Abstract
Background
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous clinicopathological entity, and its molecular classification into germinal center B cell-like (GCB) and activated B cell-like (ABC) subtypes using gene expression profile analysis has been shown to have prognostic significance. Recent attempts have been made to find an association between immunohistochemical findings and molecular subgroup, although the clinical utility of immunohistochemical classification remains uncertain.
Methods
The clinicopathological features and follow-up data of 68 cases of surgically resected gastrointestinal DLBCL were analyzed. Using the immunohistochemical findings on tissue microarray, the cases were categorized into GCB and non-GCB subtypes according to the algorithms proposed by Hans, Muris, Choi, and Tally.
Results
The median patient age was 56 years (range, 26-77 years). Of the 68 cases included, 39.7% (27/68) involved the stomach, and 60.3% (41/68) involved the intestines. The GCB and non-GCB groups sorted according to Hans, Choi, and Tally algorithms, but not the Muris algorithm, were closely concordant (Hans vs. Choi, κ=0.775, P<0.001; Hans vs. Tally, κ=0.724, P<0.001; Choi vs. Tally, κ=0.528, P<0.001). However, there was no prognostic difference between the GCB and non-GCB subtypes, regardless of the algorithm used. On univariate survival analyses, international prognostic index risk group and depth of tumor invasion both had prognostic significance.
Conclusion
The Hans, Choi, and Tally algorithms might represent identical DLBCL subgroups, but this grouping did not correlate with prognosis. Further studies may delineate the association between immunohistochemical subgroups and prognosis.
Keywords: Diffuse large B-cell lymphoma, Gastrointestinal tract, Immunohistochemistry, Prognosis
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL), the most common form of non-Hodgkin lymphoma, is a heterogeneous entity encompassing a range of clinical and morphological features [1]. Although the development of standard anthracyclin-based chemotherapeutic regimen [2, 3] and adjunctive rituximab immunotherapy [4, 5] has dramatically improved patient survival, up to 40% of patients eventually die of disease. To predict the prognosis of DLBCL patients, the international prognostic index (IPI), which uses a number of clinical and laboratory parameters, is widely employed [6]. However, the outcome of patients placed in the same risk group on the basis of IPI is still somewhat variable, making the discovery of additional prognostic factors an important goal [7].
DLBCL can be subdivided into germinal center B cell-like (GCB) and activated B cell-like (ABC) types, based on gene expression profiling (GEP), and each is associated with a distinct prognosis [8, 9]. This was also confirmed in subsequent studies conducted after the introduction of rituximab for the treatment of DLBCL [10]. However, it is not practical to use GEP in routine clinical practice.
Recently, several algorithms have been proposed for distinguishing these subgroups, based on a panel of immunohistochemical stains for the germinal center B-cell markers (CD10, BCL6, GCET1, and LMO2) and post-germinal center B-cell markers (MUM1/IRF4 and FOXP1) [11-15]. Although the immunohistochemical staining method is relatively simple and readily accessible compared with GEP and gives comparable results, it remains unclear whether the immunohistochemical classification can predict patient survival [16-19]. The aim of this study was to investigate the prognostic significance of immunohistochemical subgroups in gastrointestinal DLBCL and to evaluate the level of concordance between the different algorithms.
MATERIALS AND METHODS
Case selection
Cases involving surgically resected gastrointestinal DLBCL performed in the Asan Medical Center between January 1996 and March 2011 were included in this evaluation. Cases treated using CHOP or rituximab-CHOP (R-CHOP) regimens were included, while we excluded cases, in which no chemotherapy or non-CHOP-based regimens were administered. Cases of DLBCL arising in indolent B-cell lymphomas and posttransplantation lymphoproliferative disorders were also excluded. The medical records were reviewed, and clinical parameters such as age, gender, performance status, serum lactate dehydrogenase (LDH) concentration, and sites of involvement were documented. The depth of invasion was stratified according to the T stage of individual organs, [20] and tumor perforation was considered to represent serosal involvement. On the basis of this data, disease stage according to the Lugano classification system and risk group according to the IPI at presentation were assessed.
Pathological review and tissue microarray construction
Representative sections of the resected specimens were reviewed and reassessed by two pathologists (H.S.H. and J.H.). Representative paraffin-embedded tissue blocks of the selected cases were chosen after review. Two independent tumor cores (1 mm in diameter) were obtained using a trephine apparatus from the tissue blocks. The extracted cores were then consecutively embedded in void paraffin blocks. Four recipient blocks containing 210 individual cores were made. A 4-µm-thick representative section from each recipient block was stained with hematoxylin and eosin (H&E) using the standard protocol.
