Even though weight was available for most patients (1798), but its use is relative to other measures such as height, thus it was not considered as well. In the multivariate analysis to estimate the excess risk of death attributed to HIV co-infection, we adjusted for gender, age at MDR-TB, and year of, diagnosis, TB or MDR-TB history and resistance to some anti-TB drugs. A p-value below 0.05 was taken to indicate a statistically significant effect of the covariate.
The estimates of excess hazards ratios (EHRs) to measure the excess or net mortality due to the effect of the studied explanatory variables are shown in the last column of Table 2. For comparative purposes, the results obtained from fitting logistic and proportional hazards regression models on the odds ratio (OR) and hazards ratio (HR) scales are also shown. The excess mortality rate was higher in HIV co-infected, than in HIV-negative, patients (adjusted excess hazard ratio, 5.6 [95% CI, 3.2–9.7]). Not knowing resistance patterns of TB bacteria in a patient to ethambutol and kanamycin increased excess mortality (3.7 [2.1–6.2] and 4.9 [1.8–13.3]), respectively vs. knowing the patterns. On the other hand, not knowing whether the patient’s TB isolates were resistant or not to ofloxacin or ciprofloxacin decreased excess mortality (0.3 [0.1–0.7]) vs. knowing.
Table 2.
Comparative covariate effect estimates using the three methods.
| Categories | Odds Ratio (95% CI) | Hazards Ratio (95% CI) | Excess Hazard Ratio (95% CI) |
|---|---|---|---|
| Sex | |||
| Male | 1 | 1 | 1 |
| Female | 1.164 (0.881, 1.537) | 1.140 (0.908, 1.433) | 1.468 (0.985,2.189) |
| Age | |||
| Age 18–24 | 1 | 1 | 1 |
| Age 25–34 | 1.181 (0.757, 1.842) | 1.155 (0.796, 1.676) | 0.758 (0.440,1.308) |
| Age 35–44 | 1.506 (0.965, 2.351) | 1.371 (0.946, 1.989) | 1.014 (0.589,1.746) |
| Age >45 | 1.072 (0.660, 1.740) | 1.067 (0.707, 1.611) | 0.500 (0.213, 1.166) |
| Year of diagnosis | |||
| 1996 – 2000 | 1 | 1 | 1 |
| 2001 | 1.003 (0.513, 1.960) | 1.065 (0.597, 1.903) | 1.315 (0.355, 4.873) |
| 2002 | 1.270 (0.678, 2.381) | 1.254 (0.732, 2.151) | 1.657 (0.500, 5.497) |
| 2003 | 2.004 (1.076, 3.729) | 1.687 (0.993, 2.865) | 2.554 (0.790, 8.257) |
| 2004 | 1.024 (0.537, 1.954) | 1.058 (0.608, 1.841) | 0.837 (0.237, 2.954) |
| TB/MDR-TB history | |||
| No | 1 | 1 | 1 |
| Yes | 1.982 (1.071, 3.666) | 1.628 (0.949, 2.791) | 1.697 (0.673, 4.283) |
| HIV status | |||
| Negative | 1 | 1 | 1 |
| Positive | 4.279 (3.103, 5.899) | 2.778 (2.133, 3.619) | 5.571 (3.187, 9.739) |
| Unknown | 1.418 (1.016, 1.980) | 1.301 (0.971, 1.742) | 1.459 (0.720, 2.957) |
| Ethambutol Resistance | |||
| Known | 1 | 1 | 1 |
| Unknown | 2.467 (1.867, 3.261) | 1.907 (1.510, 2.409) | 3.654 (2.142, 6.234) |
| Kanamycin Resistance | |||
| Known | 1 | 1 | 1 |
| Unknown | 2.906 (1.538, 5.487) | 2.208 (1.305, 3.736) | 4.872 (1.782, 13.321.) |
| Ofloxacin or Ciprofloxacin Resistance | |||
| Known | 1 | 1 | 1 |
| Unknown | 0.528 (0.274, 1.016) | 0.616 (0.362, 1.046) | 0.307 (0.128, 0.736) |
Excess mortality did not depend on gender of the patients (1.5 [1.0–2.2] between females and males); neither did it depend on whether or not the patient had prior TB or MDR-TB disease (1.7 [0.7–4.3]). Excess mortality decreased with increasing age, though the trend effect was neither linear nor significant (0.8 [0.4 – 1.3], 1.0 [0.6 – 1.7], 0.5 [0.2 – 1.2]), respectively for ages 25–34, 35–44 and >=45 vs. 18–24 years. The patients who were diagnosed with MDR-TB in 2003 experienced twice as much risk of mortality (2.6 [0.8–8.3]) compared to those who were diagnosed in 1996–2000, but this finding was not significant. The two alternative methods also found significant associations between high mortality in MDR-TB patients with HIV infection.
