Abstract
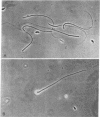
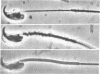
Several methods have been devised for the isolation and labeling of structural components of spermatozoa. Rodent spermatozoa were cleaved rapidly and specifically at the junction of the heads and tails by treatment with various proteases, and the separate components were isolated by density-gradient centrifugation. Treatment with reducing agents released the mitochondrial membranes from the midpiece, exposing the underlying tail structures.
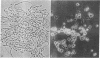
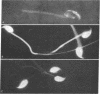
Mouse spermatozoa were found to contain about 107 sites per cell that bind concanavalin A; most of the sites appear to be on the head, for fluorescein-labeled conjugates of concanavalin A were bound mainly to the acrosomal region. Binding of concanavalin A resulted in rapid agglutination of spermatozoa; mixed agglutinates could be formed with somatic cells, as well as with spermatozoa of other species. Fluorescent probes (naphthalenesulfonic acids) bound to the sperm plasma-membrane and caused an immediate loss of motility. In contrast, ethidium bromide bound to the nuclear structures, but did not cause immediate immobilization. These isolation and probing procedures should facilitate detailed chemical analysis of the major components of mammalian spermatozoa.
Keywords: fluorescent probes, sperm motility, agglutination, lectins, subcellular fractions
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON N. G. The mass isolation of whole cells from rat liver. Science. 1953 Jun 5;117(3049):627–628. doi: 10.1126/science.117.3049.627. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Hartree E. F. Lysosomal enzymes in the acrosome and their possible role in fertilization. J Reprod Fertil. 1970 Apr;21(3):501–515. doi: 10.1530/jrf.0.0210501. [DOI] [PubMed] [Google Scholar]
- Anderson W. A. Cytochemistry of sea urchin gametes. II. Ruthenium red staining of gamete membranes of sea urchins. J Ultrastruct Res. 1968 Aug;24(3):322–333. doi: 10.1016/s0022-5320(68)90068-3. [DOI] [PubMed] [Google Scholar]
- Azzi A., Chance B., Radda G. K., Lee C. P. A fluorescence probe of energy-dependent structure changes in fragmented membranes. Proc Natl Acad Sci U S A. 1969 Feb;62(2):612–619. doi: 10.1073/pnas.62.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP M. W., SMILES J. DIFFERENTIATION OF THE ACROSOME IN LIVING MAMMALIAN SPERMATOZOA AND SPERMADTIDS BY FLUORESCENCE MICROSCOPY. J Reprod Fertil. 1963 Oct;6:297–303. doi: 10.1530/jrf.0.0060297. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Gitler C., Rubalcava B., Caswell A. Fluorescence changes of ethidium bromide on binding to erythrocyte and mitochondrial membranes. Biochim Biophys Acta. 1969;193(2):479–481. doi: 10.1016/0005-2736(69)90208-9. [DOI] [PubMed] [Google Scholar]
- Gwatkin R. B., Hutchison C. F. Capacitation of hamster spermatozoa by beta-glucuronidase. Nature. 1971 Jan 29;229(5283):343–344. doi: 10.1038/229343b0. [DOI] [PubMed] [Google Scholar]
- HARTREE E. F., SRIVASTAVA P. N. CHEMICAL COMPOSITION OF THE ACROSOMES OF RAM SPERMATOZOA. J Reprod Fertil. 1965 Feb;9:47–60. doi: 10.1530/jrf.0.0090047. [DOI] [PubMed] [Google Scholar]