Immunohistochemistry
Immunohistochemical staining for CD10, BCL2, BCL6, MUM1/IRF4, FOXP1, GCET1, and LMO2 were performed on 4-µm-thick sections from the tissue microarray block using the BenchMark XT autostainer (Ventana Medical Systems, Tucson, Arizona). Briefly, these sections were deparaffinized by xylene and ethanol. After epitope retrieval (heating for 30 minutes in ethylene diamine tetraacetic acid buffer, pH 8.0), samples were incubated with diluted primary antibodies for 1 hour at room temperature. The slides were washed again and incubated with multimer-labeled anti-mouse or rabbit IgG (Ventana), after which staining was developed using the UltraView staining kit (Ventana) according to the manufacturer's protocol. Finally, samples were counterstained with hematoxylin. The manufacturers, clones, and dilution titer of the primary antibodies are summarized in Table 1. The staining results were interpreted by two individual pathologists (H.S.H. and J.H.), and any discrepancies were reviewed to achieve a consensus opinion. If two separate cores from the same case had different signals, these were averaged before evaluation.
Table 1.
Primary antibodies used for immunohistochemical staining and their dilution.
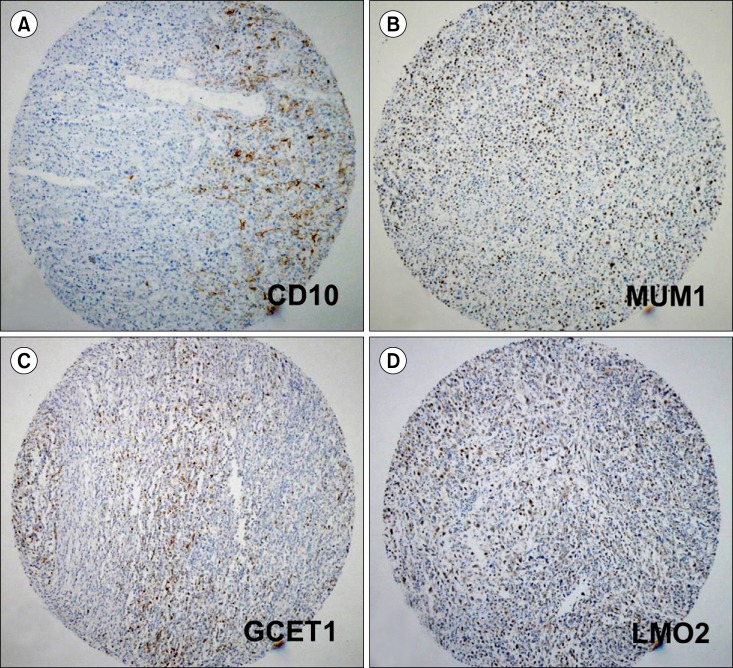
The criteria by which a positive signal was defined were the same as those used in previous studies (Fig. 1) [11-15]. For the interpretation of CD10, BCL2, and BCL6 immunohistochemical staining, the proportion of cells with a positive signal, rather than the signal intensity, was considered to be more informative as staining intensity may vary with tissue fixation and processing. However, for FOXP1, only strong nuclear staining in a significant proportion of tumor cells was considered to be a positive signal.
Fig. 1.
Examples of partially positive staining for each immunohistochemical marker. (A) CD10 staining showing a positive reaction in a small proportion of the tumor cells (about 20%), which is considered to be a negative result in all 4 algorithms (×100). (B) MUM1 staining exhibiting reactivity in about 40% of tumor cells, which is considered to be positive in the Hans and Tally algorithms but negative in the Choi algorithm (×100). (C) GCET1 positive staining in some tumor cells (about 30%), which is considered to be a negative result in the Choi algorithm (×100). (D) LMO2 positive staining in scattered tumor cells with anaplastic nuclei (about 20%) (×100).
Immunohistochemical algorithms
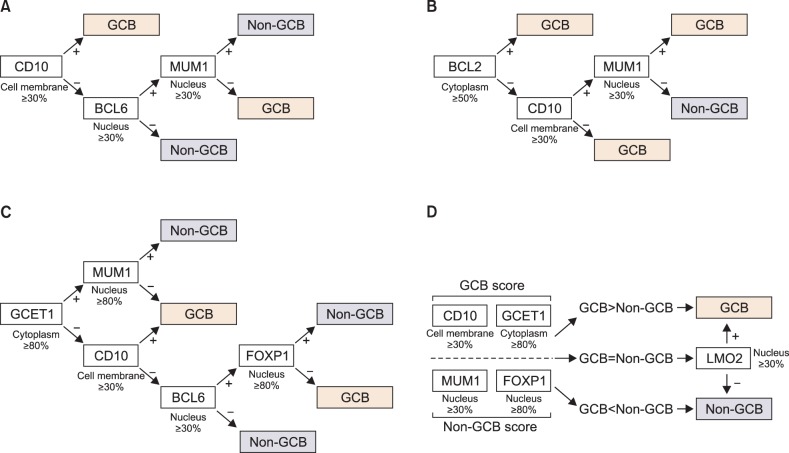
Using the results of immunohistochemistry, the submitted cases were classified into GCB or non-GCB subtypes based on the previously established algorithms. The classifications were automatically performed using programmed formulas in Microsoft Excel 2003 (Microsoft Corporation, Redmond, Washington). The diagrammatic representation of algorithms and the criteria for positive immunohistochemical staining are shown in Fig. 2.