Discussion
The epidemic levels of multidrug resistant tuberculosis (MDR-TB) are causes of concern in settings of a high HIV prevalence [5]. Our findings confirm previous studies on risk factors for MDR-TB mortality and outcomes in high HIV prevalent South Africa [23,26]. The main conclusion from these studies and the present one is that MDR-TB patients who are HIV-infected have higher mortality compared to HIV-uninfected MDR-TB patients. For our results, the comparison is obtained after accounting the general population in South Africa. This finding is not limited to MDR-TB patients; drug-susceptible TB patients have been shown to have significantly higher rates of relapse of TB when they are HIV-infected [12].
The net mortality rates in the analyzed MDR-TB patients depended on resistance ethambutol, fluoroquinolones antibiotics and second-line anti-TB injectable drugs. However, gender of patient, age and period at diagnosis and previous TB or MDR-TB infection did not affect the excess death rates. Thus, in order to prevent progression of MDR-TB to extensively drug resistant TB (XDR-TB), all MDR-TB patients must be screened for high level resistance to TB or MDR- TB drugs [1]. Having data on drug resistance profile among MDR-TB patients would help to inform best medication and care.
The findings may not be startling. However, comparatively, the results from using relative survival model show markedly higher effects on mortality among HIV co-infected MDR-TB patients than using standard methods such as logistic and proportional hazards models (Odds ratio, 4.3; hazards ratio, 2.8; excess hazards ratio, 5.6). The relative survival method measures the risk of death among HIV infected MDR-TB compared with mortality in the general population of South Africa. The results from the other methods have shown evidence indicating mortality among MDR-TB being high. However, the relative survival results show much higher mortality among HIV infected MDR-TB patients when compared to the general mortality. Thus, other methods may have underestimated the gravity of the situation among MDR-TB patients with HIV infection.
The data for our study was undertaken at the time when the antiretroviral treatment (ART) was not publicly available in South Africa. The increasing trend in mortality over the period of study may be an indication of the maturity of HIV progression to AIDS (and hence increased mortality in the absence of ARVs) among MDR-TB patients. However, in 2004 mortality data of patients from Kwazulu-Natal was not obtained; thus removing from the data high risk MDR-TB patients. The Kwazulu-Natal province has one of the highest rates of drug resistant TB and HIV in the country. The antiretroviral treatment was rolled out in 2004 and South Africa has repeatedly changed policies on TB and HIV treatments. Previously, voluntary HIV testing and counseling (VCT) was the entry point for patients to receive ART, and given the stigma that was associated with HIV, most patients were not willing to be tested to know their HIV infection [27]. This is reflected by 32% of the patients in our study with unknown HIV status. The patients with unknown HIV status may as well have been infected with HIV. Limited availability of ARVs in this period might have adversely impacted on the mortality levels of this cohort of MDR-TB patients compared to the MDR-TB patients in this era of free ARVs. In order to increase patients’ access to ART, HIV testing has been made routine for individuals who are at risk, including TB patients [28,29].