- JANOWSKY D. S., ROSENAU W., MOON H. D. ISOLATION OF IMMUNOLOGICALLY COMPETENT LYMPHOCYTES FROM SENSITIZED MOUSE SPLEENS. Proc Soc Exp Biol Med. 1964 Jan;115:77–79. doi: 10.3181/00379727-115-28835. [DOI] [PubMed] [Google Scholar]
- McClure W. O., Edelman G. M. Fluorescent probes for conformational states of proteins. 3. The activation of chymotrypsinogen. Biochemistry. 1967 Feb;6(2):567–572. doi: 10.1021/bi00854a026. [DOI] [PubMed] [Google Scholar]
- McClure W. O., Edelman G. M. Fluorescent probes for conformational states of proteins. I. Mechanism of fluorescence of 2-p-toluidinylnaphthalene-6-sulfonate, a hydrophobic probe. Biochemistry. 1966 Jun;5(6):1908–1919. doi: 10.1021/bi00870a018. [DOI] [PubMed] [Google Scholar]
- NEUBERT D., LEHNINGER A. L. The effect of thiols and disulfides on water uptake and extrusion by rat liver mitochondria. J Biol Chem. 1962 Mar;237:952–958. [PubMed] [Google Scholar]
- Rubalcava B., Martínez de Muñoz D., Gitler C. Interaction of fluorescent probes with membranes. I. Effect of ions on erythrocyte membranes. Biochemistry. 1969 Jul;8(7):2742–2747. doi: 10.1021/bi00835a008. [DOI] [PubMed] [Google Scholar]
- SRIVASTAVA P. N., ADAMS C. E., HARTREE E. F. ENZYMIC ACTION OF ACROSOMAL PREPARATIONS ON THE RABBIT OVUM IN VITRO. J Reprod Fertil. 1965 Aug;10:61–67. doi: 10.1530/jrf.0.0100061. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Taylor E. W. Properties of the protein subunit of central-pair and outer-doublet microtubules of sea urchin flagella. J Cell Biol. 1968 Aug;38(2):304–315. doi: 10.1083/jcb.38.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambaugh R., Buckley J. Identification and subcellular localization of the enzymes effecting penetration of the zona pellucida by rabbit spermatozoa. J Reprod Fertil. 1969 Aug;19(3):423–432. doi: 10.1530/jrf.0.0190423. [DOI] [PubMed] [Google Scholar]
- Vaidya R. A., Bedford J. M., Glass R. H., Morris J. M. Evaluation of the removal of tetracycline fluorescence from spermatozoa as a test for capacitation in the rabbit. J Reprod Fertil. 1969 Aug;19(3):483–489. doi: 10.1530/jrf.0.0190483. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Martonosi A. Sarcoplasmic reticulum. 8. Use of 8-anilino-1-naphthalene sulfonate as conformational probe on biological membranes. Arch Biochem Biophys. 1969 Aug;133(1):153–163. doi: 10.1016/0003-9861(69)90499-8. [DOI] [PubMed] [Google Scholar]
- Wang J. L., Cunningham B. A., Edelman G. M. Unusual fragments in the subunit structure of concanavalin A. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1130–1134. doi: 10.1073/pnas.68.6.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Edelman G. M. Fluorescent probes for conformational states of proteins. IV. The pepsinogen-pepsin conversion. J Biol Chem. 1971 Mar 10;246(5):1185–1191. [PubMed] [Google Scholar]
- Weltman J. K., Arnold J. H. The interaction of a hydrophobic probe with human blood cells. Proc Soc Exp Biol Med. 1969 Jun;131(2):546–549. doi: 10.3181/00379727-131-33922. [DOI] [PubMed] [Google Scholar]
- Wood B. T., Thompson S. H., Goldstein G. Fluorescent antibody staining. 3. Preparation of fluorescein-isothiocyanate-labeled antibodies. J Immunol. 1965 Aug;95(2):225–229. [PubMed] [Google Scholar]
- Zaneveld L. J., Srivastava P. N., Williams W. L. Relationship of a trypsin-like enzyme in rabbit spermatozoa to capacitation. J Reprod Fertil. 1969 Nov;20(2):337–339. doi: 10.1530/jrf.0.0200337. [DOI] [PubMed] [Google Scholar]