Fig. 2.
Summary of the (A) Hans, (B) Muris, (C) Choi, and (D) Tally algorithms, and criteria for a positive signal for individual immunohistochemical markers (below or to the right of the white-filled box). Note that the positive criterion for MUM1/IRF4 in the Choi algorithm (more than 80%) is different from that of the other algorithms (more than 30%).
Statistical analysis
The definition of complete remission (CR) was standardized and assessed clinically and radiologically [21]. To evaluate the concordance between the algorithms described above, the cases categorized by individual algorithms were analyzed using Pearson's chi-square test. The concordance rates and kappa coefficients were calculated to determine the degree of agreement between the algorithms.
Progression-free survival (PFS) was defined as the period from the day of initial diagnosis to the day of recurrence, death, or the last follow-up. Similarly, the overall survival (OS) was defined as the period from the day of initial diagnosis to death or the last follow-up. The follow-up period was limited to 5 years. The PFS and OS distributions with respect to individual markers and immunohistochemical subtypes were evaluated by Kaplan-Meier survival analysis, and the differences between survival curves were evaluated using the log-rank test. The above statistical evaluations were performed using the SPSS Statistics 17.0 software (SPSS Inc., Chicago, Illinois). Differences were considered to be statistically significant if the P<0.05.
RESULTS
Baseline characteristics
The overall clinical characteristics of the 68 patients are summarized in Table 2. The mean age of the patients was 55.5 years (range, 26-77 years). Twenty-four of the 68 patients were older than 60 years (35.3%), and there were more male patients than female patients (M/F, 1.61:1). Ten and 19 patients showed B symptoms and high serum LDH levels at presentation, respectively. All of the patients had a good performance status (ECOG performance status ≤1), and most had a low Lugano stage and IPI scores (Lugano stage I or II1, 67.6%; Low IPI risk group, 70.6%) at presentation. In most cases (70.6%), the tumor extended beyond the proper muscle and involved the adipose tissue or serosa. Thirty (44.1%) of the 68 patients received R-CHOP chemotherapy, and in contrast to previous reports, there was no statistically significant difference in the remission rate between patients receiving R-CHOP and those receiving CHOP only (P=0.436).
Table 2.
Baseline characteristics of assigned cases (N=68).
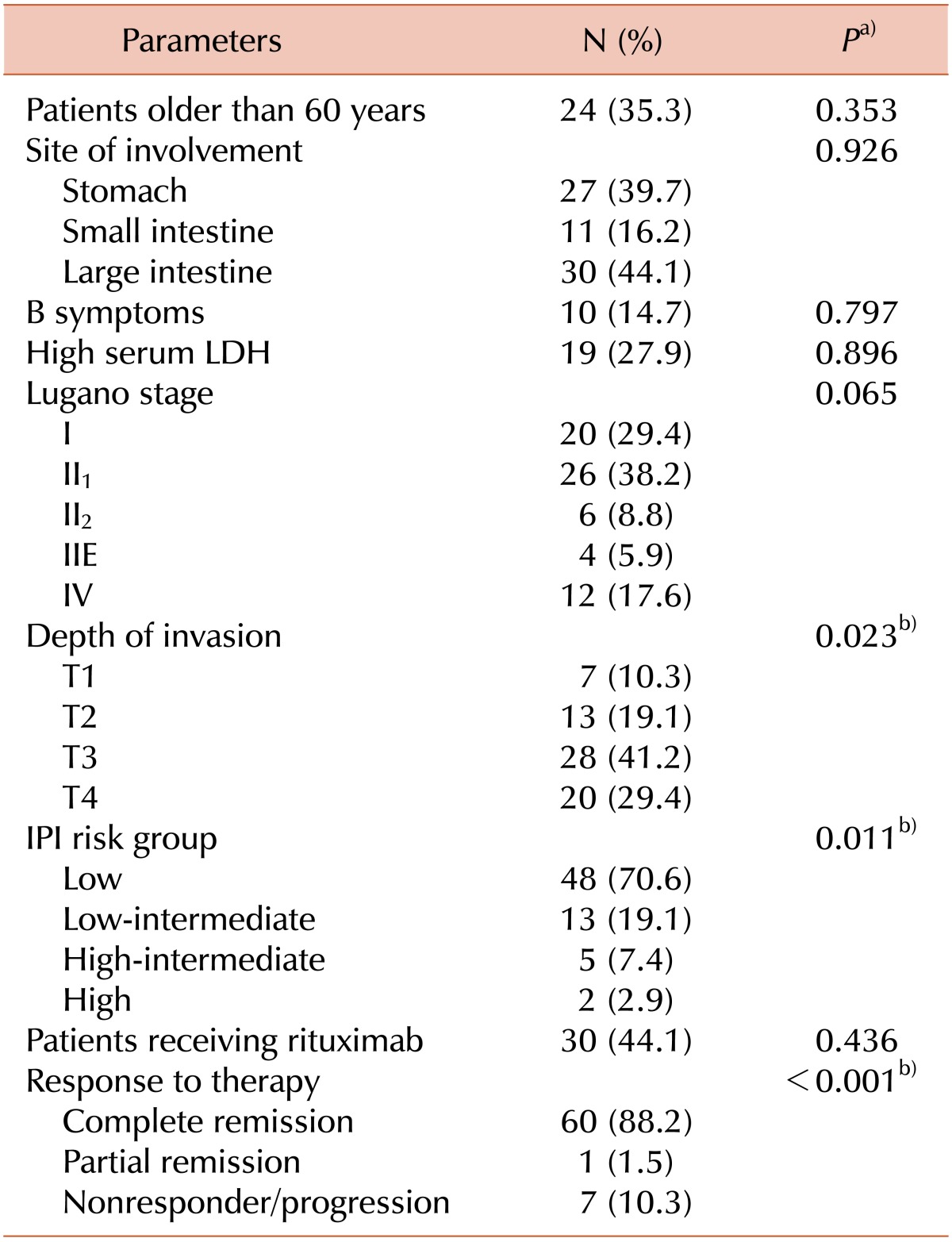
a)Log-rank test. b)Statistically significant parameters.