However, a limitation of this study is that the effect of ARV use on survival in MDR-TB patients was not addressed as the parent study pre-dated the ARV rollout. Since then, a number of studies have given evidence to the widely held extrapolation that ARV is necessary for MDR-TB outcomes; see for example the STRIDE [21] and SAPIT [30] trials. In recognition of the dual epidemic of HIV and TB, current national guidelines and policies are geared towards integration of HIV and TB services [29,31]. There is an increased support for HIV and TB interventions including provisions of ART for TB and HIV co-infected patients; HIV counseling and testing for TB patients; and routine screening for TB on HIV patients [32,33]. All these require better understanding of the association between HIV infection and anti-TB drug resistance, care and control of the MDR-TB epidemic.
A further limitation of the study concerns the interpretations of the results. In order to calculate expected survival, we use general population mortality rates, where we took probabilities of dying within a year. Due to imputations of these probabilities in some years, the expected survival rates may have been under- or over- estimated. The number of deaths observed may have been underestimated in KwaZulu-Natal province, especially for the year 2004 due to inaccessibility of the patients’ records during follow-up. This might have affected the excess mortality risk estimated for the same year. We have used regression techniques to explain survival times in terms of differences in risk exposures. We did not exhaustively address the problem of possible confounding variables such as smoking, alcohol consumption, poor nutrition, lack of education, poverty, over-crowding and stress [3–9]. We opted for parsimony in our analyses by including relevant explanatory variables that were measured and collected.
In biomedical studies, accurate empirical evidence is needed, and we have shown that relative survival methods are valuable tools that can be used to provide reliable and robust estimate of MDR-TB related mortality when the cause of death cannot be accurately determined. The fact that we get the same substantive policy answers when using classical methods is irrelevant. The main point is that now we are using an appropriate method, which can be taken as validating the logistic and Cox proportional hazards models in this context. However, for cause-specific survival, relative survival has many advantages over the standard proportional hazards model, which is still the preferred model when considering overall survival. In situations where death certification is inaccurate, and one wants disease-specific survival, then relative survival and competing risks models are used, the former taken as gold standard in these situations. Relative survival methods should be used in future studies for MDR-TB and HIV co-infection, after controlling for ART, which was neither collected nor performed in this study.
Acknowledgments
Funding
The original study on which the present article is based was sponsored by a grant from the Burroughs Wellcome Fund to the Johns Hopkins University School of Nursing. This study was also partially supported by the Fogarty International Center at the National Institutes of Health by grant number 5R25TW007506 [22]. The South African National Pilot and DOTS-Plus projects are supported by the TB directorate, Department of Health in South Africa [23]. The funders had neither role in the original study design and data collection nor any role in the analysis, interpretation and preparation of the present manuscript.
Footnotes
Author Contributions
SOMM conceived and designed the manuscript, interpreted the results and wrote the manuscript; LJM conducted the data management, statistical analysis the data, and contributed to the writing of the manuscript; JLL helped with the clarification of the data and the accuracy of the results; and MLvdW provided the data and read the manuscript for important substantive content.
References
- 1.Prasad R. Multidrug and extensively drug-resistant TB (M/XDR-TB): problems and solutions. Indian J Tuberc. 2010;57:180–191. [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report. WHO Press; Geneva: 2012. [Google Scholar]
- 3.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 4.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis & management. Indian J Med Res. 2005;121:550–567. [PubMed] [Google Scholar]
- 5.Cox HS, McDermid C, Azevedo V, Muller O, Coetzee D, et al. Epidemic levels of drug resistant tuberculosis (MDR and XDR-TB) in a high HIV prevalence setting in Khayelitsha, South Africa. PLoS One. 2010;5:e13901. doi: 10.1371/journal.pone.0013901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shean KP, Willcox PA, Siwendu SN, Laserson KF, Gross L, et al. Treatment outcome and follow-up of multidrug-resistant tuberculosis patients, West Coast/Winelands, South Africa, 1992–2002. Int J Tuberc Lung Dis. 2008;12:1182–1189. [PubMed] [Google Scholar]