Abbreviations: IPI, international prognostic index; LDH, lactate dehydrogenase.
The follow-up time ranged from 4.7 to 171.6 months (median, 45.9 months). Sixty patients (88.2%) achieved CR; 96.3% of gastric primary DLBCL cases and 82.9% of intestinal DLBCL cases archived CR. One patient with partial remission achieved long-term remission when treated using a ifosfamide-based regimen. The disease progressed in a further 7 cases (10.3%), and eventually, 8 patients (11.8%) died, with a median follow-up period of 11.3 months (range, 4.7-24.9 months), due to progressive disease in 7 cases and pulmonary small cell carcinoma in 1 case. The overall 5-year survival rate was 88.2% (60/68); 85.2% and 90.2% for cases involving the stomach and intestines, respectively.
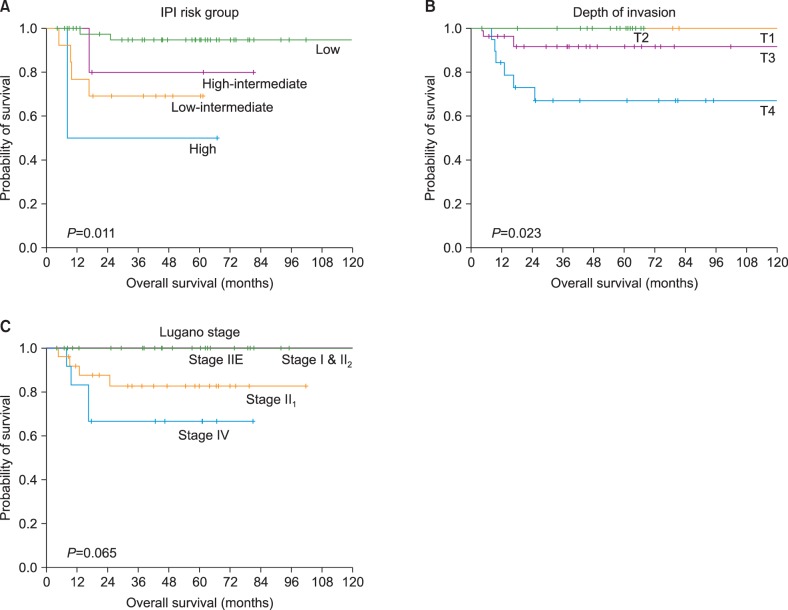
When univariate survival analyses for OS were performed, IPI risk group (P=0.011) (Fig. 3A) and tumor invasion depth (P=0.023) (Fig. 3B) proved to be significant predictive factors for patient survival. Other parameters such as Lugano stage (Fig. 3C), B symptoms, and high-serum LDH were not significantly associated with survival.
Fig. 3.
Kaplan-Meier survival analyses with respect to the (A) IPI risk group, (B) depth of tumor invasion and (C) Lugano stage.
Immunochemistry and immunohistochemical classifications
The results of immunostaining for individual proteins and immunohistochemical classification according to the different algorithms are shown in Tables 3 and 4, respectively. Cross-tabulation analyses between pairs of algorithms revealed that 3 of the 4 algorithms (Hans, Choi, and Tally) agreed substantially with each other (Hans vs. Choi, κ=0.775, P<0.001; Hans vs. Tally, κ=0.724, P<0.001; Choi vs. Tally, κ=0.528, P<0.001). However, though statistically significant, the Muris algorithm showed only a relatively weak agreement with each of the other algorithms (vs. Hans, κ=0.351, P<0.001; vs. Choi, κ=0.393, P=0.001; vs. Tally, κ=0.216, P=0.004; Table 5). All the algorithms (except Muris) gave similar case distributions, with identical results in 54 (79.4%) of 68 cases. However, the concordance of the Muris algorithm with the other 3 algorithms did not reach this level (vs. Hans, 63.2%; vs. Choi, 67.6%; vs. Tally, 52.9%).
Table 3.
Scoring of the different immunohistochemistry stains using individual antibodies.
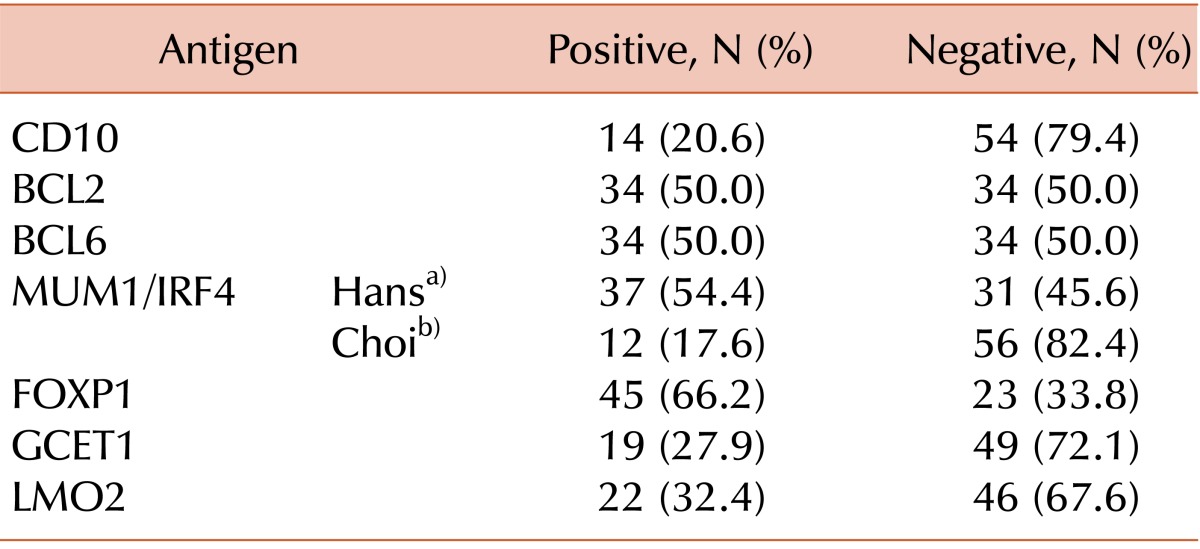
a)Results according to the Hans algorithm criteria (nuclear staining ≥30%). b)Results according to the Choi algorithm criteria (nuclear staining ≥80%).
Table 4.
Cross-table analysis of the distribution of the GCB and non-GCB subtypes according to 3 different algorithms.
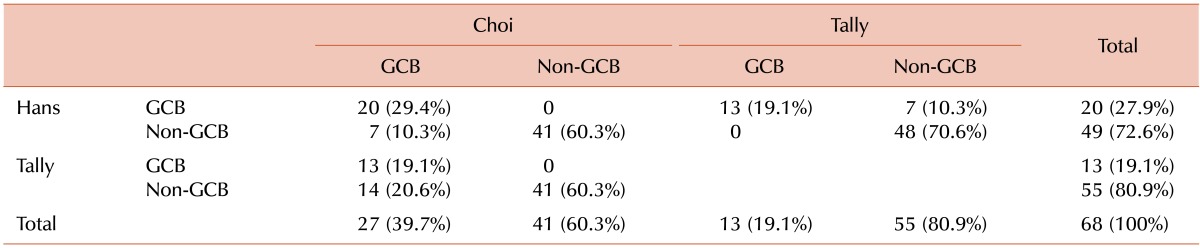
P<0.001 in all the 3 chi-square analyses.
Abbreviation: GCB, germinal center B-cell-like.
Table 5.
The concordance rate and degrees of agreement between all the 4 algorithms in the gastrointestinal diffuse large B-cell lymphomas.

a)Substantial agreement. b)Moderate agreement.
The 7 discordant cases between the Hans and Choi algorithms were interpreted as a non-GCB subtype by the former and a GCB subtype by the latter. Most of these cases showed partial MUM1/IRF4 immunoreactivity (about 30-50%), which was considered as positive in the Hans algorithm, but negative in the Choi algorithm. All the cases expressed one or more newly developed GCB markers (GCET1 and LMO2) but also expressed FOXP1 in 4 cases (Table 6).
Table 6.
Immunohistochemistry profiles of discrepant cases: Hans versus Choi algorithms.