- 7.Weyer K, Brand J, Lancaster J, Levin J, van der Walt M. Determinants of multidrug-resistant tuberculosis in South Africa: results from a national survey. S Afr Med J. 2007;97:1120–1128. [PubMed] [Google Scholar]
- 8.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and metaanalysis. PLoS One. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 10.Soleman N, Chandramohan D, Shibuya K. Verbal autopsy: current practices and challenges. Bull World Health Organ. 2006;84:239–245. doi: 10.2471/blt.05.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171–177. [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly PM, Cumming RG, Kaldor JM. HIV and tuberculosis in rural sub- Saharan Africa: a cohort study with two year follow-up. Trans R Soc Trop Med Hyg. 1999;93:287–293. doi: 10.1016/s0035-9203(99)90025-1. [DOI] [PubMed] [Google Scholar]
- 13.Stare J. An individual measure of relative survival. Appl Statist. 2005;54:115–126. [Google Scholar]
- 14.Pohar M, Stare J. Making relative survival analysis relatively easy. Comput Biol Med. 2007;37:1741–1749. doi: 10.1016/j.compbiomed.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Pohar M, Stare J. Relative survival analysis in R. Comput Methods Programs Biomed. 2006;81:272–278. doi: 10.1016/j.cmpb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 16.EDERER F, AXTELL LM, CUTLER SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 17.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 18.Fairley L, Forman D, West R, Manda S. Spatial variation in prostate cancer survival in the Northern and Yorkshire region of England using Bayesian relative survival smoothing. Br J Cancer. 2008;99:1786–1793. doi: 10.1038/sj.bjc.6604757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods LM, Rachet B, Cooper N, Coleman MP. Predicted trends in longterm breast cancer survival in England and Wales. Br J Cancer. 2007;96:1135–1138. doi: 10.1038/sj.bjc.6603668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 21.Brinkhof MW, Boulle A, Weigel R, Messou E, Mathers C, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6:e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farley JE, Ram M, Pan W, Waldman S, Cassell GH, et al. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a cohort of South African patients with high HIV prevalence. PLoS One. 2011;6:e20436. doi: 10.1371/journal.pone.0020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Walt M, Lancaster J, Odendaal R, Davis JG, Shean K, et al. Serious treatment related adverse drug reactions amongst anti-retroviral naïve MDR-TB patients. PLoS One. 2013;8:e58817. doi: 10.1371/journal.pone.0058817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson BA, Phillips HE. Adult mortality (age 15–64) based on death notification data in South Africa: 1997–2004. Pretoria, Statistics South Africa; Private Bag X 44, Pretoria 0001, South Africa: 2006. [Google Scholar]
- 25.World Health Organization. Life tables for World Health Organization Member States. [Google Scholar]
- 26.Brust JC, Lygizos M, Chaiyachati K, Scott M, van der Merwe TL, et al. Culture conversion among HIV co-infected multidrug-resistant tuberculosis patients in Tugela Ferry, South Africa. PLoS One. 2011;6:e15841. doi: 10.1371/journal.pone.0015841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kigozi NG, Heunis JC, Chikobvu P, van den Berg H, van Rensburg HC, et al. Predictors of uptake of human immunodeficiency virus testing by tuberculosis patients in Free State Province, South Africa. Int J Tuberc Lung Dis. 2010;14:399–405. [PubMed] [Google Scholar]
- 28.Tuberculosis Strategic Plan for South Africa. The Draft National Infection Prevention And Control Policy For Tb, Mdrtb And Xdrtb. Department of Health; Republic of South Africa: 2007–2011. [Google Scholar]
- 29.Multi-Drug Resistant Tuberculosis. A Policy Framework on Decentralised and Deinstitutionalised Management for South Africa. Department of Health; Republic of South Africa: 2011. [Google Scholar]
- 30.WHO. Antiretroviral treatment as prevention (TASP) of HIV and TB. Geneva: WHO; 2012. [Google Scholar]
- 31.Havlir D, Ive P, Kendall M, Luetkemeyer A, Swindells S, et al. International randomised trial of immediate vs. early ART in HIV+ patients treated for TB: ACTG 5221 STRIDE Study. 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2001. [Google Scholar]
- 32.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, et al. Timing of initiation of Antiretroviral drugs during tuberculosis therapy. N Eng J Med. 2011;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdool Karim SS, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374:921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]