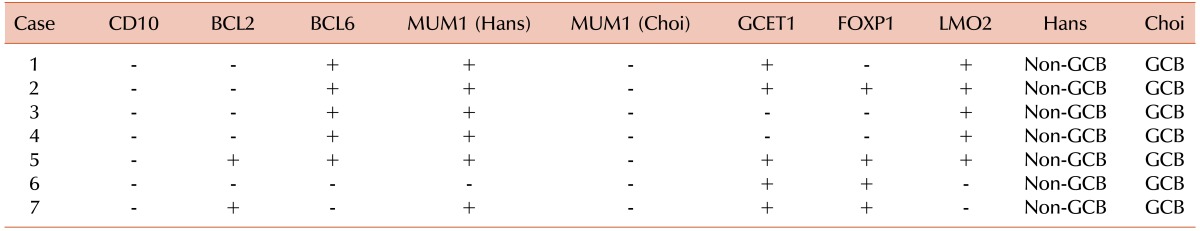
Abbreviation: GCB, germinal center B cell-like.
Similarly, the 7 discrepant cases between the Hans and Tally algorithms were interpreted as a GCB subtype by the former and a non-GCB subtype by the latter. All the cases expressed at least one of the conventional GCB markers, CD10 (6 of 7 cases) and/or BCL6 (5 of 7 cases). However, GCET and LMO2 were only expressed in a single case and in 2 cases, respectively (Table 7). In addition, partial immunoreactivities for MUM1/IRF4 were observed in 4 cases, and FOXP1 expression was observed in 5 cases.
Table 7.
Immunohistochemistry profiles of discrepant cases: Hans versus Tally algorithms.
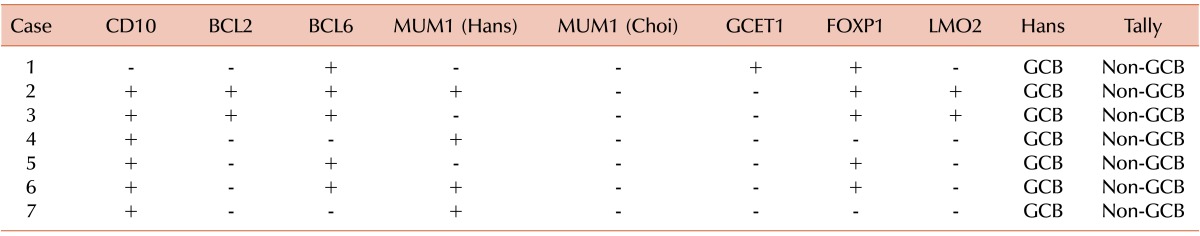
Abbreviation: GCB, germinal center B cell-like.
The 14 discordant cases between the Choi and Tally algorithms were the same as those described above. All of these cases were classified as GCB by the Choi algorithm but as non-GCB by the Tally algorithm.
Prognostic significance of immunohistochemical classifications
The results of the univariate survival analyses for the individual markers and immunohistochemical classifications are summarized in Table 8. None of the immunohistochemical classifications were significantly associated with OS. However, cases of non-GCB type disease, as defined by the Tally algorithm, exhibited worse PFS with marginal significance (P<0.1). No single immunohistochemical marker was significantly associated with OS or PFS. Nevertheless, BCL2 and LMO2 expression had a borderline association with unfavorable OS and favorable PFS, respectively (P<0.1). In localized disease, the non-GCB subgroup defined by the Hans algorithm also had a borderline association with worse PFS (P<0.1). For the single markers, BCL6 expression was also associated with favorable OS and PFS, but again with marginal significance. No other single marker was significantly associated with OS and PFS.
Table 8.
Univariate survival analyses of immunohistochemical markers and algorithms.
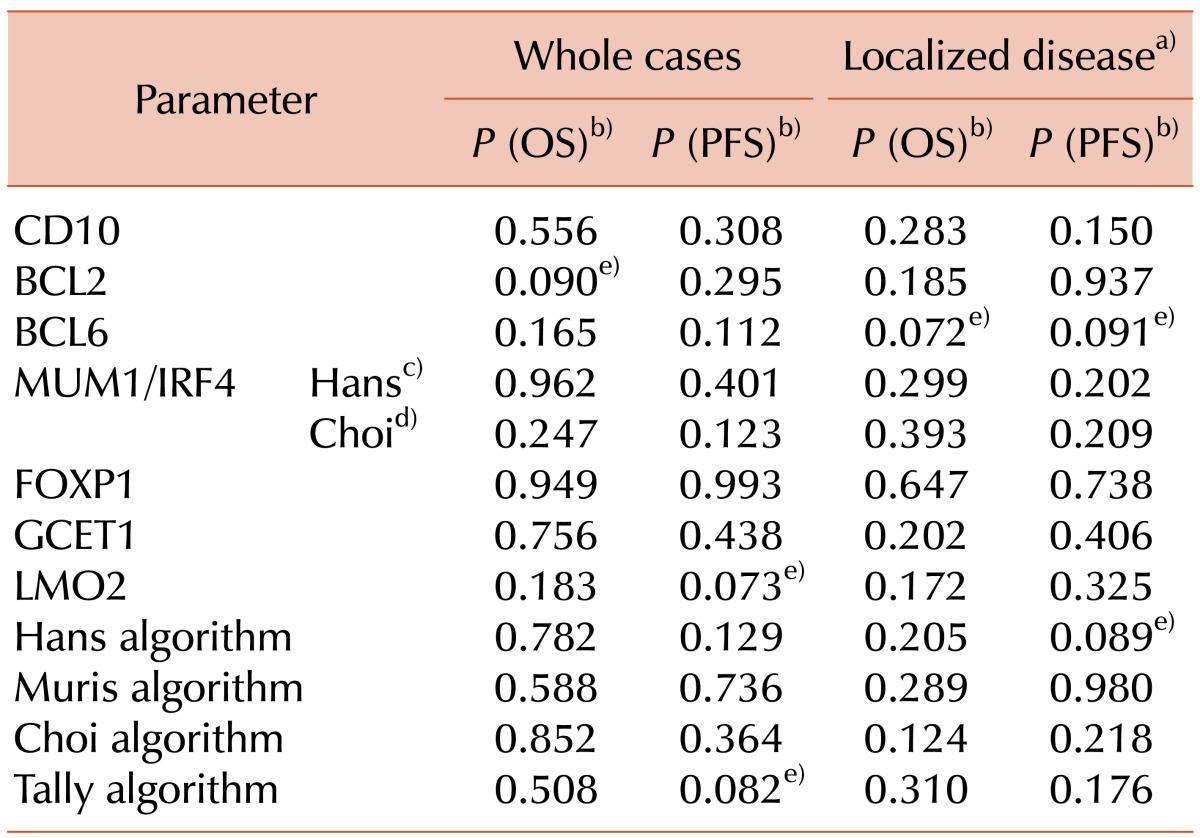
a)From Lugano stage I to IIE. b)Log-rank test. c)Results according to the criterion for Hans algorithm (nuclear staining ≥30%). d)Results according to the criterion for Choi algorithm (nuclear staining ≥80%). e)Borderline significant parameter (P<0.1).
Abbreviations: OS, overall survival; PFS, progression-free survival.
DISCUSSION
The categorization of DLBCL based on molecular subtype was proposed several years ago and is an attractive approach for both clinicians and researchers, as it is a reproducibly predictive factor for patient survival, and also a key determinant in the choice of chemotherapeutic regimen, for example, those including bortezomib [22, 23]. However, the clinical application of molecular subtype classification is limited because GEP is time-consuming, expensive, and involves a complex analysis. As described earlier, the proposed methods using immunohistochemistry can generally predict molecular subtype. However, its utility in predicting patient survival remains uncertain.
The cross-tabulation analyses against the immunohistochemical algorithms revealed that there was substantial concordance among those proposed by Hans, Choi, and Tally but was relatively weak between these and the Muris algorithm. Detailed comparisons revealed that the discordant cases between the 3 algorithms occurred either as a result of additional, new immunohistochemical markers (GCET1, FOXP1, and LMO2) or of differences in the way that a positive score is defined (MUM1/IRF4). Despite this, the high concordance rate among the 3 algorithms indirectly suggests that they may delineate the same subgroup of DLBCL. Although we have not performed GEP to confirm this, previous researches reproducibly demonstrated a significant correlation between molecular and immunohistochemical subtypes [11, 13, 15].
The limitations of our study are its retrospective character, small sample size, higher proportion of cases with low-stage disease (Lugano stage I or II1, 67.6%), relatively localized disease (Ann Arbor stage I or II, 82.4%), low IPI score (low-risk group, 70.6%) and better patient survival than in other studies (5-year survival rate, 88.6%). Despite the concordant results obtained using the different algorithms, our findings fail to demonstrate any prognostic value for immunohistochemical subtypes, in agreement with the previous study [18]. Taken together, these limitations might have obscured the potential prognostic value of the immunohistochemical subtypes. Further evaluation of other DLBCL cases, such as those not involving surgery or in which the cancer has been spread to the lymph nodes, is needed to better establish the prognostic value of immunohistochemical subtypes. Furthermore, the addition of novel germinal center or post-germinal center markers may further improve the prognostic value of the algorithms.
Previous studies have demonstrated that the expression of some immunohistochemical markers, mainly BCL2, BCL6, and LMO2, is associated with patient survival. Specifically, BCL6 and/or LMO2 expression is associated with a good prognosis, while BCL2 expression is associated with a poor prognosis [24-29]. Our data demonstrated that these markers have a marginal but not significant association with prognosis. As with the immunohistochemical subtypes, this discrepancy may be attributable to the relatively high proportion of cases in this study involving low-stage disease, a high survival rate, and surgical resection. Their prognostic value might be better evaluated if more resected cases were included.
We also found that the invasion depth of the tumor is significantly associated with patient survival. In fact, the invasion depth in gastrointestinal lymphoma has not generally been considered important because most patients with gastrointestinal DLBCL have initially undergone chemotherapy rather than surgery. Thus, the relationship between this factor and patient prognosis has not previously been reported. However, a recent multicenter study found that primary surgical resection was associated with a favorable prognosis in cases of intestinal DLBCL [30], encouraging the use of surgical resection as the primary treatment after diagnosis. If primary surgery for gastrointestinal DLBCL becomes more widely used, the evaluation of invasion depth in resected specimens might become more useful for predicting patient survival.
Footnotes
This study was partly supported by a grant (no. 2011-090) from the Asan Institute for Life Sciences, Seoul, Korea.
No potential conflicts of interest relevant to this article were reported.
References
- 1.Stein H, Warnke RA, Chan WC, et al. Diffuse large B-cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC; 2008. pp. 233–237. [Google Scholar]
- 2.Armitage JO, Fyfe MA, Lewis J. Long-term remission durability and functional status of patients treated for diffuse histiocytic lymphoma with the CHOP regimen. J Clin Oncol. 1984;2:898–902. doi: 10.1200/JCO.1984.2.8.898. [DOI] [PubMed] [Google Scholar]
- 3.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 4.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 6.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 7.Lossos IS, Morgensztern D. Prognostic biomarkers in diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:995–1007. doi: 10.1200/JCO.2005.02.4786. [DOI] [PubMed] [Google Scholar]
- 8.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 9.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 10.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 12.Muris JJ, Meijer CJ, Vos W, et al. Immunohistochemical profiling based on Bcl-2, CD10 and MUM1 expression improves risk stratification in patients with primary nodal diffuse large B cell lymphoma. J Pathol. 2006;208:714–723. doi: 10.1002/path.1924. [DOI] [PubMed] [Google Scholar]
- 13.Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15:5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, Banham AH, Leppa S. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod Pathol. 2009;22:1094–1101. doi: 10.1038/modpathol.2009.73. [DOI] [PubMed] [Google Scholar]
- 15.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29:200–207. doi: 10.1200/JCO.2010.30.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuis J, Gaulard P, Hemery F, et al. Respective prognostic values of germinal center phenotype and early (18)fluorodeoxyglucose-positron emission tomography scanning in previously untreated patients with diffuse large B-cell lymphoma. Haematologica. 2007;92:778–783. doi: 10.3324/haematol.10895. [DOI] [PubMed] [Google Scholar]
- 17.Veelken H, Vik Dannheim S, Schulte Moenting J, Martens UM, Finke J, Schmitt-Graeff A. Immunophenotype as prognostic factor for diffuse large B-cell lymphoma in patients undergoing clinical risk-adapted therapy. Ann Oncol. 2007;18:931–939. doi: 10.1093/annonc/mdm012. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011;117:4836–4843. doi: 10.1182/blood-2010-12-322362. [DOI] [PubMed] [Google Scholar]
- 19.Rayman N, Lam KH, van der Holt B, et al. Prognostic relevance of immunohistochemical subclassification of diffuse large B-cell lymphoma in two prospective phase III clinical trials. Clin Lymphoma Myeloma Leuk. 2011;11:23–32. doi: 10.3816/CLML.2011.n.003. [DOI] [PubMed] [Google Scholar]
- 20.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging handbook. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 21.Cheson BD. New response criteria for lymphomas in clinical trials. Ann Oncol. 2008;19(Suppl 4):iv35–iv38. doi: 10.1093/annonc/mdn191. [DOI] [PubMed] [Google Scholar]
- 22.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan J, Martin P, Furman RR, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29:690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 24.Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 25.Chen YW, Hu XT, Liang AC, et al. High BCL6 expression predicts better prognosis, independent of BCL6 translocation status, translocation partner, or BCL6-deregulating mutations, in gastric lymphoma. Blood. 2006;108:2373–2383. doi: 10.1182/blood-2006-05-022517. [DOI] [PubMed] [Google Scholar]
- 26.Iqbal J, Neppalli VT, Wright G, et al. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:961–968. doi: 10.1200/JCO.2005.03.4264. [DOI] [PubMed] [Google Scholar]
- 27.Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006;107:4207–4213. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natkunam Y, Zhao S, Mason DY, et al. The oncoprotein LMO2 is expressed in normal germinal-center B cells and in human B-cell lymphomas. Blood. 2007;109:1636–1642. doi: 10.1182/blood-2006-08-039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uccella S, Placidi C, Marchet S, et al. Bcl-6 protein expression, and not the germinal centre immunophenotype, predicts favourable prognosis in a series of primary nodal diffuse large B-cell lymphomas: a single centre experience. Leuk Lymphoma. 2008;49:1321–1328. doi: 10.1080/10428190802087447. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Kang HJ, Kim JS, et al. Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood. 2011;117:1958–1965. doi: 10.1182/blood-2010-06-288480. [DOI] [PubMed] [Google Scholar]